Can Wearable Device Promote Physical Activity and Reduce Pain in People with Chronic Musculoskeletal Conditions?
Abstract
1. Introduction
2. Methodology
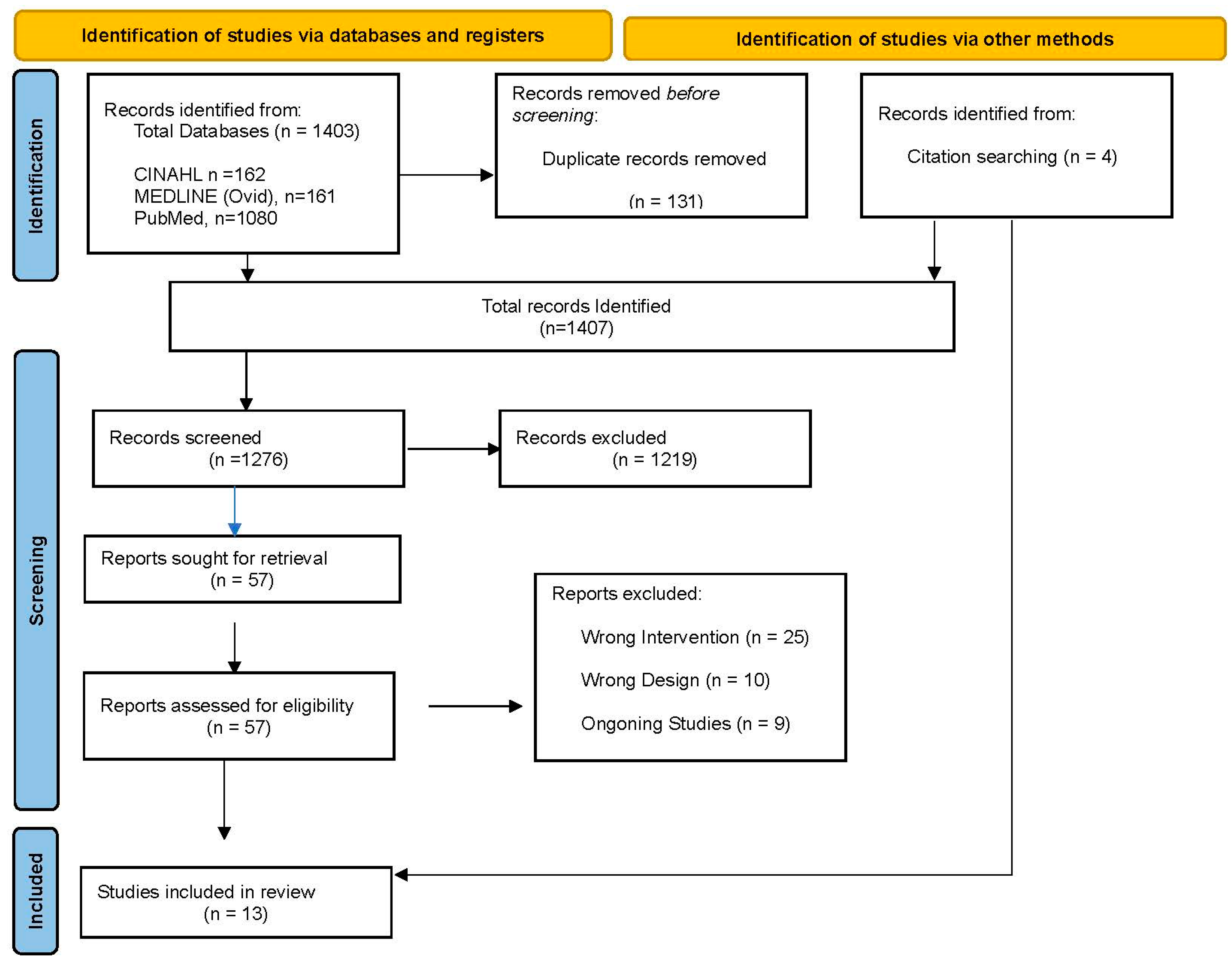
2.1. Search Strategy
2.2. Inclusion Criteria, Participants, and Type of Intervention
2.3. Exclusion Criteria
- Articles/reviews/studies not published in English languages;
- Studies of musculoskeletal pain in people with suspected cancer, pregnancy-related pain problems, palliative patients, and vulnerable patients (e.g., experienced trauma, cognitive impairment, dementia, terminal illness);
- Systematic review studies with meta-synthesis or protocols were excluded;
- Conference abstracts, scoping reviews, literature reviews, research letters or commentarial notes, or any other type of publication not being a report of a clinical study.
2.4. Data Extraction
- The country of origin;
- The participant study size and duration;
- Study design;
- Study objective;
- Inclusion and exclusion criteria;
- Interventions;
- Adverse events;
- Findings.
2.5. Assessment of Study Quality
3. Results
3.1. General Characteristics
3.2. Quality of Studies
3.3. Wearable Technology Characteristics
3.4. Intervention
3.5. Outcome Measures (Physical Activity)
3.6. Pain
3.7. Adherence and Adverse Events
4. Discussion
4.1. Strength of the Review
4.2. Limitations and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMS | The Arthritis Impact Measurement Scale |
| AS | Ankylosing Spondylitis |
| CDC | Centers for Disease Control and Prevention |
| CINAHL | Cumulative Index to Nursing and Allied Health Literature |
| FM | Fibromyalgia |
| GBD | Global Burden of Diseases |
| HOOS | Hip injury and Osteoarthritis Outcome Score |
| JIA | Juvenile Idiopathic Arthritis |
| KOOS | Knee Injury and OA Outcome Score |
| LBP | Low back pain |
| LPA | Light physical activity |
| MET | Metabolic equivalent of task |
| MMAT | Mixed-Method Appraisal Tool |
| MSK | Musculoskeletal |
| MVPA | Moderate/vigorous physical activity |
| NICE | National Institute for Health and Care Excellence |
| NRA | Numerical Pain Scale |
| OA | Osteoarthritis |
| PA | Physical Activity |
| PCS | Pain Catastrophizing Scale |
| PhGA | Physician global assessment |
| PICOS | Population, intervention, comparison, outcome, and study |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analyses |
| PROMS | Patient Reported Outcomes Measurement Information System |
| RA | Rheumatoid arthritis, |
| RCT | Randomised controlled trial |
| RMDQ | Roland–Morris Disability Questionnaire |
| SLE | Systemic lupus erythematosus |
| SpA | Spondylarthritis |
| TENS | Transcutaneous electrical nerve stimulation |
| VAS | Visual Analogue Scale |
| VPA | Vigorous-intensity physical activity |
| WAT | Wearable Technology |
| WHO | World Health Organization |
References
- World Health Organization. Musculoskeletal Health. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 3 June 2024).
