Minimally Invasive Nephrectomy for the Management of Polycystic Kidney Disease: The Hilum-First Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.1.1. Positioning
2.1.2. Port Placement for Laparoscopy
- A 12 mm camera port placed in the periumbilical region on the side of the planned nephrectomy.
- A 12 mm port placed under the costal arch and another two or three 5 mm ports placed in the mid and lower flank on the side of the nephrectomy.
- Ports were inserted under vision, and pneumoperitoneum was maintained using CO2 to 15 mmHg.
2.1.3. The Hilum-First Technique
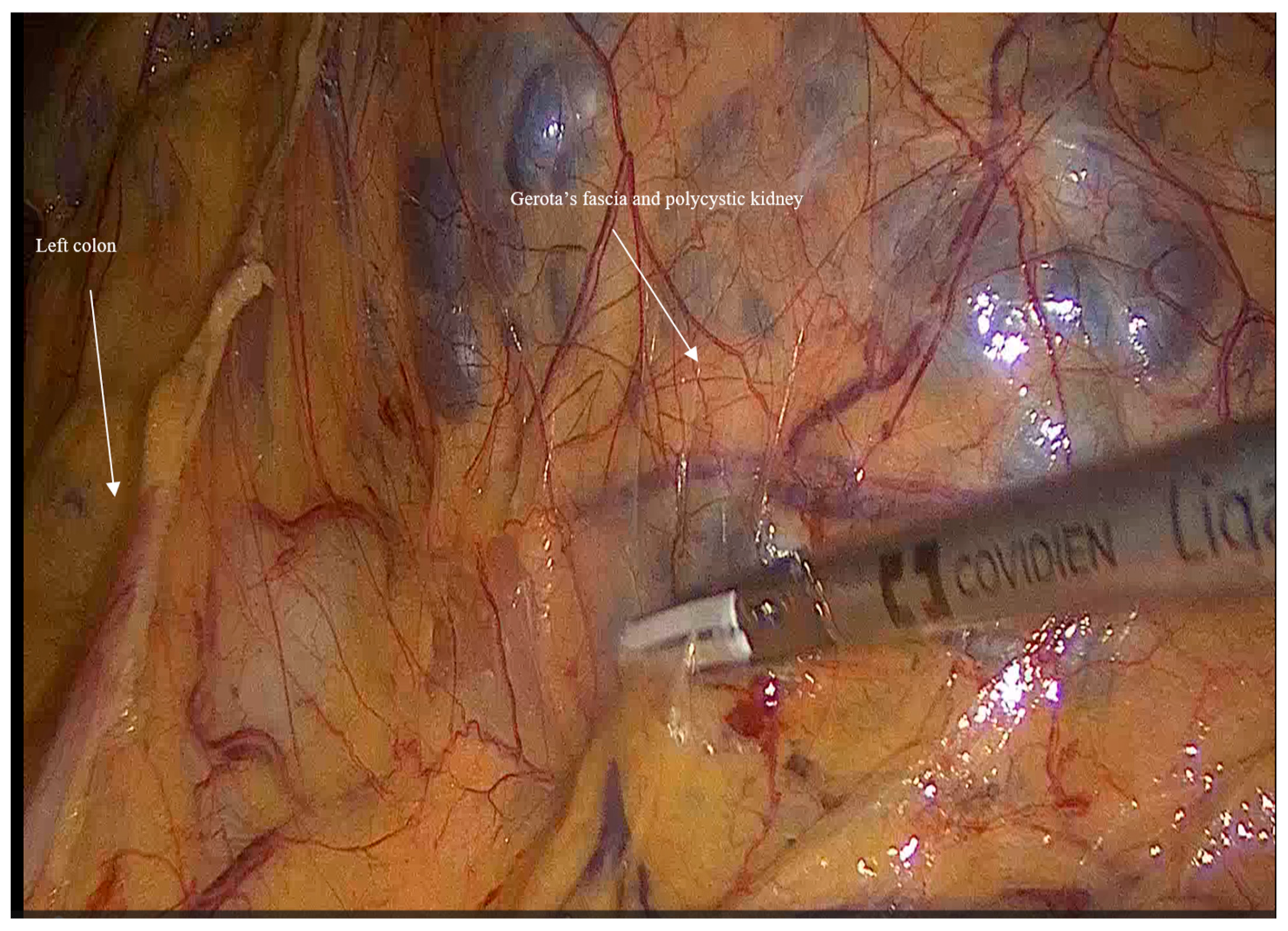
- Mobilization: Initially, the left colon was mobilized laterally, and Gerota’s fascia was identified. Careful dissection was performed to reveal the kidney Figure 1.
