Dementia Prevalence in the CARhES Cohort: Importance of Socioeconomic Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Definition of Dementia Cases
2.2. Risk Factors for Dementia
2.3. Statistical Analysis
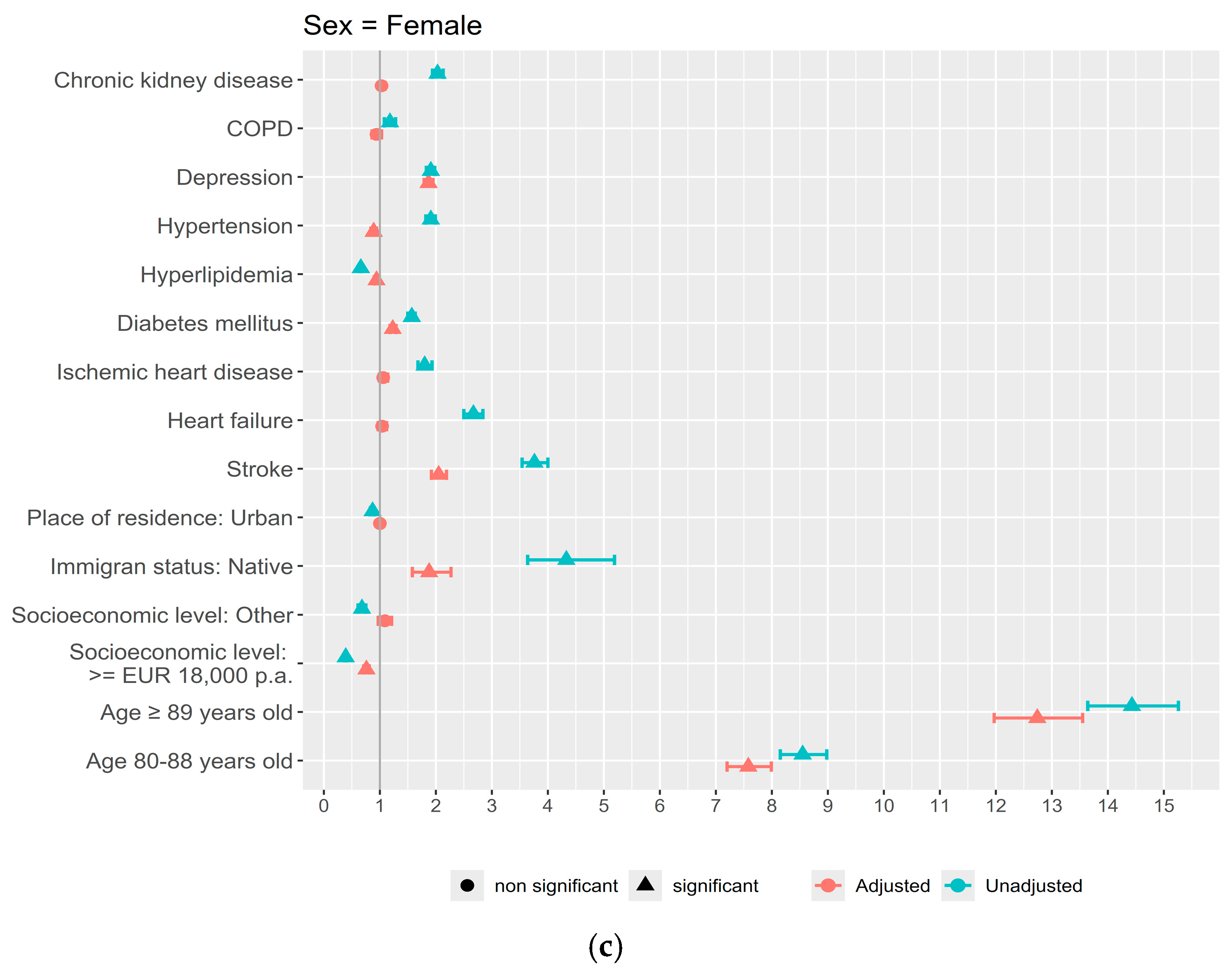
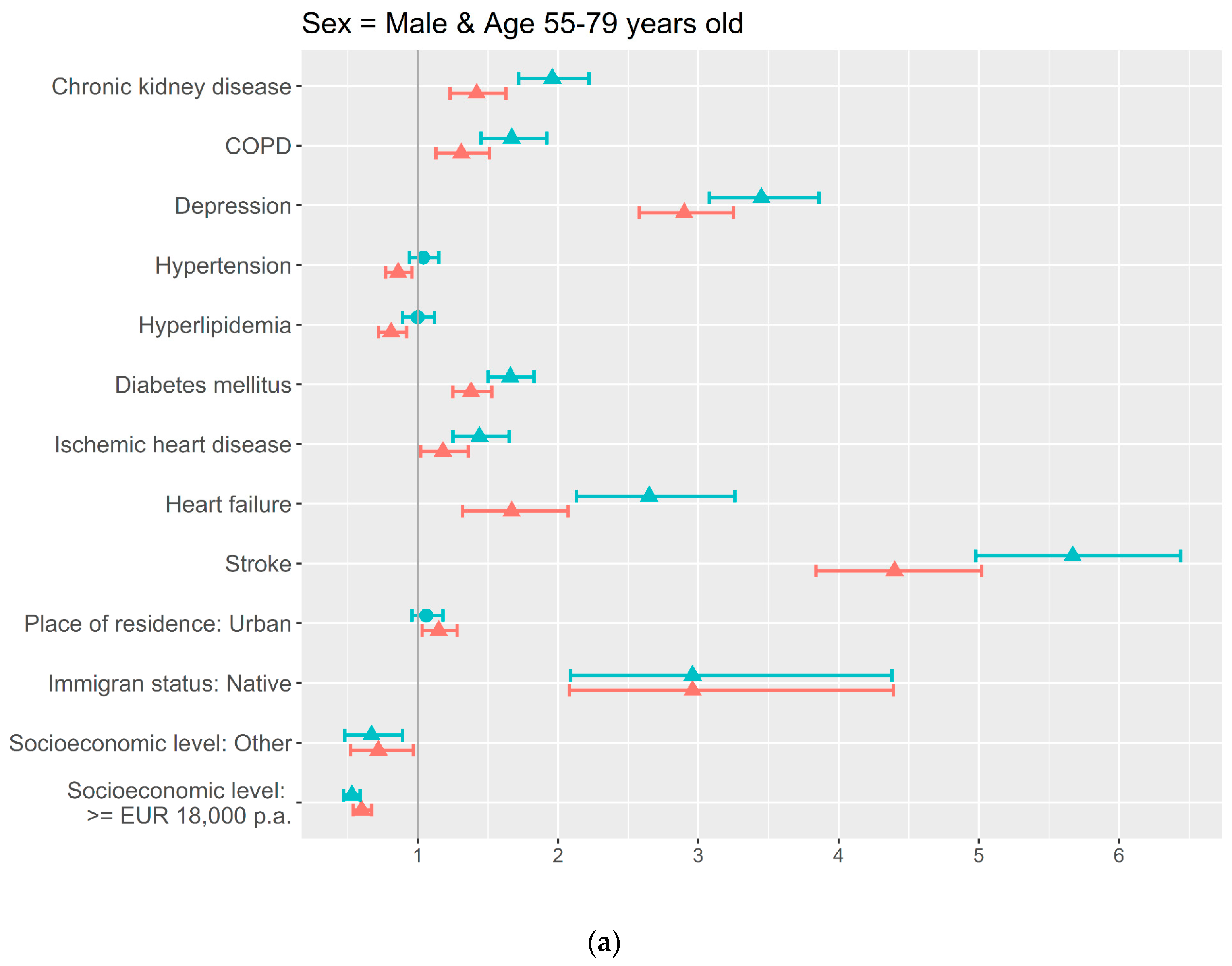
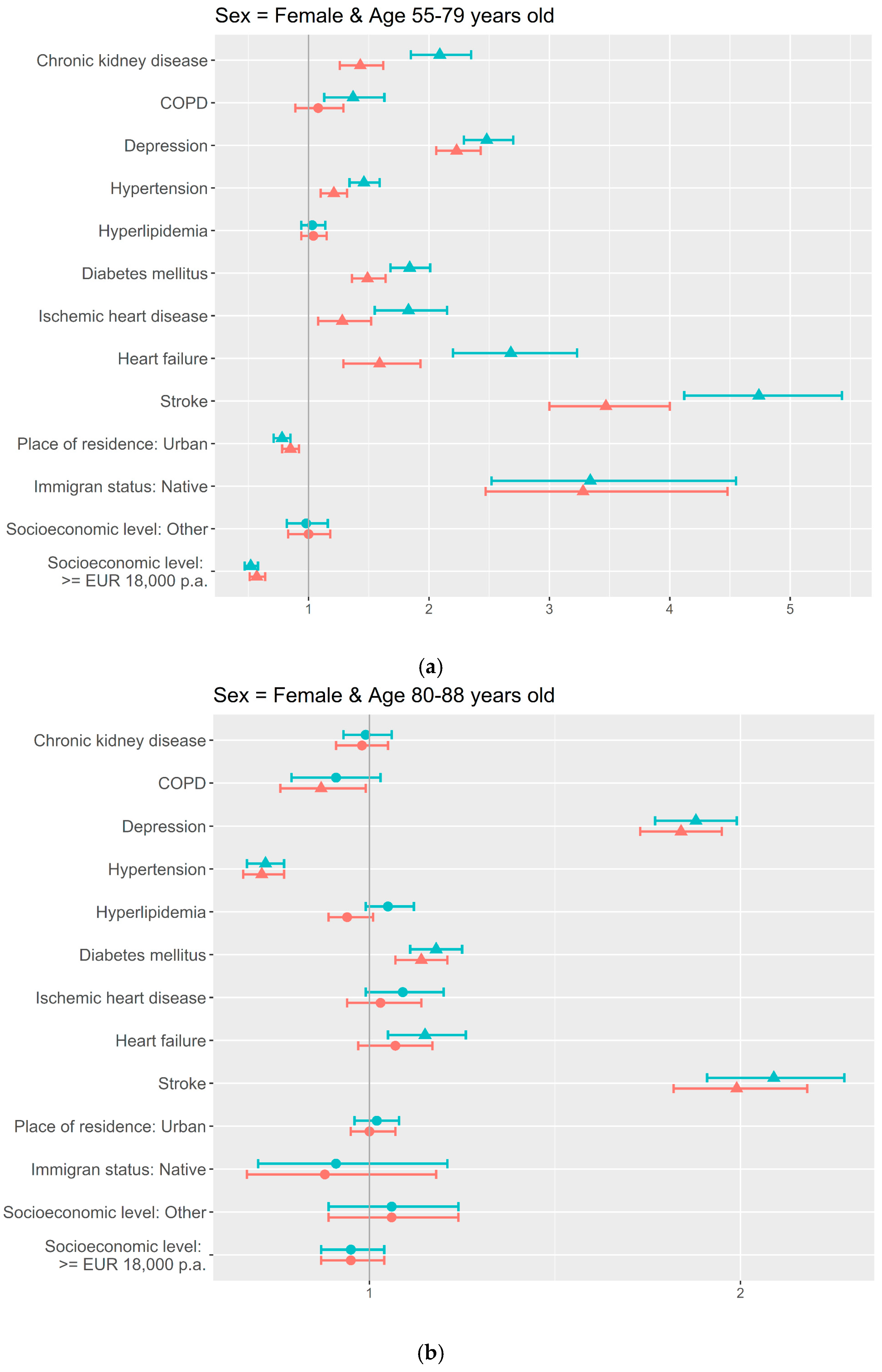
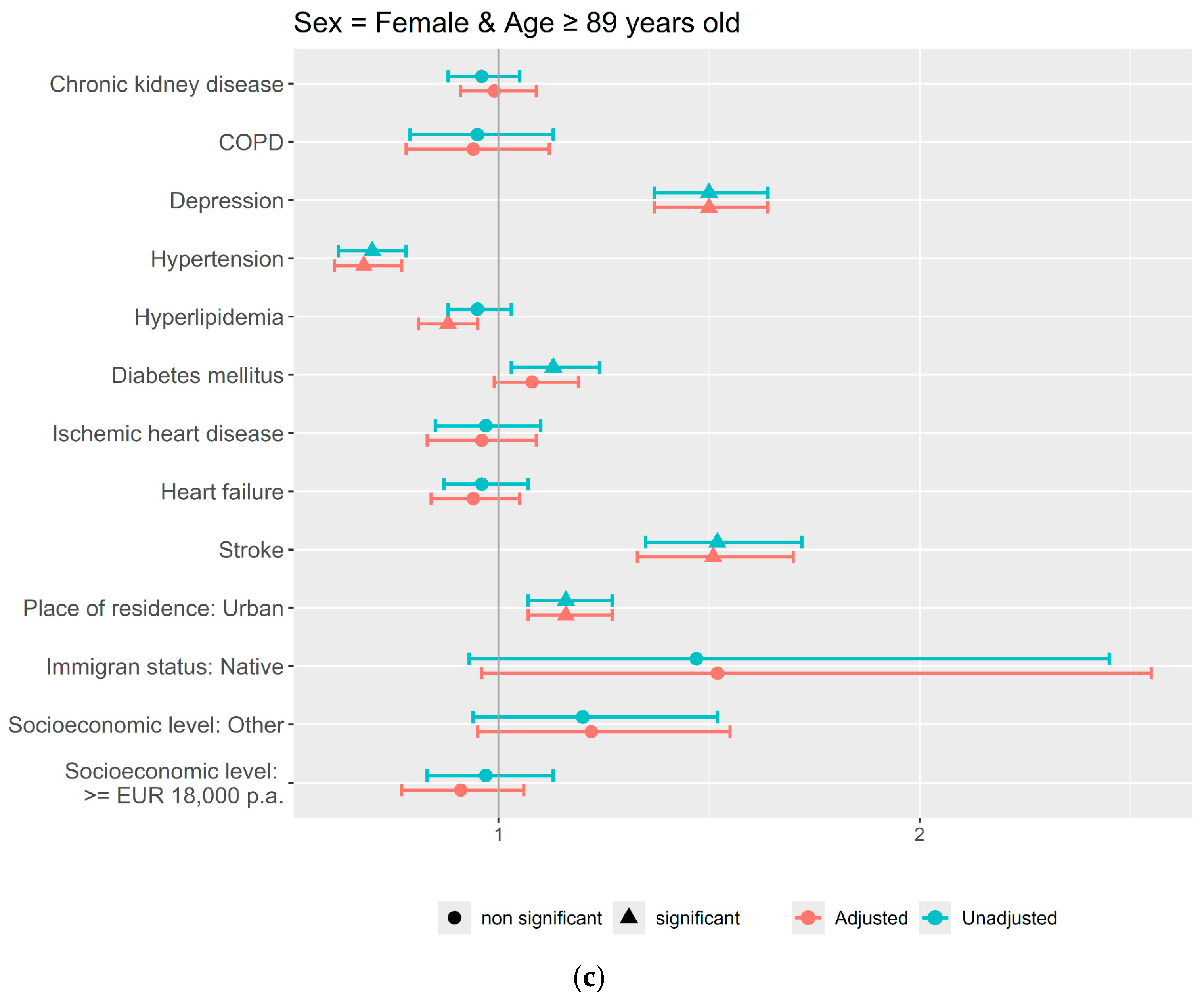
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CARhES | CArdiovascular Risk factors for HEalth Services research |
| CVD | Cardiovascular Disorder |
| BIGAN | Big Data Sanitario de Aragón |
| ICD | International Classification of Diseases |
| MBDSD | Minimum Basic Data Set Database |
| ED | Emergency Database |
| CIAP | Primary Care Database |
| ATC | Anatomical Therapeutic Chemical |