- GBD 2021 Other Musculoskeletal Disorders Collaborators. Global, regional, and national burden of other musculoskeletal disorders, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e670–e682. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Kang, S.; Benedetto, E.; Myers, H.; Blackburn, S.; Smith, S.; Dunn, K.M.; Hay, E.; Rees, J.; Beard, D.; et al. Development and initial cohort validation of the Arthritis Research UK Musculoskeletal Health Questionnaire (MSK-HQ) for use across musculoskeletal care pathways. BMJ Open 2016, 6, e012331. [Google Scholar] [CrossRef]
- Hewitt, S.; Sephton, R.; Yeowell, G. The Effectiveness of Digital Health Interventions in the Management of Musculoskeletal Conditions: Systematic Literature Review. J. Med. Internet Res. 2020, 22, e15617. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Mallick-Searle, T.; Sharma, K.; Toal, P.; Gutman, A. Pain and Function in Chronic Musculoskeletal Pain-Treating the Whole Person. J. Multidiscip. Healthc. 2021, 14, 335–347. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Pinheiro, M.B.; Fairhall, N.; Walsh, S.; Franks, T.C.; Kwok, W.; Bauman, A.; Sherrington, C. Evidence on Physical Activity and the Prevention of Frailty and Sarcopenia Among Older People: A Systematic Review to Inform the World Health Organization Physical Activity Guidelines. J. Phys. Act. Health 2020, 17, 1247–1258. [Google Scholar] [CrossRef]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.K.; Pergolizzi, J.V.; Christo, P.J. Management of Musculoskeletal Pain: An Update with Emphasis on Chronic Musculoskeletal Pain. Pain Ther. 2021, 10, 181–209. [Google Scholar] [CrossRef]
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sànchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016, 56 (Suppl. 2), 243. [Google Scholar] [CrossRef]
- Tavakkol, R.; Kavi, E.; Hassanipour, S.; Rabiei, H.; Malakoutikhah, M. The global prevalence of musculoskeletal disorders among operating room personnel: A systematic review and meta-analysis. Clin. Epidemiol. Glob. Health 2020, 8, 1053–1061. [Google Scholar] [CrossRef]
- Williams, A.; Kamper, S.J.; Wiggers, J.H.; O’Brien, K.M.; Lee, H.; Wolfenden, L.; Yoong, S.L.; Robson, E.; McAuley, J.H.; Hartvigsen, J.; et al. Musculoskeletal conditions may increase the risk of chronic disease: A systematic review and meta-analysis of cohort studies. BMC Med. 2018, 16, 167. [Google Scholar] [CrossRef]
- Petit, A.; Begue, C.; Richard, I.; Roquelaure, Y. Factors influencing physiotherapists’ attitudes and beliefs toward chronic low back pain: Impact of a care network belonging. Physiother. Theory Pract. 2019, 35, 437–443. [Google Scholar] [CrossRef]
- De la Corte-Rodriguez, H.; Roman-Belmonte, J.M.; Resino-Luis, C.; Madrid-Gonzalez, J.; Rodriguez-Merchan, E.C. The Role of Physical Exercise in Chronic Musculoskeletal Pain: Best Medicine-A Narrative Review. Healthcare 2024, 12, 242. [Google Scholar] [CrossRef]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpinski, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Denche-Zamorano, A.; Pereira-Payo, D.; Collado-Mateo, D.; Adsuar-Sala, J.C.; Tomas-Carus, P.; Parraca, J.A. Physical Function, Self-Perceived Physical Fitness, Falls, Quality of Life and Degree of Disability According to Fear and Risk of Falling in Women with Fibromyalgia. J. Funct. Morphol. Kinesiol. 2024, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Jahid, M.; Khan, K.U.; Rehan-Ul-Haq Ahmed, R.S. Overview of Rheumatoid Arthritis and Scientific Understanding of the Disease. Mediterr. J. Rheumatol. 2023, 34, 284–291. [Google Scholar] [CrossRef]
- Nuesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Juni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence, (NICE). Chronic Pain (Primary and Secondary) in Over 16s: Assessment of All Chronic Pain and Management of Chronic Primary Pain; NICE: London, UK, 2021; pp. 1–40. [Google Scholar]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar] [PubMed]
- Jordan, K.P.; Kadam, U.T.; Hayward, R.; Porcheret, M.; Young, C.; Croft, P. Annual consultation prevalence of regional musculoskeletal problems in primary care: An observational study. BMC Musculoskelet. Disord. 2010, 11, 144. [Google Scholar] [CrossRef]
- Kirsch Micheletti, J.; Blafoss, R.; Sundstrup, E.; Bay, H.; Pastre, C.M.; Andersen, L.L. Association between lifestyle and musculoskeletal pain: Cross-sectional study among 10,000 adults from the general working population. BMC Musculoskelet. Disord. 2019, 20, 609. [Google Scholar] [CrossRef]
- Brickwood, K.; Watson, G.; O’Brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. [Google Scholar] [CrossRef]
- Ferguson, T.; Olds, T.; Curtis, R.; Blake, H.; Crozier, A.J.; Dankiw, K.; Dumuid, D.; Kasai, D.; O’Connor, E.; Virgara, R.; et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: A systematic review of systematic reviews and meta-analyses. Lancet Digit. Health 2022, 4, e615–e626. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, M.M.; Alon, G.; Pai, V.M.; Conroy, R.S. Wearable technologies for active living and rehabilitation: Current research challenges and future opportunities. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319839607. [Google Scholar] [CrossRef]
- Mahmood, A.; Kim, H.; Kedia, S.; Dillon, P. Wearable Activity Tracker Use and Physical Activity Among Informal Caregivers in the United States: Quantitative Study. JMIR Mhealth Uhealth 2022, 10, e40391. [Google Scholar] [CrossRef]
- Kos, M.; Pijnappel, E.N.; Buffart, L.M.; Balvers, B.R.; Kampshoff, C.S.; Wilmink, J.W.; van Laarhoven, H.W.M.; van Oijen, M.G.H. The association between wearable activity monitor metrics and performance status in oncology: A systematic review. Support Care Cancer 2021, 29, 7085–7099. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Donno, L.; Cimolin, V.; Galli, M. Cervical Range of Motion Assessment through Inertial Technology: A Validity and Reliability Study. Sensors 2023, 23, 6013. [Google Scholar] [CrossRef] [PubMed]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PM&R 2018, 10, S220–S232. [Google Scholar]
