HMGB-1 Increases Proinflammatory Reaction via TLR4 in Human Granulosa Cells of Endometriosis
Abstract
1. Introduction
2. Materials and Methods
2.1. hGL5 Cell Culture
2.2. CCK-8 Assay
2.3. Apoptosis Assay
2.4. Western Blot
2.5. ELISA for Estradiol
2.6. qRT-PCR for HMGB-1 and TLR4
2.7. ELISA for IL-1β and IL-6
2.8. Statistical Analysis
3. Results
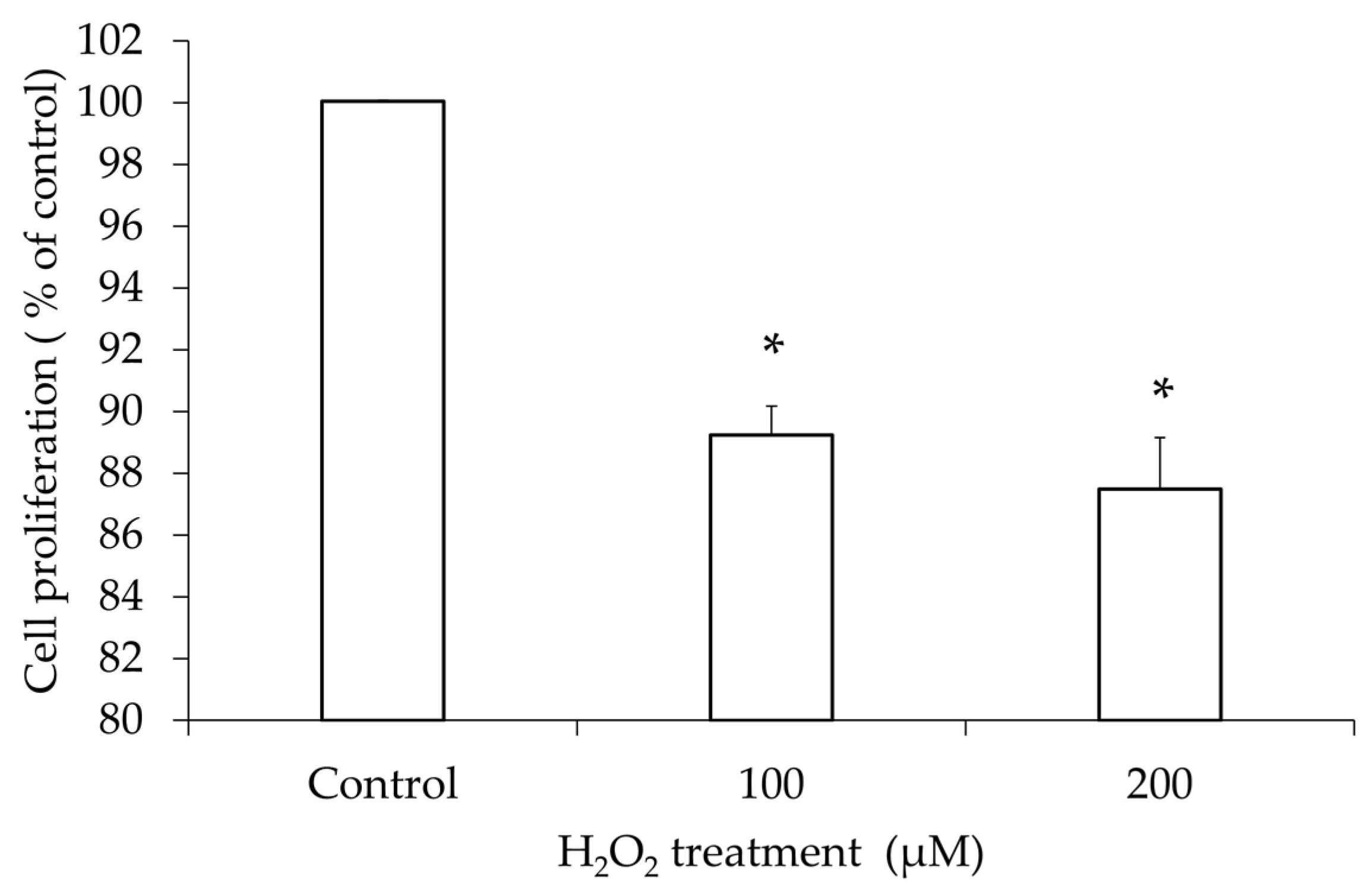
3.1. hGL5 Cell Proliferation After H2O2 Treatment
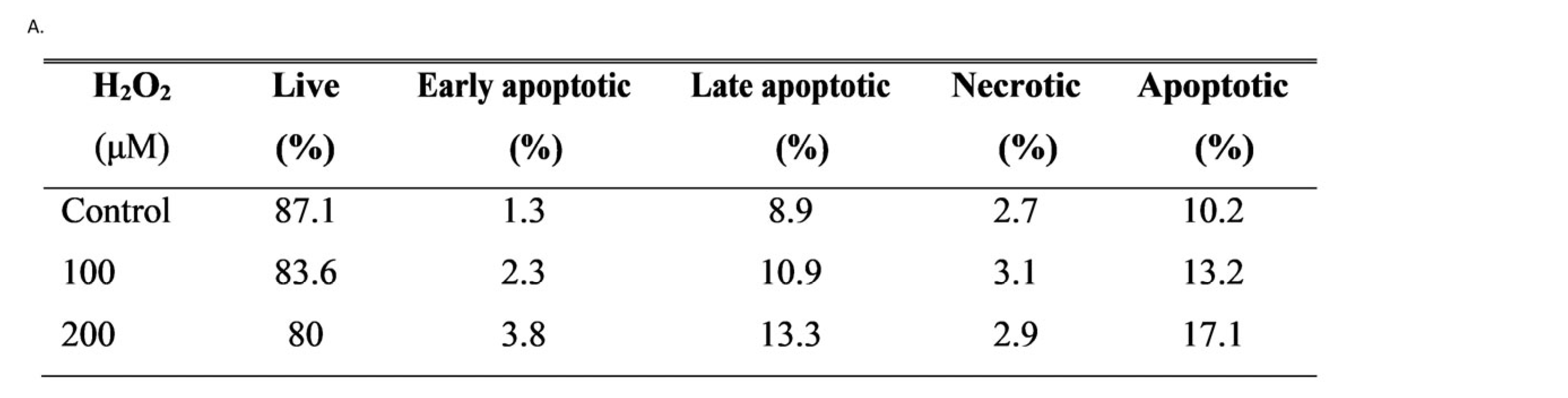
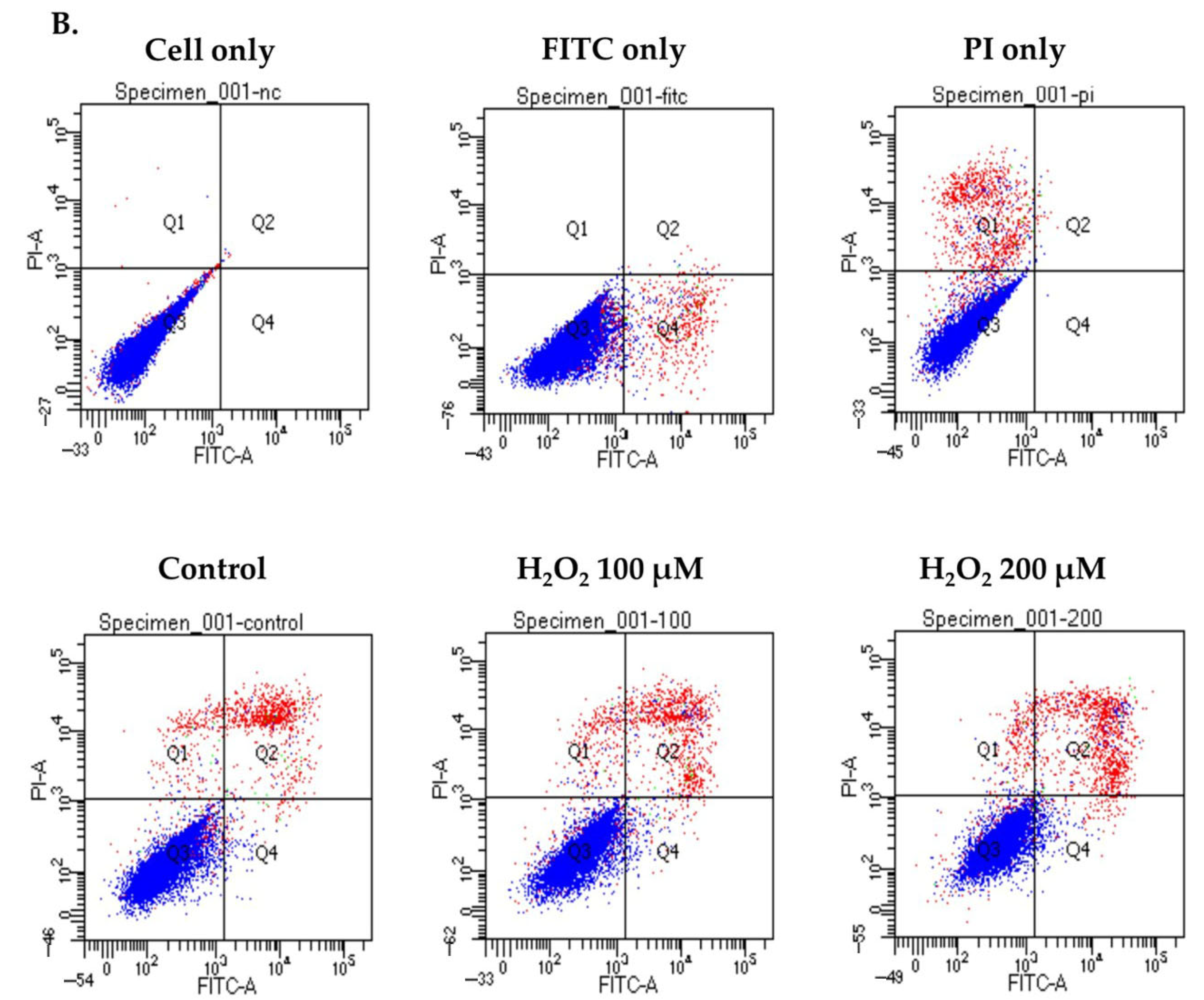
3.2. Apoptosis in hGL5 Cell After H2O2 Treatments
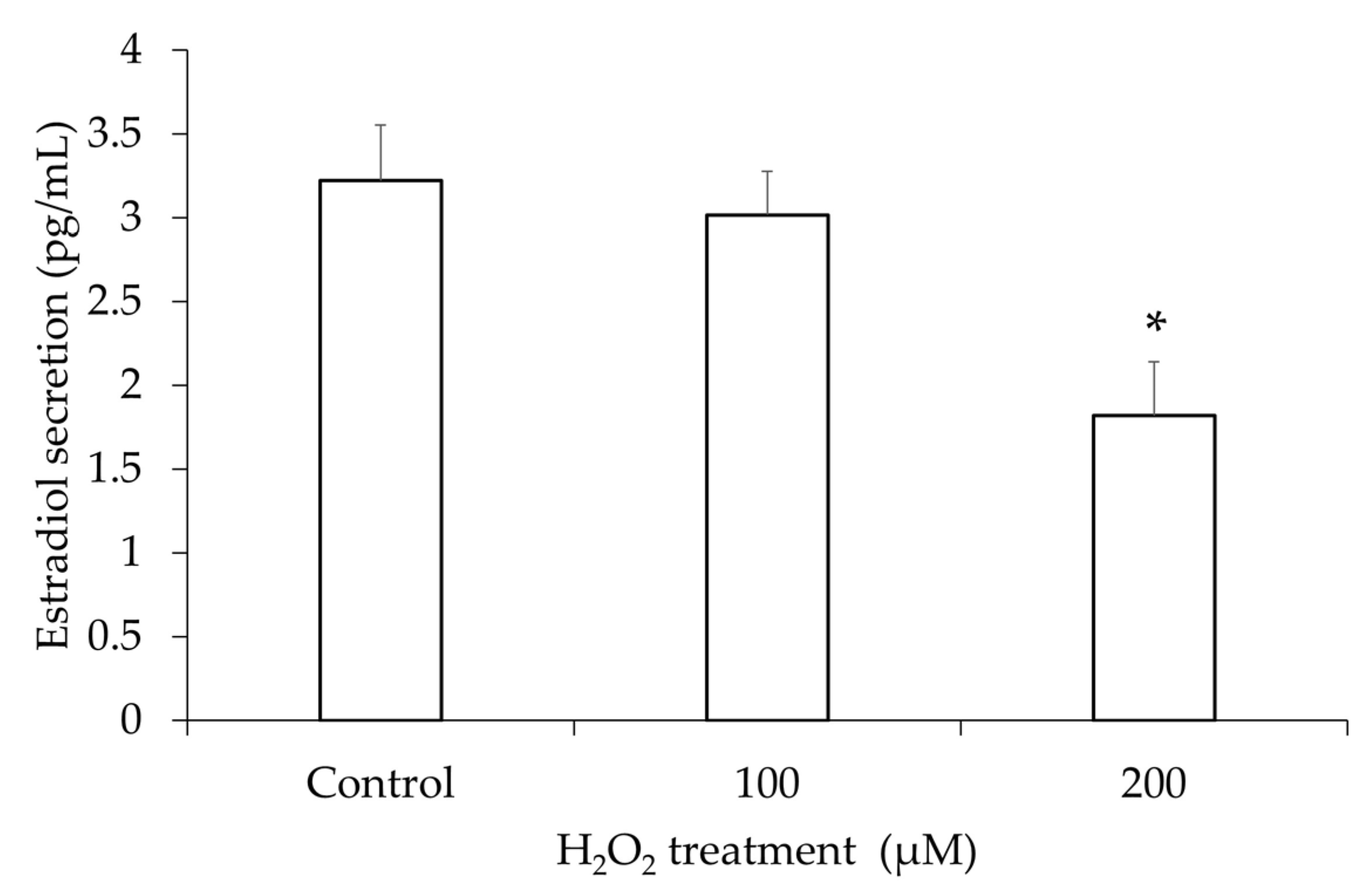
3.3. Estradiol Secretion in the hGL5 Cell Supernatants After H2O2 Treatment
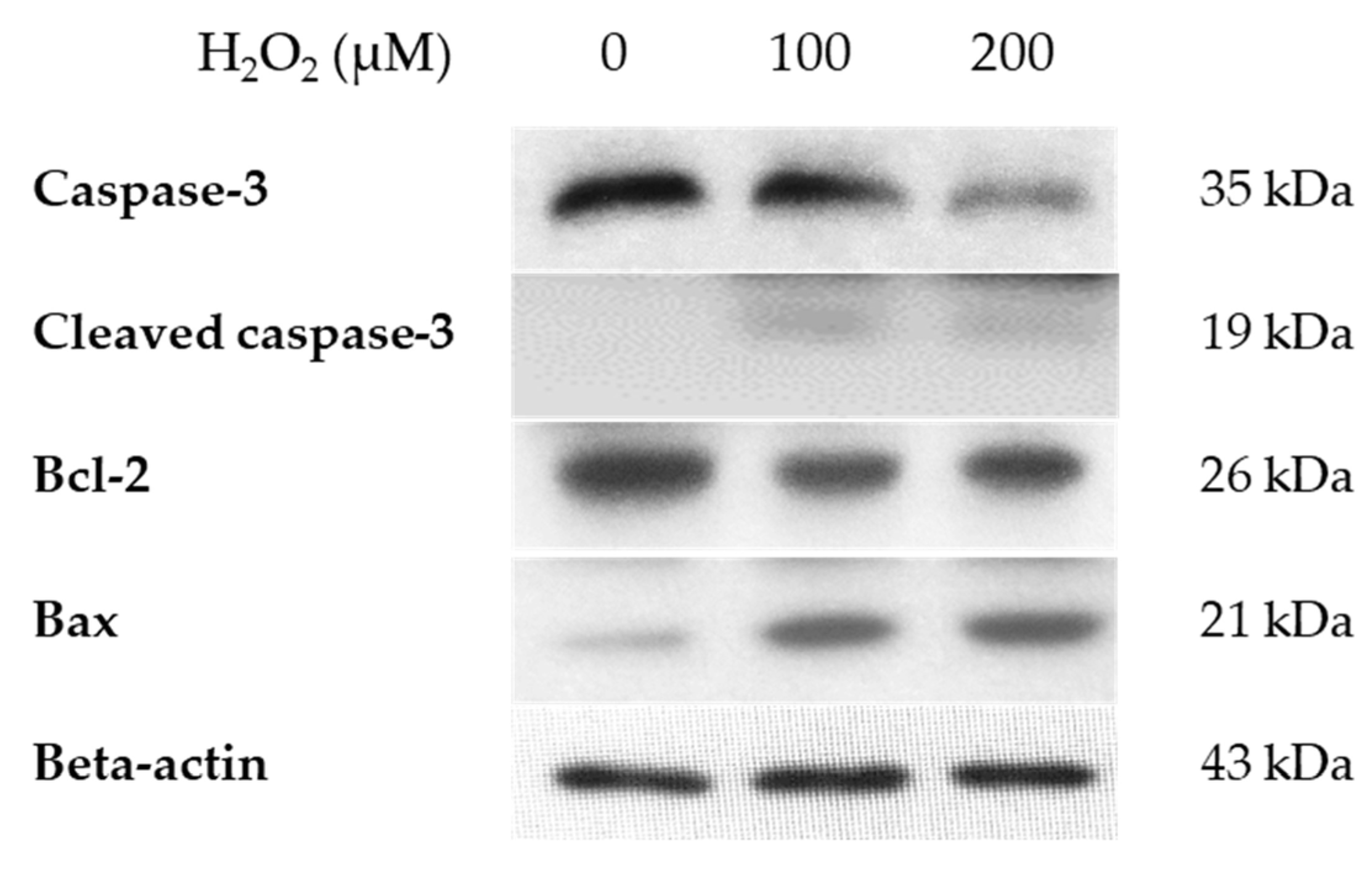
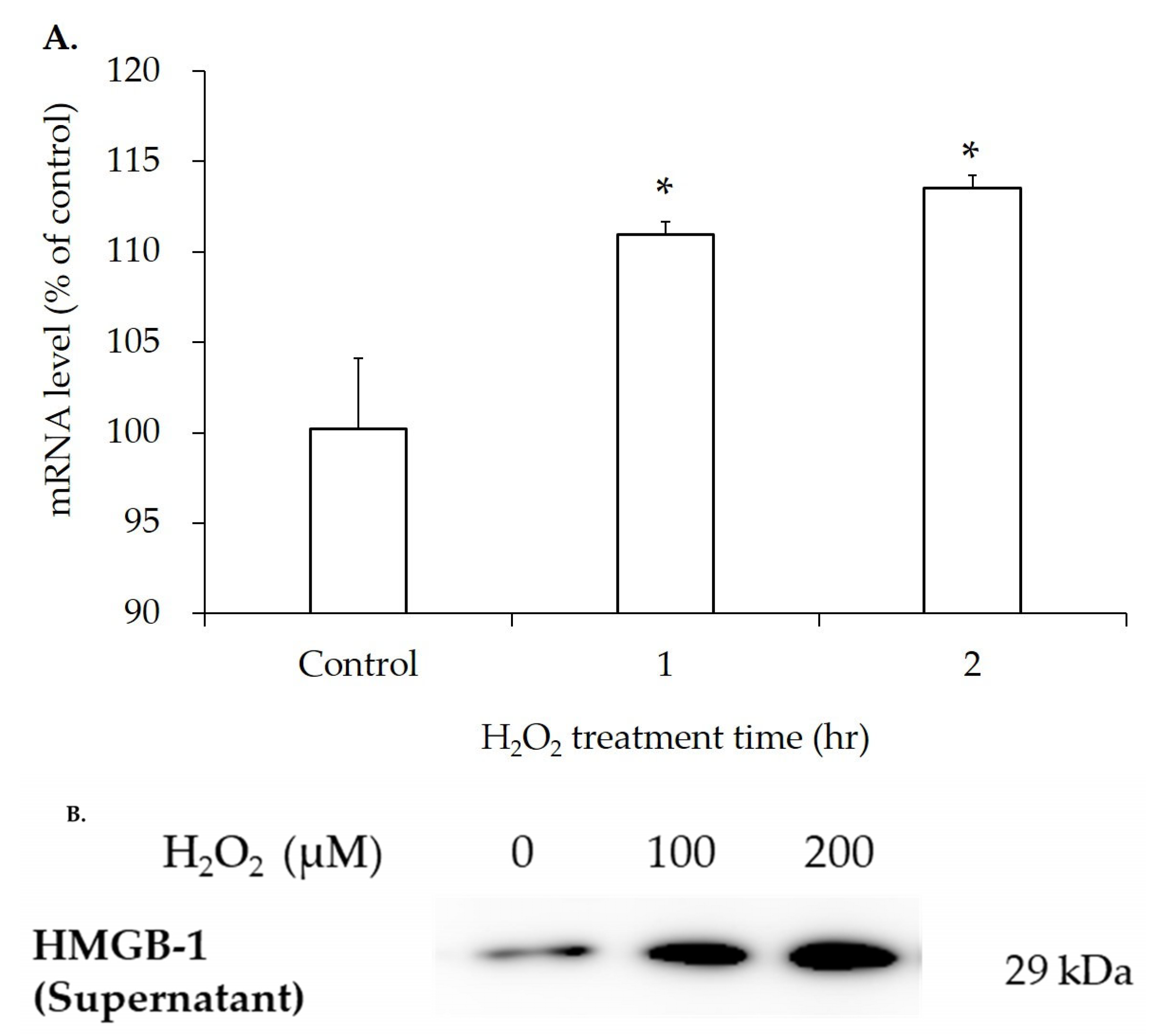
3.4. mRNA and Protein Expression of HMGB-1 After H2O2 Treatment
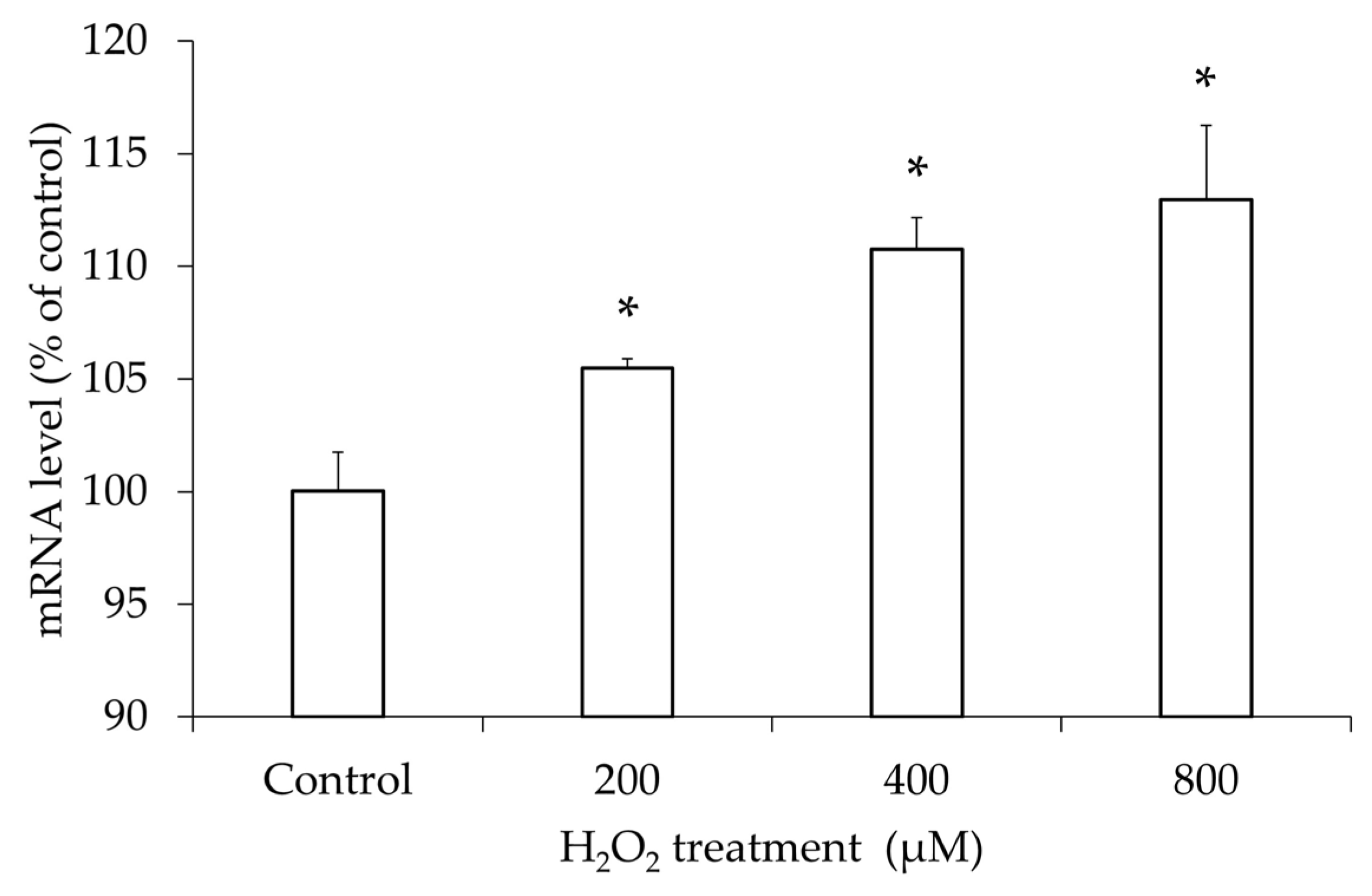
3.5. Increased TLR4 mRNA Expression in hGL5 After H2O2 Treatment
3.6. Activation of NF-κB Pathway After H2O2 Treatment