References
- World Health Organization. Dementia. WHO Dementia Fact Sheets. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 25 February 2025).
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S.; on behalf of the American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Clinical Cardiology; and Council on Hypertension. Comprehensive Management of Cardiovascular Risk Factors for Adults with Type 2 Diabetes: A Scientific Statement from the American Heart Association. Circulation 2022, 145, 722–759. [Google Scholar] [CrossRef] [PubMed]
- González, H.M.; Tarraf, W.; Harrison, K.; Windham, B.G.; Tingle, J.; Alonso, A.; Griswold, M.; Heiss, G.; Knopman, D.; Mosley, T.H. Midlife cardiovascular health and 20-year cognitive decline: Atherosclerosis Risk in Communities Study results. Alzheimers Dement. 2018, 14, 579–589. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Goodwin, V.A.; Low, M.S.A.; Quinn, T.J.; Cockcroft, E.J.; Shepherd, V.; Evans, P.H.; Henderson, E.J.; Mahmood, F.; Ni Lochlainn, M.; Needham, C.; et al. Including older people in health and social care research: Best practice recommendations based on the INCLUDE framework. Age Ageing 2023, 52, afad082. [Google Scholar] [CrossRef] [PubMed]
- Van Dalen, J.W.; Brayne, C.; Crane, P.K.; Fratiglioni, L.; Larson, E.B.; Lobo, A.; Lobo, E.; Marcum, Z.A.; van Charante, E.P.M.; Qiu, C.; et al. Association of Systolic Blood Pressure With Dementia Risk and the Role of Age, U-Shaped Associations, and Mortality. JAMA Intern. Med. 2022, 182, 142–152. [Google Scholar] [CrossRef]
- Baranyi, G.; Buchanan, C.R.; Conole, E.L.S.; Backhouse, E.V.; Maniega, S.M.; Hernández, M.d.C.V.; Bastin, M.E.; Wardlaw, J.; Deary, I.J.; Cox, S.R.; et al. Life-course neighbourhood deprivation and brain structure in older adults: The Lothian Birth Cohort 1936. Mol. Psychiatry 2024, 29, 3483–3494. [Google Scholar] [CrossRef] [PubMed]
- Hazan, J.; Liu, K.Y.; Isaacs, J.D.; Mukadam, N. Dementia diagnosis rates and the impact of ethnicity, rurality and deprivation. Aging Ment. Health 2025, 29, 138–144. [Google Scholar]
- Gobierno de Aragón. Datasets—Aragón Open Data. Instituto Aragonés de Estadística 2022. Available online: https://opendata.aragon.es/datos/catalogo/dataset/cifras-de-poblacion-revision-del-padron-municipal (accessed on 25 February 2025).