- Saygin, D.; Rockette-Wagner, B.; Oddis, C.; Neiman, N.; Koontz, D.; Moghadam-Kia, S.; Aggarwal, R. Consumer-based activity trackers in evaluation of physical activity in myositis patients. Rheumatology 2022, 61, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Degroote, L.; Hamerlinck, G.; Poels, K.; Maher, C.; Crombez, G.; De Bourdeaudhuij, I.; Vandendriessche, A.; Curtis, R.G.; DeSmet, A. Low-Cost Consumer-Based Trackers to Measure Physical Activity and Sleep Duration Among Adults in Free-Living Conditions: Validation Study. JMIR Mhealth Uhealth 2020, 8, e16674. [Google Scholar] [CrossRef] [PubMed]
- Haghi, M.; Thurow, K.; Stoll, R. Wearable Devices in Medical Internet of Things: Scientific Research and Commercially Available Devices. Healthc. Inform. Res. 2017, 23, 4–15. [Google Scholar] [CrossRef]
- Kandola, A.; Ashdown-Franks, G.; Hendrikse, J.; Sabiston, C.M.; Stubbs, B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019, 107, 525–539. [Google Scholar] [CrossRef]
- Labat, G.; Hayotte, M.; Bailly, L.; Fabre, R.; Brocq, O.; Gerus, P.; Breuil, V.; Fournier-Mehouas, M.; Zory, R.; D’Arripe-Longueville, F.; et al. Impact of a Wearable Activity Tracker on Disease Flares in Spondyloarthritis: A Randomized Controlled Trial. J. Rheumatol. 2022, 49, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Piwek, L.; Ellis, D.A.; Andrews, S.; Joinson, A. The Rise of Consumer Health Wearables: Promises and Barriers. PLoS Med. 2016, 13, e1001953. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C. No exchange, same pain, no gain: Risk-reward of wearable healthcare disclosure of health personally identifiable information for enhanced pain treatment. Health Inform. J. 2019, 25, 1675–1691. [Google Scholar] [CrossRef] [PubMed]
- Petticrew, M.; Roberts, H. Systematic reviews—Do they ’work’ in informing decision-making around health inequalities? Health Econ. Policy Law 2008, 3, 197–211. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.D.; Quatman, C.E.; Manring, M.M.; Siston, R.A.; Flanigan, D.C. How to write a systematic review. Am. J. Sports Med. 2014, 42, 2761–2768. [Google Scholar] [CrossRef]
- Taylor, J.; Jesson, J.K.; Matheson, L.; Lacey, F.M. Doing Your Literature Review—Traditional and Systematic Techniques; SAGE Publications Ltd.: London, UK, 2011; p. 192. [Google Scholar]
- Bramer, W.M.; de Jonge, G.B.; Rethlefsen, M.L.; Mast, F.; Kleijnen, J. A systematic approach to searching: An efficient and complete method to develop literature searches. J. Med. Libr. Assoc. 2018, 106, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Whittaker, A. Succeeding in Literature Reviews and Research Project Plans for Nursing Students; Learning Matters: Exeter, UK, 2017. [Google Scholar]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.N.; Gonzalez-Reyes, A.; Pluye, P. Improving the usefulness of a tool for appraising the quality of qualitative, quantitative and mixed methods studies, the Mixed Methods Appraisal Tool (MMAT). J. Eval. Clin. Pract. 2018, 24, 459–467. [Google Scholar] [CrossRef]
- Pace, R.; Pluye, P.; Bartlett, G.; Macaulay, A.C.; Salsberg, J.; Jagosh, J.; Seller, R. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int. J. Nurs. Stud. 2012, 49, 47–53. [Google Scholar] [CrossRef]
- Alzahrani, H.; Mackey, M.; Stamatakis, E.; Shirley, D. Wearables-based walking program in addition to usual physiotherapy care for the management of patients with low back pain at medium or high risk of chronicity: A pilot randomized controlled trial. PLoS ONE 2021, 16, e0256459. [Google Scholar] [CrossRef] [PubMed]
- Amorim, A.B.; Pappas, E.; Simic, M.; Ferreira, M.L.; Jennings, M.; Tiedemann, A.; Carvalho-e-Silva, A.P.; Caputo, E.; Kongsted, A.; Ferreirra, P.H. Integrating Mobile-health, health coaching, and physical activity to reduce the burden of chronic low back pain trial (IMPACT): A pilot randomised controlled trial. BMC Musculoskelet. Disord. 2019, 20, 71. [Google Scholar] [CrossRef]
- Gordon, R.; Bloxham, S. Influence of the Fitbit Charge HR on physical activity, aerobic fitness and disability in non-specific back pain participants. J. Sports Med. Phys. Fit. 2017, 57, 1669–1675. [Google Scholar] [CrossRef]
- Ostlind, E.; Eek, F.; Stigmar, K.; Sant’Anna, A.; Hansson, E.E. Promoting work ability with a wearable activity tracker in working age individuals with hip and/or knee osteoarthritis: A randomized controlled trial. BMC Musculoskelet. Disord. 2022, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Plumb Vilardaga, J.C.; Kelleher, S.A.; Diachina, A.; Riley, J.; Somers, T.J. Linking physical activity to personal values: Feasibility and acceptability randomized pilot of a behavioral intervention for older adults with osteoarthritis pain. Pilot Feasibility Stud. 2022, 8, 164–170. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wang, W.; Shi, Y.; Ji, X.; Hu, L.; Wang, L.; Yin, Y.; Xie, S.; Zhu, J.; et al. Adherence, Efficacy, and Safety of Wearable Technology-Assisted Combined Home-Based Exercise in Chinese Patients with Ankylosing Spondylitis: Randomized Pilot Controlled Clinical Trial. J. Med. Internet Res. 2022, 24, e29703. [Google Scholar] [CrossRef]
- Li, L.C.; Feehan, L.M.; Xie, H.; Lu, N.; Shaw, C.; Gromala, D.; Aviña-Zubieta, J.A.; Koehn, C.; Hoens, A.M.; English, K.; et al. Efficacy of a Physical Activity Counseling Program with Use of a Wearable Tracker in People with Inflammatory Arthritis: A Randomized Controlled Trial. Arthritis Care Res. 2020, 72, 1755–1765. [Google Scholar] [CrossRef]
- Li, L.C.; Feehan, L.M.; Xie, H.; Lu, N.; Shaw, C.D.; Gromala, D.; Zhu, S.; Aviña-Zubieta, J.A.; Hoens, A.M.; Koehn, C.; et al. Effects of a 12-Week Multifaceted Wearable-Based Program for People with Knee Osteoarthritis: Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e19116. [Google Scholar] [CrossRef]
- Li, L.C.; Sayre, E.C.; Xie, H.; Clayton, C.; Feehan, L.M. A Community-Based Physical Activity Counselling Program for People with Knee Osteoarthritis: Feasibility and Preliminary Efficacy of the Track-OA Study. JMIR Mhealth Uhealth 2017, 5, e86. [Google Scholar] [CrossRef]
- Li, L.C.; Sayre, E.C.; Xie, H.; Falck, R.S.; Best, J.R.; Liu-Ambrose, T.; Grewal, N.; Hoens, A.M.; Noonan, G.; Feehan, L.M. Efficacy of a Community-Based Technology-Enabled Physical Activity Counseling Program for People with Knee Osteoarthritis: Proof-of-Concept Study. J. Med. Internet Res. 2018, 20, e159. [Google Scholar] [CrossRef]