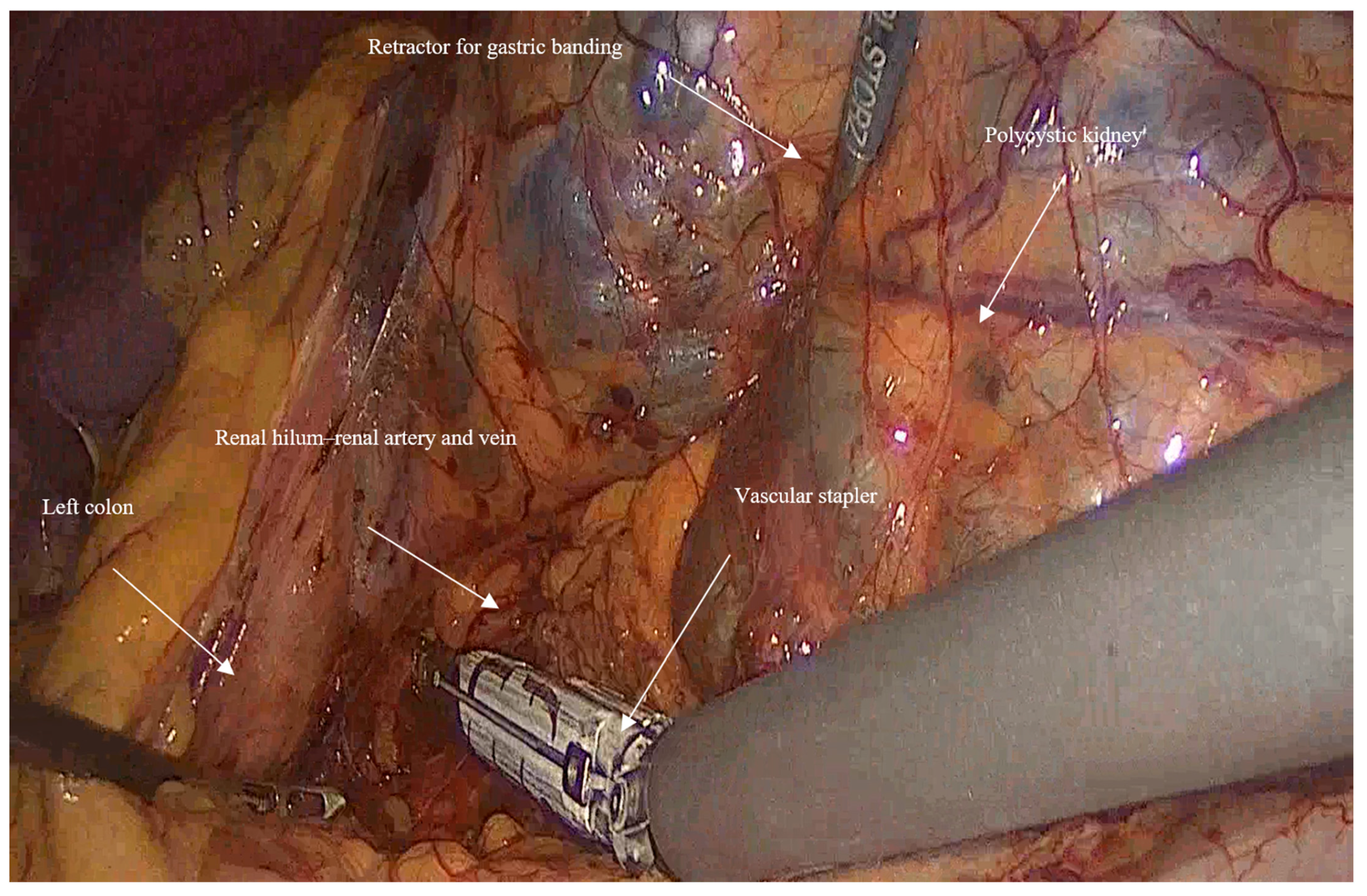
- Vascular control and ligation: The renal artery and vein were clamped together close to the hilum of the kidney using a vascular stapler (SigniaTM with Tri-StapleTM reinforced reloads, Medtronic, Minneapolis, MN, USA). Following that, a dissection of the rest of the inferior border of the kidney is completed, and then the ureter is ligated using Hem-o-lock™ clips (Weck, Research Triangle, NC, USA; Figure 2).
2.1.4. Enucleation of the Kidney
2.1.5. Extraction Technique
2.1.6. Closure
2.2. Challenges and Tips
- In huge kidney with previous infections where controlled aspiration may be hazardous, a sufficient retraction with a retractor for Gastric Banding (Karl Storz 30,623 GB), which has a 0-to-90-degree angle of its tip and can provide an efficient retraction and elevation of the huge kidney from the psoas muscle and assist in gaining access to the hilum of the kidney (Figure 3).
- When performing a right nephrectomy, the release of the lateral ligaments of the right liver is important and should be performed early in the surgery to facilitate a good exposure to the vena cava.
- For bilateral nephrectomies, a robot-assisted procedure may be better in terms of fewer incisions since it can be done using 4 robotic incisions in the midline (two above and two below the umbilicus)
3. Patient Information, Clinical Findings & Diagnostic Assessment
4. Therapeutic Intervention & Outcome
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helal, I.; Fick-Brosnahan, G.M.; Reed-Gitomer, B.; Schrier, R.W. Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012, 9, 293–300. [Google Scholar] [CrossRef]
- Rastogi, A.; Ameen, K.M.; Al-Baghdadi, M.; Al-Khader, A.A. Autosomal dominant polycystic kidney disease: Updated perspectives. Ther. Clin. Risk Manag. 2019, 15, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Grantham, J.J. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2008, 359, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.M. Surgical Management of Autosomal Dominant Polycystic Kidney Disease: Principles and Current Prac-tice. JNMA J. Nepal. Med. Assoc. 2023, 61, 485–491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gill, I.S.; Kavoussi, L.R.; Clayman, R.V.; Ehrlich, R.; Evans, R.; Fuchs, G.; Gersham, A.; Hulbert, J.C.; McDougall, E.M.; Rosenthal, T.; et al. Complications of laparoscopic nephrectomy in 185 patients: A multi-institutional review. J. Urol. 1995, 154, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.K.; Kapoor, A. Laparoscopic nephrectomy for massive polycystic kidney disease: Updated technique and outcomes. Can. Urol. Assoc. J. 2014, 8, 341–345. [Google Scholar] [CrossRef]