3.7. Increased Release of Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nguyen, X.L.; Huynh, Q.H.; Nguyen, P.N. Assessing the Clinical Characteristics and the Role of Imaging Modalities in Uterine Sarcoma: A Single-Center Retrospective Study From Vietnam. J. Clin. Ultrasound 2025, 53, 1527–1537. [Google Scholar] [CrossRef]
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Marcoux, S.; Maheux, R.; Berube, S. Laparoscopic surgery in infertile women with minimal or mild endometriosis. Canadian Collaborative Group on Endometriosis. N. Engl. J. Med. 1997, 337, 217–222. [Google Scholar] [CrossRef]
- Haney, A.F.; Jenkins, S.; Weinberg, J.B. The stimulus responsible for the peritoneal fluid inflammation observed in infertile women with endometriosis. Fertil. Steril. 1991, 56, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef]
- Da Broi, M.G.; de Albuquerque, F.O.; de Andrade, A.Z.; Cardoso, R.L.; Jordao Junior, A.A.; Navarro, P.A. Increased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016, 366, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Alberico, H.C.; Woods, D.C. Role of Granulosa Cells in the Aging Ovarian Landscape: A Focus on Mitochondrial and Metabolic Function. Front. Physiol. 2021, 12, 800739. [Google Scholar] [CrossRef]