- Aguilar-Palacio, I.; Rabanaque, M.J.; Castel, S.; Maldonado, L.; González-García, J.; Compés, L.; Malo, S. Cohort Profile: The CArdiovascular Risk factors for hEalth Services research (CARhES) cohort study. Int. J. Epidemiol. 2024, 53, dyae015. [Google Scholar]
- Muggeo, V.M.R. Estimating regression models with unknown break-points. Stat Med. 2003, 22, 3055–3071. [Google Scholar] [CrossRef]
- Venkatasubramaniam, A.; Wolfson, J.; Mitchell, N.; Barnes, T.; Jaka, M.; French, S. Decision trees in epidemiological research. Emerg. Themes Epidemiol. 2017, 14, 11. [Google Scholar] [CrossRef]
- Ding, M.; Ek, S.; Aho, E.; Jönsson, L.; Schmidt-Mende, K.; Modig, K. Prevalence of dementia diagnosis in Sweden by geographical region and sociodemographic subgroups: A nationwide observational study. Lancet Reg. Health Eur. 2024, 45, 101029. [Google Scholar] [CrossRef] [PubMed]
- Wakutani, Y.; Kusumi, M.; Wada, K.; Kawashima, M.; Ishizaki, K.; Mori, M.; Mori, N.; Ijiri, T.; Adachi, Y.; Ashida, Y.; et al. Longitudinal changes in the prevalence of dementia in a Japanese rural area. Psychogeriatrics 2007, 7, 150–154. [Google Scholar] [CrossRef]
- Gan, J.; Zeng, Y.; Huang, G.; Wang, X.D.; Lü, Y.; Niu, J.; Meng, X.; Cai, P.; Li, X.; Li, Y.; et al. The updated prevalence and risk factors of dementia in old adults in China: A cross-sectional study. J. Alzheimer’s Dis. 2024, 102, 1209–1223. [Google Scholar] [CrossRef]
- Manly, J.J.; Jones, R.N.; Langa, K.M.; Ryan, L.H.; Levine, D.A.; McCammon, R.; Heeringa, S.G.; Weir, D. Estimating the Prevalence of Dementia and Mild Cognitive Impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022, 79, 1242–1249. [Google Scholar] [CrossRef]
- Poon, A.N.; Xiang, Y.; Zavalishina, Y.; Ayanian, S.; Aitken, C.F.; Procter, A.C.; Rudan, I.; Chan, K.Y. Systematic review estimating the burden of dementia in the WHO Southeast Asia Region using Bayesian and frequentist approaches. J. Glob. Health 2020, 10, 020701. [Google Scholar] [CrossRef]
- Bacigalupo, I.; Mayer, F.; Lacorte, E.; Di Pucchio, A.; Marzolini, F.; Canevelli, M.; Di Fiandra, T.; Vanacore, N. A Systematic Review and Meta-Analysis on the Prevalence of Dementia in Europe: Estimates from the Highest-Quality Studies Adopting the DSM IV Diagnostic Criteria. J. Alzheimer’s Dis. 2018, 66, 1471–1481. [Google Scholar] [CrossRef]
- de Hoyos-Alonso, M.C.; Bonis, J.; Tapias-Merino, E.; Castell, M.V.; Otero, A. Estimated prevalence of dementia based on analysis of drug databases in the Region of Madrid (Spain). Neurologia 2016, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Saz, P.; Marcos, G.; Dia, J.L.; De-la-Camara, C.; Ventura, T.; Montañes, J.A.; Lobo-Escolar, A.; Aznar, S.; Workgroup, T.Z. Prevalence of dementia in a southern European population in two different time periods: The ZARADEMP Project. Acta Psychiatr. Scand. 2007, 116, 299–307. [Google Scholar] [CrossRef]