- Collini, A.; Benigni, R.; Ruggieri, G.; Carmellini, P.M. Laparoscopic nephrectomy for massive kidneys in polycystic kidney disease. JSLS J. Soc. Laparosc. Robot. Surg. 2021, 25, e2020.00107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elashry, O.M.; Nakada, S.Y.; Wolf, J.S., Jr.; McDougall, E.M.; Clayman, R.V. Laparoscopy for adult polycystic kidney disease: A promising alternative. Am. J. Kidney Dis. 1996, 27, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, F.R.; Iselin, C.E. Hand-assisted laparoscopic bilateral nephrectomy. Urology 2000, 56, 153. [Google Scholar] [CrossRef] [PubMed]
- Geertsema, P.; Gansevoort, R.T.; Arici, M.; Capasso, G.; Cornec-Le-Gall, E.; Furlano, M.; Fuster, D.G.; Galletti, F.; Gómez-Dos-Santos, V.; Perez-Gomez, M.V.; et al. Nephrectomy in autosomal dominant polycystic kidney disease: A consensus statement of the ERA Genes & Kidney Working Group. Nephrol. Dial. Transplant. 2025, 40, 1032–1054. [Google Scholar] [CrossRef]
- Guo, P.; Xu, W.; Li, H.; Ren, T.; Ni, S.; Ren, M. Laparoscopic nephrectomy versus open nephrectomy for patients with autosomal dominant polycystic kidney disease: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0129317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daugherty, M.; Bratslavsky, G. Surgical Techniques in the Management of Small Renal Masses. Urol. Clin. N. Am. 2017, 44, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.E.Z.; Olakkengil, K.S.; Bhattacharjya, S.; Olakkengil, S.A. Autosomal Dominant Polycystic Kidney Disease Patients Requiring Nephrectomy: Characteristics and Surgical Considerations. ANZ J. Surg. 2025, 95, 1605–1616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seshadri, P.A.; Poulin, E.C.; Pace, D.; Schlachta, C.M.; Cadeddu, M.O.; Mamazza, J. Transperitoneal laparoscopic nephrectomy for giant polycystic kidneys: A case control study. Urology 2001, 58, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Landman, J.; Andreoni, C.; McDougall, E.M.; Clayman, R.V. Laparoscopic bilateral hand assisted nephrectomy for autosomal dominant polycystic kidney disease: Initial experience. J. Urol. 2001, 166, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Benoit, T.; Peyronnet, B.; Roumiguié, M.; Verhoest, G.; Beauval, J.B.; Delreux, A.; Chauveau, D.; Malavaud, B.; Manunta, A.; Soulié, M.; et al. Laparoscopic nephrectomy for polycystic kidney: Comparison of the transperitoneal and retroperitoneal approaches. World J. Urol. 2016, 34, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Bignante, G.; Orsini, A.; Lasorsa, F.; Lambertini, L.; Pacini, M.; Amparore, D.; Pandolfo, S.D.; Del Giudice, F.; Zaytoun, O.; Buscarini, M.; et al. Robotic-assisted surgery for the treatment of urologic cancers: Recent advances. Expert Rev. Med. Devices 2024, 21, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Masterson, J.M.; Zhao, H.; Taich, L.; Naser-Tavakolian, A.; Johnson, H.; Najjar, R.; Kim, I.K.; Gupta, A. Robotic bilateral nephrectomy for large polycystic kidney disease. BJUI Compass 2023, 4, 701–708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurung, P.M.S.; Frye, T.P.; Rashid, H.H.; Joseph, J.V.; Wu, G. Robot-assisted synchronous bilateral nephrectomy for autosomal dominant polycystic kidney disease: A stepwise description of technique. Urology 2021, 153, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-M.; Chuang, Y.-W.; Yu, M.-C.; Chen, C.-H.; Yang, C.-K.; Huang, S.-T.; Lin, C.-L.; Shu, K.-H.; Kao, C.-H. Risk of cancer in patients with polycystic kidney disease: A propensity-score matched analysis of a nationwide, population-based cohort study. Lancet Oncol. 2016, 17, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Drake, A.M.; Paynter, J.A.; Yim, A.; Tempo, J.A.; Manning, T.G.; Brennan, J.; Qin, K.R. Prevalence of renal neoplasia in autosomal dominant polycystic kidney disease: Systematic review and meta-analysis. Nephron 2024, 148, 457–467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, S.D.; Lesani, O.A.; Zhao, L.C.; Johnston, W.K.; Wolf, J.S., Jr.; Clayman, R.V.; Nadler, R.B. A multi-institutional study on the safety and efficacy of specimen morcellation after laparoscopic radical nephrectomy for clinical stage T1 or T2 renal cell carcinoma. J. Endourol. 2009, 23, 1513–1518. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value (n = 16) |
|---|---|
| Sex (male:female) | 12:4 |
| Mean age (years) | 56.3 |
| Preoperative dialysis (n) | 8 |
| Preoperative kidney transplantation | 8 |
| Operative side (left:right:bilateral) | 12:3:1 |
| Mean preoperative hemoglobin levels | 12 |
| Previous abdominal surgery | 12 |