- Katz, P.; Margaretten, M.; Gregorich, S.; Trupin, L. Physical Activity to Reduce Fatigue in Rheumatoid Arthritis: A Randomized Controlled Trial. Arthritis Care Res. 2018, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heale, L.D.; Dover, S.; Goh, Y.I.; Maksymiuk, V.A.; Wells, G.D.; Feldman, B.M. A wearable activity tracker intervention for promoting physical activity in adolescents with juvenile idiopathic arthritis: A pilot study. Pediatr. Rheumatol. Online J. 2018, 16, 66–75. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Pieper, D.; Bajpai, R.; Makaruk, H.; Könsgen, N.; Neuhaus, A.L.; Semwal, M. Exercise/physical activity and health outcomes: An overview of Cochrane systematic reviews. BMC Public Health 2020, 20, 1724. [Google Scholar] [CrossRef] [PubMed]
- Naugle, K.M.; Fillingim, R.B.; Riley, J.L., III. A meta-analytic review of the hypoalgesic effects of exercise. J. Pain 2012, 13, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, I.; Pavic, J.; Filipovic, B.; Ozimec Vulinec, S.; Ilic, B.; Petek, D. Integrated Approach to Chronic Pain-The Role of Psychosocial Factors and Multidisciplinary Treatment: A Narrative Review. Int. J. Environ. Res. Public Health 2024, 21, 1135. [Google Scholar] [CrossRef]
- Leroux, A.; Rzasa-Lynn, R.; Crainiceanu, C.; Sharma, T. Wearable Devices: Current Status and Opportunities in Pain Assessment and Management. Digit. Biomark. 2021, 5, 89–102. [Google Scholar] [CrossRef]
- Naugle, K.M.; Riley, J.L., III. Self-reported physical activity predicts pain inhibitory and facilitatory function. Med. Sci. Sports Exerc. 2014, 46, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.V.; Abner, T.S.S.; Sluka, K.A. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J. Physiol. 2017, 595, 4141–4150. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; WHO: Geneva, Switzerland, 2018; pp. 3–43. [Google Scholar]
- Centers for Disease Control and Prevention, (CDC). Physical Activity Guidelines for Americans; CDC: Atlanta, GA, USA, 2018.
| Author | Study Aim | Study Design, Period, and SIZE | Type of MSK Condition and Wearable Device | Adverse Events | Inclusion/Exclusion Criteria | Intervention Details |
|---|---|---|---|---|---|---|
| Alzahrani et al. (2021) [46] Sydney, Australia | To examine the feasibility and initial efficacy of a wearable-based walking in addition to usual physiotherapy care in people with LBP at risk of chronicity. | A pilot randomised controlled trial. The intervention duration was 8 weeks. A total of 26 participants. Intervention group 12 and control group 14. | LBP. Fitbit Flex. Fitbit Flex was used as a wristband. | No adverse events were recorded. | Inclusion criteria. Aged 18 years and above, non-specific LBP patient diagnosed by a physiotherapist, not meeting the recommended PA guidelines for adults, and have access to internet. Willing and able to participate PA as determined by PAR-Q. Exclusion criteria. Patients with cardiovascular diseases, fractures, spinal nerve compromise, and pregnant women. | Participants in the experimental group received 8-week wearables-based walking intervention in addition to the usual physiotherapy care. The intervention consisted of (1) wearable device, (2) access to the 10,000 Steps website, and (3) progressive walking programme. Control group. Participants in this group received 8 weeks of usual physiotherapy. |
| Amorim et al. (2019) [47] Sydney | The aim of the study was to investigate the feasibility and preliminary efficacy of a patient-centred physical activity intervention, supported by health coaching and mobile health, to reduce care-seeking, pain, and disability in patients with chronic low back pain after treatment discharge. | Randomised controlled trial, pilot study. Intervention group (n = 34), and 31 participants completed. Control group (n = 34), and 24 participants completed 6 months intervention period and 6 months follow-up. Recruitment was between March 2016 and July 2017. A total of 90 participants were recruited, 68 participants agreed to participate. Recruitment took place in 4 public outpatient physiotherapy departments and the general community in Sydney. | LBP. Fitbit, the device was used as a wristband. | There were no between groups differences found for pain levels or activity restriction. This indicated that there were no adverse events reported. | Inclusion criteria. Patients discharged from the hospital and private practice such as GP, physiotherapy, or chiropractic but symptomatic. Speak English, persistent 12-week chronic pain of LBP. Exclusion. Pregnant patients, infectious diseases of the spine, patients with spinal surgery in the past 12 months, systemic or inflammatory disorder, comorbid health conditions, and LBP due to traffic accidents in last 12 months or ongoing litigation. | The IG was given a PA information booklet, plus one face-to-face and 12 telephone-based health coaching sessions. Also, supported by an internet-based application and Fitbit an activity tracker. Control group received PA information booklet and advice to stay active that was delivered once on the phone. |
| Gordon and Bloxham (2017) [48] Plymouth, U.K. | The aim of this study was to determine the effect of new advances in commercially available wearable technology on PA, aerobic fitness, and disability of low back pain participants. | Randomised control trial (RCT). Six-week intervention period and one-month follow-up. The study was divided into two groups: Fitbit Charge HR (FIT N. = 9) or pedometer (PED N. = 8) RCT participants. | Non-specific back pain. Fitbit Change HR and pedometer. The devices were used as wristband. | No adverse event was recorded. | The inclusion criteria for this study included patients over 18 years with NSCLBP more than 3 months and access to a computer with internet to enable syncing of the Fitbit (Fitbit Charge HR group only). All participants were deemed eligible for light moderate exercise by their general practitioner prior to commencing the programme. No exclusion criteria was identified. | Participants attended six 2 h PA and lifestyle intervention sessions and were invited back one month later for a follow-up. The FIT group were provided with Fitbit Charge HR and feedbacks on their exercise intensity each week. The PED group were provided with a pedometer and a step diary to enable them to record their daily step count. |