- Wu, Y.-T.; Beiser, A.S.; Breteler, M.M.B.; Fratiglioni, L.; Helmer, C.; Hendrie, H.C.; Honda, H.; Ikram, M.A.; Langa, K.M.; Lobo, A.; et al. The changing prevalence and incidence of dementia over time—Current evidence. Nat. Rev. Neurol. 2017, 13, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, A.A.; Wei, L.; Ma, T.; Man, K.K.C.; Lau, W.C.Y.; Brauer, R.; Almetwazi, M.; Howard, R.; Wong, I.C. Prevalence and Incidence of Dementia in People with Diabetes Mellitus. J. Alzheimers Dis. 2020, 75, 607–615. [Google Scholar] [CrossRef]
- Lopez-de-Andres, A.; Jimenez-Garcia, R.; Zamorano-Leon, J.J.; Omaña-Palanco, R.; Carabantes-Alarcon, D.; Hernández-Barrera, V.; De Miguel-Diez, J.; Cuadrado-Corrales, N. Prevalence of Dementia among Patients Hospitalized with Type 2 Diabetes Mellitus in Spain, 2011–2020: Sex-Related Disparities and Impact of the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2023, 20, 4923. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.M.; Schmeidler, J. The protected survivor model: Using resistant successful cognitive aging to identify protection in the very old. Med. Hypotheses 2018, 110, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wood Alexander, M.; Paterson, J.; Arvanitakis, Z.; Black, S.E.; Casaletto, K.B.; Christakis, M.K.; Einstein, G.; Galea, L.A.M.; Harwood, L.; Kirkham, A.; et al. Cardiovascular contributions to dementia: Examining sex differences and female-specific factors. Alzheimer’s Dement. 2025, 21, e70610. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Aggarwal, N.T.; Vila-Castelar, C.; Agarwal, P.; Arenaza-Urquijo, E.M.; Brett, B.; Brugulat-Serrat, A.; DuBose, L.E.; Eikelboom, W.S.; Flatt, J.; et al. Consideration of sex and gender in Alzheimer’s disease and related disorders from a global perspective. Alzheimers Dement. 2022, 18, 2707–2724. [Google Scholar] [CrossRef]
- Gong, J.; Harris, K.; Peters, S.A.E.; Woodward, M. Sex differences in the association between major cardiovascular risk factors in midlife and dementia: A cohort study using data from the UK Biobank. BMC Med. 2021, 19, 110. [Google Scholar] [CrossRef]
- Galbete, A.; Cambra, K.; Forga, L.; Baquedano, F.J.; Aizpuru, F.; Lecea, O.; Librero, J.; Ibáñez, B. Achievement of cardiovascular risk factor targets according to sex and previous history of cardiovascular disease in type 2 diabetes: A population-based study. J. Diabetes Complicat. 2019, 33, 107445. [Google Scholar] [CrossRef]
- Manteuffel, M.; Williams, S.; Chen, W.; Verbrugge, R.R.; Pittman, D.G.; Steinkellner, A. Influence of Patient Sex and Gender on Medication Use, Adherence, and Prescribing Alignment with Guidelines. J. Women’s Health 2014, 23, 112–119. [Google Scholar] [CrossRef]
- Jung, H.H. Blood pressure control in patients aged above and below 75 years. PLoS ONE 2024, 19, e0297103. [Google Scholar] [CrossRef]
- Baumgartner, N.W.; Capuano, A.W.; Barnes, L.L.; Bennett, D.A.; Arvanitakis, Z. Sex differences in the association between age-related decline in blood pressure and decline in cognition: A prospective cohort study. medRxiv 2025. Preprint. Available online: https://pubmed.ncbi.nlm.nih.gov/39830253/ (accessed on 7 October 2025).