- Casarini, L.; Reiter, E.; Simoni, M. beta-arrestins regulate gonadotropin receptor-mediated cell proliferation and apoptosis by controlling different FSHR or LHCGR intracellular signaling in the hGL5 cell line. Mol. Cell Endocrinol. 2016, 437, 11–21. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Jordao, A.A., Jr.; Ferriani, R.A.; Navarro, P.A. Oocyte oxidative DNA damage may be involved in minimal/mild endometriosis-related infertility. Mol. Reprod. Dev. 2018, 85, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, A.; Michalcova, K.; Roychoudhury, S.; Baldovska, S.; Tvrda, E.; Vasicek, J.; Chrenek, P.; Sanislo, L.; Kren, V. Antioxidative effect of dietary flavonoid isoquercitrin on human ovarian granulosa cells HGL5 in vitro. Physiol. Res. 2021, 70, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.H.; Chon, S.J.; Choi, Y.S.; Cho, S.; Lee, B.S.; Seo, S.K. Pathophysiology of Endometriosis: Role of High Mobility Group Box-1 and Toll-Like Receptor 4 Developing Inflammation in Endometrium. PLoS ONE 2016, 11, e0148165. [Google Scholar] [CrossRef]
- Bianchi, M.E.; Beltrame, M.; Paonessa, G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science 1989, 243 Pt 1, 1056–1059. [Google Scholar] [CrossRef]
- Kajihara, H.; Yamada, Y.; Kanayama, S.; Furukawa, N.; Noguchi, T.; Haruta, S.; Yoshida, S.; Sado, T.; Oi, H.; Kobayashi, H. New insights into the pathophysiology of endometriosis: From chronic inflammation to danger signal. Gynecol. Endocrinol. 2011, 27, 73–79. [Google Scholar] [CrossRef]
- Yun, B.H.; Kim, S.; Chon, S.J.; Kim, G.H.; Choi, Y.S.; Cho, S.; Lee, B.S.; Seo, S.K. High mobility group box-1 promotes inflammation in endometriotic stromal cells through Toll-like receptor 4/nuclear factor-kappa B. Am. J. Transl. Res. 2021, 13, 1400–1410. [Google Scholar]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef]
- Lu, B.; Wang, H.; Andersson, U.; Tracey, K.J. Regulation of HMGB1 release by inflammasomes. Protein Cell 2013, 4, 163–167. [Google Scholar] [CrossRef]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed. Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef]