| Heale et al. (2018) [57] Canada | The aim of the study is to determine the feasibility of a wearable activity tracker (the Misfit Flash™) intervention in adolescents with Juvenile Idiopathic Arthritis (JIA) and (2) estimate the variability in the effect size of an activity tracker intervention on the physical activity levels of adolescents with JIA, for use in planning a definitive trial. | Feasibility pilot observational study (a single-group pre- and post-intervention study). A total of 31 participants met the inclusion criteria. A total of 28 participated in the study, 2 withdrew because of school and extra curriculum activity commitment, and 1 had inflammatory bowel disease during the study. The intervention period was 5 weeks. | Juvenile idiopathic arthritis (JIA). Misfit Flash. This device was used as a wristband or a clash for attaching to shoes/clothes. | A total of 9 participants reported that illness, injury, or pain prevented them from being active at some point in the study period. One patient had arthritis-related knee and ankle pain in the last week of the study period. A total of 8 participants (29%) reported that the battery died and their device stopped working during the study period. A total of 4 participants’ devices stopped working after wearing them in the water, and 3 participants reported that the activity tracker disc fell out of the wrist band. | Inclusion criteria. Boys and girls aged 12–18 years who met the International League of Associations for Rheumatology (LAR) classification criteria for JIA were selected. Participants with JIA disease status were considered stable by their rheumatologist; they were unlikely to require modification to medication during the study, and they had access to a smartphone or tablet compatible with Misfit Flash. Exclusion criteria. If participants had moderate or high disease activity based on 2011 American College of Rheumatology recommendations for the treatment of JIA. Participants’ changes to their JIA medications in the 3 months prior to study enrolment had significant cardiovascular, respiratory, or metabolic comorbidity and were already using an acuity tracker at the time of the study. | Participants were required to set daily activity goal for themselves without input from the research team. Participants were asked to wear the Misfit Flash™ for 24 h per day, 7 days a week, for at least 28 consecutive days following the telephone interview 1 week after study enrolment. Participants were asked to return the device at the final study visit. |
| Katz et al. (2017) [56] United States | The effect of a pedometer-based intervention on increasing physical activity and decreasing fatigue among individuals with RA. | Randomised control trial. 20 weeks of intervention and 96 participants. Two intervention groups: IG1 (n = 34) and IG2 (n = 34). One control group (n = 28). | RA. Fitbit Zip and Jawbone up pedometer (used at baseline and 21 week). Location of device was not specified. | A participant reported a calf muscle strain at day 5 and decreased activity for a short period but completed the intervention. | Inclusion criteria. Physician-diagnosed RA. Ability to speak English or Spanish. Commitment to attend at least 3 in-person research visits. Presence of greater than minimal fatigue. Exclusion. Body mass index (BMI) < 20 kg/m2. Participating in regular exercise, and non-ambulatory or presence of a condition that would limit the ability to walk (e.g., foot deformities, lower extremity joint surgery upcoming or in past 6 months, myocardial infarction in past 6 months, stroke, congestive heart failure, or severe chronic obstructive pulmonary disease). | IG1: Pedometer + step log IG2: Pedometer + step log + goal setting. Control Education only |
| Labat et al. (2022) [33] Nice, France | To evaluate the impact of a wearable activity tracker used to encourage physical activity on disease flares in patients with spondylarthritis (SpA). | Randomised controlled trial. A total of 108 participants. Tracker (n = 55) and non-tracker (n = 53) groups Study period 36 weeks. Intervention periods 2 × 12 weeks (1st: week 1 to week 12, 2nd: week 24 to week 36). | Spondylarthritis (SA). Garmin Vivo Fit 4.0. This device was used on the wrist. | No adverse event was recorded. | Inclusion criteria. Individuals were eligible if they were over 18 years of age, understood the objectives and constraints of the study, had a diagnosis of spondylarthritis according to the Assessment of Spondylarthritis International Society criteria, lived in Nice or the surrounding 20 km, and were certified as having no contraindication to the practice of a sports activity such as swimming or Nordic walking. Exclusion criteria. Researchers excluded patients who had coronary artery disease, moderate to severe heart failure, uncontrolled hypertension, myocarditis, pericarditis or endocarditis, lung disease, any contraindication to PA, those who were unable to attend the activity venue if they were already undergoing supervised PA in a club or with a sports coach and were pregnant or breastfeeding. Exclusion criteria during the study were serious adverse events, withdrawal of consent, and protocol violation. | Patients in both groups were asked to do weekly sessions of PA. Patients in the TG were monitored by a wearable activity tracker (WAT): a bracelet (Garmin Vívofit 4) combined with weekly sending of activity reminder SMS messages. Patients in the NTG group did not receive a WAT. |
| Li et al. (2020a) [52] Canada | Assessing the effectiveness of a multifaceted counselling intervention at improving physical participation and patient outcomes. | Randomised controlled trial. Immediate group and delay group. The study period was 27 weeks. Intervention period was 8 weeks. | Rheumatoid arthritis. Fitbit. The Fitbit was worn on the wrist. | During the study 23 participants reported adverse events due to physical activity: 19 with muscle pain and 4 with ligament sprain. Falls were reported by 5 participants. | Inclusion criteria. Individuals were eligible if they had a physician-confirmed diagnosis of RA or SLE, had an email address and daily access to internet, and were able to attend an in-person session. Exclusion criteria. Individuals excluded are people who had used any physical activity wearable devices or indicated that it was unsafe to be physically active without health professional supervision, as identified by the Physical Activity Readiness Questionnaire (PAR-Q) (19). If participants did not pass the PAR-Q, a physician’s note was required to determine eligibility. | In weeks 1–8, the immediate group received education and counselling by a physiotherapist (PT), while the delayed group did not receive any intervention. In weeks 10–17, participants in the immediate group received Fitbit Flex 2 with feedbacks on attainment from FitViz, while the delay group received education and counselling by PT. Participants were assessed at baseline, weeks 9, 18, and 27. This review only looked at assessment at week 18. |