- Delgado, J.; Bowman, K.; Ble, A.; Masoli, J.; Han, Y.; Henley, W.; Welsh, S.; Kuchel, G.A.; Ferrucci, L.; Melzer, D. Blood Pressure Trajectories in the 20 Years Before Death. JAMA Intern. Med. 2018, 178, 93–99. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Capuano, A.W.; Lamar, M.; Shah, R.C.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology 2018, 91, e517–e525. [Google Scholar] [CrossRef]
- Maas, A.H.E.M.; Appelman, Y.E.A. Gender differences in coronary heart disease. Neth. Heart J. 2010, 18, 598. [Google Scholar] [CrossRef]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef]
- Dehlendorff, C.; Andersen, K.K.; Olsen, T.S. Sex Disparities in Stroke: Women Have More Severe Strokes but Better Survival Than Men. J. Am. Heart Assoc. 2015, 4, e001967. [Google Scholar] [CrossRef]
- Owais, S.B.; Bulwa, Z.B.; El Ammar, F. Differences in stroke clinical presentation among sexes. J. Stroke Cerebrovasc. Dis. 2024, 33, 107807. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.M.; Rodriguez, F.S. The role of social deprivation and depression in dementia risk: Findings from the longitudinal survey of health, ageing and retirement in Europe. Epidemiol. Psychiatr. Sci. 2023, 32, e10. [Google Scholar] [CrossRef] [PubMed]
- Letellier, N.; Ilango, S.D.; Mortamais, M.; Tzourio, C.; Gabelle, A.; Empana, J.P.; Samieri, C.; Berr, C.; Benmarhnia, T. Socioeconomic inequalities in dementia risk among a French population-based cohort: Quantifying the role of cardiovascular health and vascular events. Eur. J. Epidemiol. 2021, 36, 1015. [Google Scholar] [CrossRef]
- Wang, K.; Fang, Y.; Zheng, R.; Zhao, X.; Wang, S.; Lu, J.; Wang, W.; Ning, G.; Xu, Y.; Bi, Y. Associations of socioeconomic status and healthy lifestyle with incident dementia and cognitive decline: Two prospective cohort studies. eClinicalMedicine 2024, 76, 102831. [Google Scholar] [CrossRef]
- Deckers, K.; Cadar, D.; Van Boxtel, M.P.J.; Verhey, F.R.J.; Steptoe, A.; Köhler, S. Modifiable Risk Factors Explain Socioeconomic Inequalities in Dementia Risk: Evidence from a Population-Based Prospective Cohort Study. J. Alzheimer’s Dis. 2019, 71, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Adkins-Jackson, P.B.; George, K.M.; Besser, L.M.; Hyun, J.; Lamar, M.; Hill-Jarrett, T.G.; Bubu, O.M.; Flatt, J.D.; Heyn, P.C.; Cicero, E.C.; et al. The structural and social determinants of Alzheimer’s disease related dementias. Alzheimer’s Dement. 2023, 19, 3171–3185. [Google Scholar] [CrossRef]
- Ponjoan, A.; Garre-Olmo, J.; Blanch, J.; Fages, E.; Alves-Cabratosa, L.; Martí-Lluch, R.; Comas-Cufí, M.; Parramon, D.; Garcia-Gil, M.; Ramos, R. Epidemiology of dementia: Prevalence and incidence estimates using validated electronic health records from primary care. Clin. Epidemiol. 2019, 11, 217–228. [Google Scholar] [CrossRef]
- Perera, G.; Pedersen, L.; Ansel, D.; Alexander, M.; Arrighi, H.M.; Avillach, P.; Foskett, N.; Gini, R.; Gordon, M.F.; Gungabissoon, U.; et al. Dementia prevalence and incidence in a federation of European Electronic Health Record databases: The European Medical Informatics Framework resource. Alzheimers Dement. 2018, 14, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Bertola, L.; Suemoto, C.K.; Aliberti, M.J.R.; Gomes Gonçalves, N.; Pinho PJde, M.R.; Castro-Costa, E.; Lima-Costa, M.F.; Ferri, C.P. Prevalence of Dementia and Cognitive Impairment No Dementia in a Large and Diverse Nationally Representative Sample: The ELSI-Brazil Study. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 1060–1068. [Google Scholar] [CrossRef] [PubMed]



| Population N = 323,973 | Individuals without Dementia N = 307,194 (94.8%) | Individuals with Dementia N = 16,779 (5.2%) | p-Value * | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Age | 71.6 | 10.6 | 70.9 | 10.3 | 83.5 | 7.3 | <0.001 | |