- Yu, Y.; Tang, D.; Kang, R. Oxidative stress-mediated HMGB1 biology. Front. Physiol. 2015, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, X.; Lv, Y. HMGB1 Mediated Inflammation and Autophagy Contribute to Endometriosis. Front. Endocrinol. 2021, 12, 616696. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, R.; Rocco, J.; Rojas, C.; Sovino, H.; Poch, A.; Kohen, P.; Alvarado-Diaz, C.; Devoto, L. Physiologic activation of nuclear factor kappa-B in the endometrium during the menstrual cycle is altered in endometriosis patients. Fertil. Steril. 2012, 97, 645–651. [Google Scholar] [CrossRef]
- Uyar, A.; Torrealday, S.; Seli, E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil. Steril. 2013, 99, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Ting, A.Y.; Xu, J.; Stouffer, R.L. Differential effects of estrogen and progesterone on development of primate secondary follicles in a steroid-depleted milieu in vitro. Hum. Reprod. 2015, 30, 1907–1917. [Google Scholar] [CrossRef]
- Carpintero, N.L.; Suarez, O.A.; Mangas, C.C.; Varea, C.G.; Rioja, R.G. Follicular steroid hormones as markers of oocyte quality and oocyte development potential. J. Hum. Reprod. Sci. 2014, 7, 187–193. [Google Scholar] [CrossRef]