| Li et al. (2020b) [53] Canada | This study aimed to examine the effect of a 12-week, multifaceted, wearable-based programme on physical activity and patient outcomes in patients with knee OA. | Randomised controlled trial with a delay-control design. The study period was 39 weeks. Intervention was 12 weeks. A total of 51 were randomised into two groups. Immediate group (n = 26) and delay group (n = 25). | Knee OA. Fitbit Flex-2 SenseWear. It was used as a wristband. | There were tracked adverse events (falls as well as cardiovascular and musculoskeletal events) related to their physical activity in the follow-up questionnaire at weeks 13, 26, and 39. During the programme, 10 participants reported adverse events because of physical activity. A total of 7 reported muscle pain, 2 fell while being physically active, and 1 had a vertebral compression fracture. | Inclusion. Patients who had a confirmed diagnosis of knee osteoarthritis or were aged ≥50 years. Patients not using disease-modifying antirheumatic drugs, not on a waiting list for knee or hip replacement surgery, have email address and access to internet, and able to attend education classes. Exclusion. Patients who have used wearable device previously. Participants who have received steroid and hyaluronate injection in a knee in the last 6 months. Patients on medication that will impair PA and at risk of exercising as identified by the Physical Activity Readiness Questionnaire. | The intervention has 3 components: (1) an in-person session with 20 min of group education and 30 min of individual counselling with a PT, (2) the use of a Fitbit Flex-2 wristband, and (3) PT counselling by phone to review physical activity goals (20–30 min). In weeks 1–12, the immediate group received the intervention, while the delayed group received monthly emails of arthritis news that were unrelated to PA. Participants were assessed baseline, weeks 13, 26, and 39. This review only looked at assessment qt week 13. |
| Li et al. (2018) [55] Canada | The study aimed to assess the efficacy of a technology-enabled counselling intervention for improving physical activity in people with either a physician-confirmed diagnosis of knee osteoarthritis or having passed two validated criteria for early osteoarthritis. | Randomised control trial. A total of 61 participants participated in a 6-month intervention. Two groups: immediate group (n = 30) and delayed group (n = 31). | Knee osteoarthritis. Fitbit Flex-2 was used as a wristband by participants. | Participants reported adverse events relating to falls or cardiovascular and musculoskeletal events. | Inclusion. Physician-confirmed diagnosis of knee OA. Or passed 2 criteria for early OA. Age 50 years or older and having experienced pain or discomfort in or around the knee during the previous year lasting 28 or more separate or consecutive. Exclusion. Diagnosis of inflammatory arthritis, connective tissue diseases, fibromyalgia, or gout. Used disease-modifying antirheumatic drugs or gout medications. Knee arthroplasty. On a waitlist to receive knee or hip arthroplasty. Any surgery in the back, hip, knee, foot, or ankle joint in the past 12 months. Acute knee injury in the past 6 months. Received a steroid injection or hyaluronate injection in a knee in the last 6 months. BMI of 40 kg/m2 or higher. No email address or daily access to a personal computer with internet access. Unable to attend the required education session in person. Using medications that impaired activity tolerance (e.g., beta-blockers) and had an inappropriate level of risk for increasing their unsupervised physical activity. | Intervention included three components: education, Fitbit Flex, and a bi-weekly telephone call for activity counselling for 2 months, while the delayed group received monthly email of arthritis news that were unrelated to PA during these 2 months. Control group (delay group). Received the same intervention 2 months later. Participants were assessed at baseline, 2 months, 4 months, and 6 months. This review only looked at assessment at 2 months. |
| Li et al. (2017) [54] Canada | Assessing the feasibility of a strategy that combines the use of wearables and telephone counselling by a physical therapist for improving PA behaviour in people with knee OA. | Community-based feasibility randomised controlled trial. 34 enrolled for the study. Study period is 9 weeks. Intervention period is 4 weeks. Two groups: immediate group (n = 17) and delayed group (n = 17). | Knee osteoarthritis. Fitbit Flex. It was located on the wrist. | No adverse events associated with the intervention was reported by participants during the study, | Inclusion criteria. Patients who have been confirmed by a physician to have knee OA or passed 2 criteria for early OA. Should be 50 years or older, Experiencing pain or discomfort in or around the knee during the previous year lasting 28 or more consecutive days. Exclusion criteria. Patients who have been diagnosis of inflammatory arthritis, connective tissue diseases, fibromyalgia, or gout, patients using disease-modifying antirheumatic drugs or gout medications, patients with knee arthroplasty, and patients who are on the waitlist to receive total knee arthroplasty. Patients who have acute knee injury in the past 6 months, patients who did not have an email address or daily access to a personal computer with internet access, and who has a body mass index of 40 kg/m2 or more. Also, patients receiving steroid injection in the last 6 months, and had received hyaluronate injection in a knee in the last 6 months. Patients using medications that impaired activity tolerance. Finally, patients with an inappropriate level of risk for increasing their unsupervised physical activity. | The intervention engaged participants attending a 1.5 h session, where they received a standardised group education session about PA, a Fitbit Flex, and weekly counselling with a PT by telephone. Control group (delay group) received the same intervention 2 months later. It is not clear what the control did during the one-month wait. Participants were assessed at baseline, 1 month, and 2 months. This review only looked at assessment at 1 month. |