| N | % | N | % | N | % | |||
| 55–79 years old | 238,193 | 73.5 | 234,060 | 76.2 | 4133 | 24.6 | <0.001 | |
| 80–89 years old | 65,434 | 20.2 | 56,923 | 18.5 | 8511 | 50.7 | ||
| ≥90 years old | 20,346 | 6.3 | 16,211 | 5.3 | 4135 | 24.6 | ||
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | <0.001 | |
| Sex | Women | 175,496 | 54.2 | 163,797 | 53.3 | 11,699 | 69.7 | <0.001 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | <0.001 | |
| Socioeconomic level | <EUR 18,000 | 215,030 | 66.4 | 201,187 | 65.5 | 13,843 | 82.5 | <0.001 |
| ≥EUR 18,000 | 97,664 | 30.2 | 95,223 | 31.0 | 2441 | 14.6 | ||
| Other | 11,271 | 3.5 | 10,777 | 3.5 | 494 | 2.9 | ||
| Missing | 8 | 0 | 7 | 0 | 1 | 0.0 | ||
| Immigrant status | Native | 310,451 | 95.8 | 293,857 | 95.7 | 16,594 | 98.9 | <0.001 |
| Missing | 8 | 0 | 7 | 0 | 1 | 0.0 | ||
| Place of residence | Urban | 226,427 | 69.9 | 215,031 | 70.0 | 11,396 | 67.9 | <0.001 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Stroke | 15,312 | 4.7 | 12,950 | 4.2 | 2362 | 14.1 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Heart failure | 14,183 | 4.4 | 12,492 | 4.1 | 1691 | 10.1 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Ischemic heart disease | 27,464 | 8.5 | 25,639 | 8.4 | 1825 | 10.9 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Depression | 54,653 | 16.9 | 49,459 | 16.1 | 5194 | 31 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| COPD | 23,317 | 7.2 | 21,978 | 7.2 | 1339 | 8.0 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Chronic kidney disease | 38,905 | 12.0 | 35,476 | 11.6 | 3429 | 20.4 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Cirrhosis | 8877 | 2.7 | 8589 | 2.8 | 288 | 1.7 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Osteoporosis | 48,648 | 15.0 | 45,051 | 14.7 | 3597 | 21.4 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Arthritis | 8971 | 2.8 | 8526 | 2.8 | 445 | 2.7 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Obesity | 50,722 | 15.7 | 48,782 | 15.9 | 1940 | 11.6 | <0.001 | |
| Missing | 1043 | 0.3 | 861 | 0.3 | 182 | 1.1 | ||
| Hyperlipidemia | 237,929 | 73.4 | 226,911 | 73.9 | 11,018 | 65.7 | <0.001 | |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Hypertension | 215,378 | 66.5 | 202,571 | 65.9 | 12,807 | 76.3 | <0.001 | |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Diabetes | 79,214 | 24.5 | 74,091 | 24.1 | 5123 | 30.5 | <0.001 | |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo, E.; Malo, S.; Aguilar-Palacio, I.; Castel-Feced, S.; Maldonado, L.; De la Cámara, C.; Rabanaque, M.J. Dementia Prevalence in the CARhES Cohort: Importance of Socioeconomic Level. J. Clin. Med. 2025, 14, 7375. https://doi.org/10.3390/jcm14207375
Lobo E, Malo S, Aguilar-Palacio I, Castel-Feced S, Maldonado L, De la Cámara C, Rabanaque MJ. Dementia Prevalence in the CARhES Cohort: Importance of Socioeconomic Level. Journal of Clinical Medicine. 2025; 14(20):7375. https://doi.org/10.3390/jcm14207375
Chicago/Turabian StyleLobo, Elena, Sara Malo, Isabel Aguilar-Palacio, Sara Castel-Feced, Lina Maldonado, Concepción De la Cámara, and María José Rabanaque. 2025. "Dementia Prevalence in the CARhES Cohort: Importance of Socioeconomic Level" Journal of Clinical Medicine 14, no. 20: 7375. https://doi.org/10.3390/jcm14207375
APA StyleLobo, E., Malo, S., Aguilar-Palacio, I., Castel-Feced, S., Maldonado, L., De la Cámara, C., & Rabanaque, M. J. (2025). Dementia Prevalence in the CARhES Cohort: Importance of Socioeconomic Level. Journal of Clinical Medicine, 14(20), 7375. https://doi.org/10.3390/jcm14207375