- Sreerangaraja Urs, D.B.; Wu, W.H.; Komrskova, K.; Postlerova, P.; Lin, Y.F.; Tzeng, C.R.; Kao, S.H. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int. J. Mol. Sci. 2020, 21, 3592. [Google Scholar] [CrossRef]
- Fang, F.; Jiang, D. IL-1beta/HMGB1 signalling promotes the inflammatory cytokines release via TLR signalling in human intervertebral disc cells. Biosci. Rep. 2016, 36, e00379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, H.; Chen, F.; Wang, Y.; Ma, J.; Wang, G. The HMGB1-RAGE/TLR-TNF-alpha signaling pathway may contribute to kidney injury induced by hypoxia. Exp. Ther. Med. 2019, 17, 17–26. [Google Scholar] [CrossRef]
- Malutan, A.M.; Drugan, T.; Ciortea, R.; Mocan-Hognogi, R.F.; Bucuri, C.; Rada, M.P.; Mihu, D. Serum anti-inflammatory cytokines for the evaluation of inflammatory status in endometriosis. J. Res. Med. Sci. 2015, 20, 668–674. [Google Scholar] [CrossRef]
- Malutan, A.M.; Drugan, T.; Costin, N.; Ciortea, R.; Bucuri, C.; Rada, M.P.; Mihu, D. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent. Eur. J. Immunol. 