| Comorbidities HTN ESRD DM Dyslipidemia Gout AFib IHD OSA | 12 8 3 5 1 1 1 1 |
| Preoperative presentations Abdominal and flank pain Preparation for transplant Recurrent UTIs Hemorrhagic renal cyst, hematuria Recto-urethral fistula Shortness of breath | 8 7 6 4 1 1 |
| Parameter | Value (n = 16) |
|---|---|
| Mean duration of procedure (minutes) | 159.6 |
| Estimated blood loss | Minimal (up to 50 mL) |
| Blood transfusion (units) 0 1 | 14 2 |
| Mean kidney weight according to pathology reports (grams) | 1177.2 (364–2170) |
| Mean kidney volume according to pathology reports (cm3) | 2428.4 (570–4320) |
| Histopathology Malignancy (papillary RCC, T1NxMx) | 1 |
| Median duration of postoperative hospital stays (days) | 4 (3–10) |
| Mean postoperative hemoglobin levels (g/dL) | 11.2 |
| Postoperative complications during the in-hospital postoperative period No complications Thrombosis of AV fistula requiring radiological intervention Ileus Hyperkalemia requiring dialysis | 12 2 1 1 |
| 30-day readmission SSI Subcutaneous hematoma Pneumonia | 3 1 1 1 |
| Median time from nephrectomy to transplant (days) | 132 |
| Clavien–Dindo Grade | n |
|---|---|
| Grade I | 2 |
| Grade II | 2 |
| Grade IIIa | 2 |
| Grade IIIb | 0 |
| Grade IVa | 1 |
| Grade IVb | 0 |
| Grade V | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shweiki, A.; Khalayleh, H.; Rivin, M.; Shabaneh, S.; Khalaileh, A.; Imam, A. Minimally Invasive Nephrectomy for the Management of Polycystic Kidney Disease: The Hilum-First Technique. J. Clin. Med. 2025, 14, 7485. https://doi.org/10.3390/jcm14217485
Shweiki A, Khalayleh H, Rivin M, Shabaneh S, Khalaileh A, Imam A. Minimally Invasive Nephrectomy for the Management of Polycystic Kidney Disease: The Hilum-First Technique. Journal of Clinical Medicine. 2025; 14(21):7485. https://doi.org/10.3390/jcm14217485
Chicago/Turabian StyleShweiki, Amir, Harbi Khalayleh, Michael Rivin, Suha Shabaneh, Abed Khalaileh, and Ashraf Imam. 2025. "Minimally Invasive Nephrectomy for the Management of Polycystic Kidney Disease: The Hilum-First Technique" Journal of Clinical Medicine 14, no. 21: 7485. https://doi.org/10.3390/jcm14217485
APA StyleShweiki, A., Khalayleh, H., Rivin, M., Shabaneh, S., Khalaileh, A., & Imam, A. (2025). Minimally Invasive Nephrectomy for the Management of Polycystic Kidney Disease: The Hilum-First Technique. Journal of Clinical Medicine, 14(21), 7485. https://doi.org/10.3390/jcm14217485