2015, 40, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Veillat, V.; Lavoie, C.H.; Metz, C.N.; Roger, T.; Labelle, Y.; Akoum, A. Involvement of nuclear factor-kappaB in macrophage migration inhibitory factor gene transcription up-regulation induced by interleukin- 1 beta in ectopic endometrial cells. Fertil. Steril. 2009, 91, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Francisco, A.M.C.; Patel, B.G.; Cline, J.M.; Zou, E.; Berga, S.L.; Taylor, R.N. IL-1beta Stimulates Brain-Derived Neurotrophic Factor Production in Eutopic Endometriosis Stromal Cell Cultures: A Model for Cytokine Regulation of Neuroangiogenesis. Am. J. Pathol. 2018, 188, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. IkappaB kinases: Key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef] [PubMed]

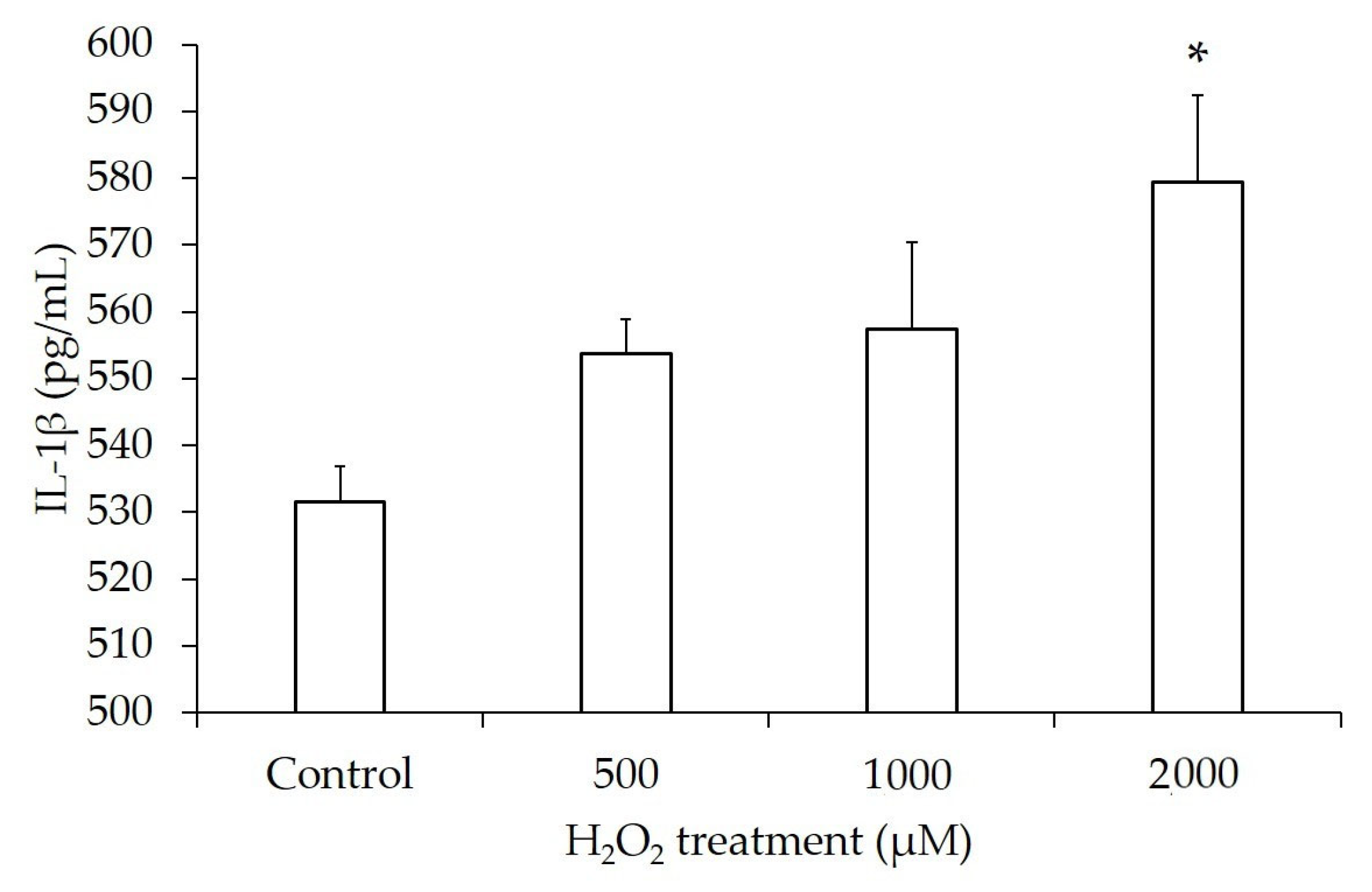
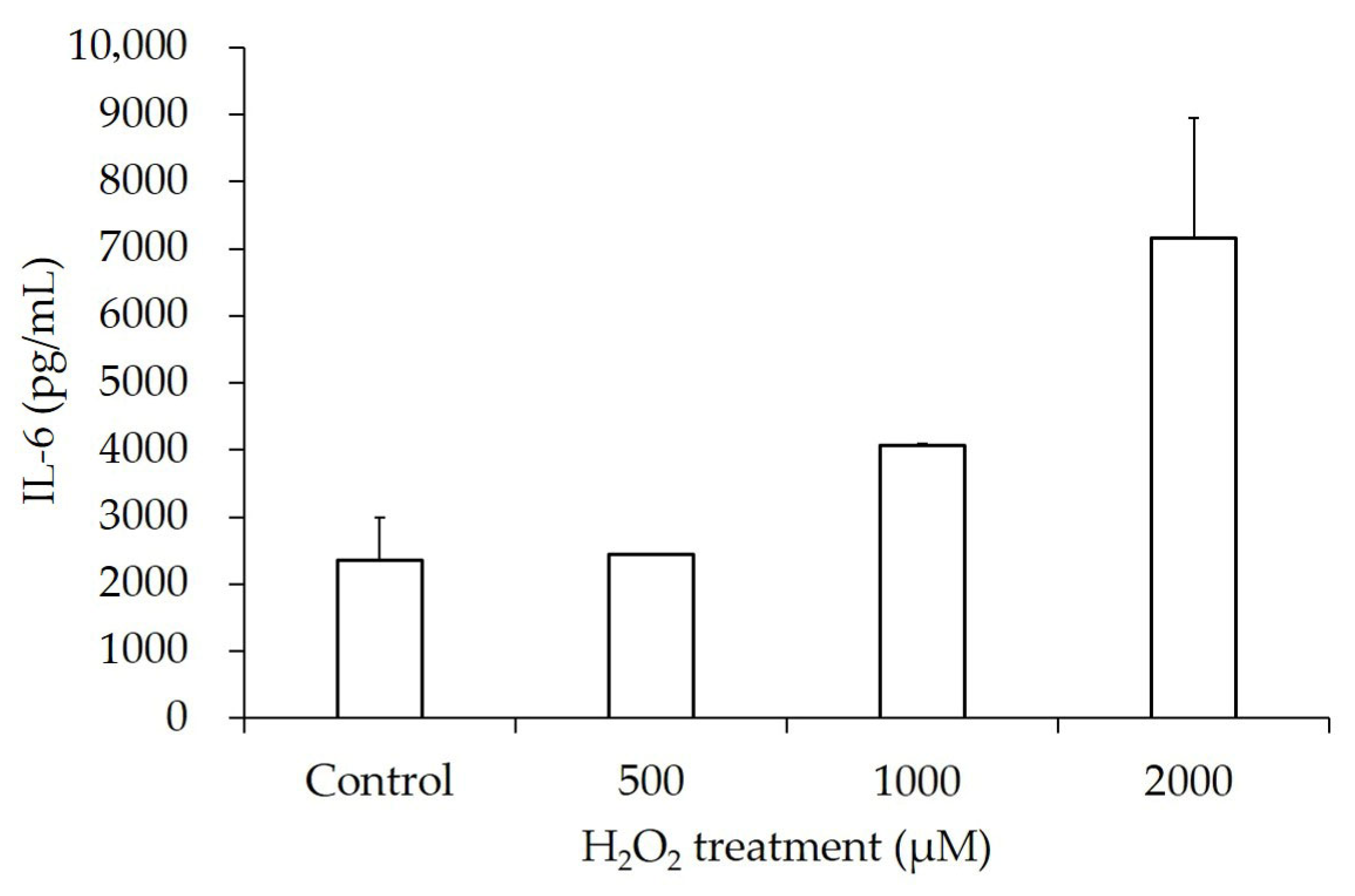
| H2O2 Concentration | IL-1β (pg/mL) | p-Value | IL-6 (pg/mL) | p-Value |
|---|---|---|---|---|
| Control | 531.62 ± 10.40 | 2355.05 ± 1270.04 | ||
| 500 µM | 553.68 ± 10.40 | 0.08 | 2445.90 ± 25.02 | 0.46 |
| 1000 µM | 557.35 ± 26.00 | 0.16 | 4075.57 ± 51.92 | 0.10 |
| 2000 µM | 579.41 ± 26.00 | <0.05 | 7163.67 ± 3570.18 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.I.; Kim, K.H.; Cho, S.; Choi, Y.S.; Lee, B.S.; Chon, S.J.; Yun, B.H. HMGB-1 Increases Proinflammatory Reaction via TLR4 in Human Granulosa Cells of Endometriosis. J. Clin. Med. 2025, 14, 7532. https://doi.org/10.3390/jcm14217532
Kim HI, Kim KH, Cho S, Choi YS, Lee BS, Chon SJ, Yun BH. HMGB-1 Increases Proinflammatory Reaction via TLR4 in Human Granulosa Cells of Endometriosis. Journal of Clinical Medicine. 2025; 14(21):7532. https://doi.org/10.3390/jcm14217532
Chicago/Turabian StyleKim, Hye In, Kyung Hee Kim, SiHyun Cho, Young Sik Choi, Byung Seok Lee, Seung Joo Chon, and Bo Hyon Yun. 2025. "HMGB-1 Increases Proinflammatory Reaction via TLR4 in Human Granulosa Cells of Endometriosis" Journal of Clinical Medicine 14, no. 21: 7532. https://doi.org/10.3390/jcm14217532
APA StyleKim, H. I., Kim, K. H., Cho, S., Choi, Y. S., Lee, B. S., Chon, S. J., & Yun, B. H. (2025). HMGB-1 Increases Proinflammatory Reaction via TLR4 in Human Granulosa Cells of Endometriosis. Journal of Clinical Medicine, 14(21), 7532. https://doi.org/10.3390/jcm14217532