| Östlind et al. (2022) [49] Sweden | The aims of this study were to examine the effect of self-monitoring PA with a WAT on work ability, PA, and work productivity among individuals of working age with hip and/or knee OA. | Cluster-randomised control trial. Supported Osteoarthritis Self-Management Programme SOASP. 160 participants. Two groups: intervention (n = 86) and control (n = 74). Intervention period was 12 weeks. | Hip/knee osteoarthritis. Fitbit Flex-2 was the wearable device used, and it was worn on the wrist. | There were no serious adverse events reported in this study. | Inclusion criteria. Patients should work for 20 h weekly, live in Southern Sweden with hip and/or knee OA, aged 18–67 years, and understand and write Swedish. Access to smartphone or computer and wear WAT for 12 weeks. | The participants in the intervention group were asked to wear the Fitbit for 12 weeks, from morning until bedtime. They were also asked to monitor their activity by using the app once a day. Asking them to use the app once per day facilitated self-monitoring and allowed for synchronisation of the data from the device to the app. Supported Osteoarthritis Self-Management Program (SOASP) was offered to both groups. |
| Plumb Vilardage et al. (2022) [50] Formatting… | The aim of this study was to examine the feasibility and acceptability of delivering Engage-PA to older adults with OA pain. Also, to examine the changes in arthritis-related pain and functioning, physical activity, psychological distress, psychological flexibility, and valued living before and after patients engaged in the intervention. | Randomised pilot feasibility and acceptability trial. 39 participants. Two groups: intervention group (n = 19) and control (n = 20). Study period 52 weeks. Intervention period 12 weeks. | Knee/hip osteoarthritis. Garmin Vivo Fit 4.0. It was located on the wrist. | No adverse was recorded. | Inclusion criteria. Adults aged 65 or older. Diagnosis of OA in the knee and/or hip. English speaking, and ability to participate in telephone sessions. Ability to ambulate even if assisted by a cane or walker and rating worst pain and pain interference within the last week as a 3 or greater out of 10. Exclusion criteria. Planned surgery (including joint replacement surgery) during the study duration that would affect or limit mobility for more than 3 weeks. Surgery requiring limited mobility within the past 3 months, and myocardial infarction within the past 3 months. Falls within the past 3 months that led to immediate medical treatment, and current enrolment in cardiac rehabilitation. Presence of a serious psychiatric condition. Reported or suspected moderate cognitive impairment. Indication by a medical provider that exercise should only be medically supervised, and presence of other unmanaged medical condition (e.g., hypertension, diabetes, asthma, neurodegenerative condition) that might lead to unsafe participation as outlined in the Physical Activity Readiness Questionnaire Plus (PAR-Q-2020 an evidence-based measure for patient-determined safety for engaging in physical activity) subsequently verified by electronic medical record review and/or via communication with patients’ treating medical team. | IG1. |
| Study workbook, two 45 min telephone delivered treatment session, and a fitness tracker Garmin Vivoft 4 for 6 weeks. IG2. Usual care plus a fitness tracker Garmin Vivoft 4 with handout for 6 weeks. | ||||||
| Wang et al. (2022) [51] China | Investigating the adherence, efficacy, and safety of a wearable technology-assisted combined home-based exercise programme in AS. | Randomised pilot-controlled clinical trial. Intervention period of 16 weeks. A total of 54 participants. Two groups: intervention (n = 26) and control (n = 28). Intervention period is 16 weeks. | Ankylosing Spondylitis (AS). Mio FUSE Heart Rate Monitor wristband (Medisana GmbH). This device was located on the wrist. | The incidences of adverse events observed in the intervention group was 12% and control group 0%. The 3 participants completed the intervention and no adverse event occurred during the trial on both groups. | Inclusion criteria. Patients’ disease should comply with the criteria for AS (1984 Modified New York criteria). Participants should be aged 18–60 years, stable drug treatment in the preceding month, and Ankylosing Spondylitis Disease Activity Score (ASDAS) between 1.3 and 3.5. Exclusion criteria. Patients with cardiovascular disease or clinical status at high risk, screened with the American Heart Association/ACSM Health/Fitness, cervical vertebral bridges, surgery within the preceding 6 months, biological agents (tumour necrosis factor inhibitor therapy, etc.) used in the preceding 3 months, regular exercise in the preceding 3 months and factors leading to the inability to receive regular exercise rehabilitation (such as language impairment, difficulty in understanding, and limited movements). | Intervention group. The IG combined usual care plus exercise programme consisting of in-person counselling sessions, supervised training sessions, and aerobic and functional home-based exercise plus wearable device wristband (Medisana GmbH). Control group. Usual care. |
| Authors. Methodological Quality Criteria | Alzahrani et al., 2021 [46] | Amorim et al., 2019 [47] | Gordon & Bloxham et al., 2017 [48] | Heale et al., 2018 [57] | Katz et al., 2010 [56] | Labat et al., 2022 [33] | Li et al., 2020a [52] | Li et al., 2020b [53] | Li et al., 2018 [28] | Li et al., 2017 [54] | Ostlind, et al., 2022 [49] | PlumbVilardaga et al., 2022 [50] | Wang et al., 2022 [51] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Are there clear research question? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Do the collected data allow to address the research question? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Is randomisation appropriately performed? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are the groups comparable at baseline? | Yes | Yes | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Are there complete outcome data? | Yes | Yes | No | N/A | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes |
| Are outcome assessors blinded to the intervention provided? | Yes | No | No | N/A | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Did the participants adhere to the assigned intervention? | No | Yes | Yes | N/A | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Author/Methodological Quality Criteria | Heale et al., 2018 [57] |
|---|---|
| Are the participant representative of the target population? | Yes |
| Are the measurement appropriate regarding both the outcome and intervention (exposure)? | No |
| Are there complete outcome data? | No |
| Are the confounders accounted for in the design and analysis? | No |
| During the study period, is the intervention administered | Yes |
| Authors | Intervention Baseline | Post-Intervention | Changes | Control Baseline | Post-Control | Changes | Between-Group Differences |
|---|---|---|---|---|---|---|---|
| STEPS PER DAY | |||||||
| Alzahrani et al. (2021) [46] | 12,998 | 15,647 (9 weeks) 13,770 (26 weeks) | 2649 (20.4%) 772 (5.9%) | 13,563 | 11,978 (9 weeks) 11,600 (26 weeks) | −1585 (−11.7%) −1963 (−14.5%) | p < 0.001 (9 week) p = 0.056 (26 week) |
| Amorim et al. (2019) [47] | 7373 | 7379 (6 months) | 6 (0.08%) | 7240 | 7020 (6 months) | −220 (−3.04%) | p = 0.347 |
| Gordon and Bloxham (2017) [48] | 8620 | 10,586 (6 weeks) | 1966 (23.0%) | 5856 | 7580 (6 weeks) | 1724 (29.4%) | NS |
| Katz et al. (2017) [56] | Pedometer 4223 Pedometer + target 5019 | 5655 (21 weeks) 6675 | 1432 (33.9%) 1656 (33.0%) | 5572 | 4609 (21 weeks) | −963 (−13.9%) | p < 0.05 p < 0.05 |
| Li et al. (2020a) [52] | 5900 | 6673 (8 weeks) | 773 (13.1%) | 5605 | 5819 (8 weeks) | 214 (3.82%) | Not statistically significant |
| Li et al. (2020b) [53] | 6294 | 7133.3 (12 weeks) | 839.3 = 13.3% | 7030.1 | 6232.7 (12 weeks) | −797.4 (−11.3%) | Not statistically significant |
| Li et al., 2018 [55] | 7069.2 | 8217.4 (2 months) | 1148.2 (16.2%) | 7556.6 | 6713.6 (2 months) | −843 (−11.15%) | p = 0.02 |
| PlumbVilardaga et al., 2022 [50] | 35,712 | 38,268 | 2556 (7.1.6%) | 28,166 | 36,407 | 8241 (29.25%) | 0.627 |
| METABOLIC EQUIVALENT TASK (MET) | |||||||
| Ostlind et al., 2022 [49] | 3167 | 3421 min/weekly (3 months) 3319 min/weekly (6 months) 2774 (min/weekly 12 months) | 254 (8.02%) min/weekly 152 (4.80%) min/weekly −393 (12.41%) min/weekly | 2654 min/weekly | 2864 min/weekly (3 months) 2918 min/weekly (6 months) 2636 min/weekly (12 months) | 210 (8%) min/weekly 264 (10%) min/weekly −18 (−0.7%) min/weekly | NS NS NS |
| TIME SPENT IN LPA (MIN) | |||||||
| Alzahrani et al., 2021 [46] | 269.39 | 314.77 (9 weeks) 269.76 (26 weeks) | 45.38 (16.85%) 0.37 (0.14%) | 301.65 | 244.24 (9 weeks) 271.48 (26 weeks) | −57.41 (−19%) −30.17 (−10%) | p < 0.001 p = 0.350 |
| Amorim et al., 2019 [47] | 283.6 | 295.1 | 11.5 (4.06%) | 276.7 | 277.3 | 0.6 (0.22%) | p = 0.378 |
| TIME SPENT IN MVPA (MIN) | |||||||
| Alzahrani et al., 2021 [46] | 80.93 (MPA) 0.36 (VPA) | 103.13 (9 weeks) 84.41 (26 weeks) 1.0 (9 weeks) 1.21 (26 weeks) | 22.2 (27.43%) 4.41 (4.3%) 0.64 (178%) 0.85 (236%) | 68.16 (MPA) 0.29 (VPA) | 84.95 (9 weeks) 76.09 (26 weeks) 0.84 (9 weeks) 0.95 (26 weeks) | 16.8 (24.6%) 7.93 (11.63%) 0.55 (190%) 0.66 (223%) | p = 0.012 p = 0.086 p = 0.778 p = 0.573 |
| Amorim et al., 2019 [47] | 28.9 | 26.8 | −2.1 (−7.27%) | 28.6 | 24.2 | −4.4 (−15.4%) | p = 0.334 |
| Heale et al., 2018 [57] | 3.722 | 3.905 (5 weeks) | 0.18 (4.83%) | ||||
| Li et al., 2020a [52] | 37.8 | 44.7 (9 weeks) | 6.9 (18.25%) | 31.6 | 31.6 (9 weeks) | Nil | p < 0.05 |
| Li et al., 2020b [53] | 31.0 | 37.7 (13 weeks) | 6.7 (21.61%) | 71.3 | 49.4 (13 weeks) | −21.9 (−30.7%) | Not statistically significant |
| Li et al., 2018 [55] | 62.1 | 75.5 (2 months) | 13.4 (21.6%) | 65.3 | 50.0 (2 months) | −15.3 (−23.4%) | p = 0.02 |
| Li et al., 2017 [54] | 41.3 | 64.2 (1 month) | 22.9 (55.45%) | 66.5 | 56 (1 month) | −10.5 (−16%) | p < 0.05 |
| Authors | Pain Outcome | Intervention Baseline | Post-Intervention | Changes | Control Baseline | Post-Control | Changes | Between-Group Differences |
|---|---|---|---|---|---|---|---|---|
| Alzahrani et al., 2021 [46] | Visual Analogue Scale (VAS) Pain Catastrophizing Scale (PCS) | 4 18.50 | 3 (9 weeks) 1 (26 weeks) 13.0 (9 weeks) 14.55 (26 weeks) | 1 (25%) 3 (75%) 5.5 (29.73%) 3.9 (21.35%) | 5 17 | 3 (9 weeks) 3 (26 weeks) 9.50 (9 weeks) 10.91 (26 weeks) | 2 (40%) 2 (40%) 7.5 (44.12%) 6.09 (35.8%) | p = 0.273 p = 0.013 p = 0.006 p = 0.151 |
| Amorim et al., 2019 [47] | Numerical rating scale | 5.3 | 3.8 | 1.5 = 28.3% | 5.1 | 4.0 | 1.1 (21.6%) | p = 0.815 |
| Gordon and Bloxham, 2017 [48] | [48] Oswestry Disability Questionnaire | 19% | 13% | Non-significant reduction | ||||
| Heale et al., 2018 [57] | Visual Analogue Scale (VAS) | 1.319 | 1.890 | −0.57 = −43.3% | ||||
| Katz et al., 2017 [56] | PROMIS | Pedometer 61.7 Pedometer + Target 61.1 | 59.2 (21 weeks) 55.9 (21 weeks) | 2.5 (4.05%) 5.2 = 8.51% | 59.8 | 57.6 (21 weeks) | 2.2 (3.68%) | p = 0.35 |
| Labat et al., 2022 [33] | 0.7 (moderate flares) 0.6 (persistent flares) | 0.5 (12 weeks) 0.4 (12 weeks) 0.4 (36 weeks) | 0.2 = 28.6% 0.2 = 33.33% | 1.0 (moderate flares) 0.6 (persistent flares) | 0.5 (12 weeks) 0.5 (12 weeks) | 0.5 = 50% 0.1 = 16.67% | p = 0.87 p = 0.80 | |
| Li et al., 2020b [53] | KOOS | 72.6 | 73.1 (12 weeks) | 0.5 (0.69%) | 65.1 | 65.9 (12 weeks) | 0.8 (1.23%) | Not statistically significant |
| Li et al., 2018 [55] | KOOS Higher = better | 66.2 | 70.9 | 4.7 (7.1%) | 65.1 | 64.8 | −0.3 (−0.46%) | Not significant |
| Li et al., 2017 [54] | KOOS | 74.2 | 71.4 (1 month) 79.1 (2 months) | −2.8 (−3.77%) 4.9 (6.6%) | 68.6 | 71.6 (1 month) 74.0 (2 months) | 3 (4.37%) 5.4 (7.87%) | No significant effect |
| Plumb-Vilardaga et al., 2022 [50] | Arthritis Impact Measurement M, Scale (AIMS) | 13.72 | 11.78 | 1.94 = 14.14% | 14.9 | 14.58 | 0.32 = 2.15% | p = 0.044 |
| Wang et al., 2022 [51] | Spondylarthritis International Society Health Index (ASAS HI) | 14 | 9 (16 weeks) | 5 (36.71%) | 18 | 8 (16 weeks) | 10 (55.6%) | Significantly beneficial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eboreime, K.O.; Hughes, J.G.; Lee, R.; Luo, J. Can Wearable Device Promote Physical Activity and Reduce Pain in People with Chronic Musculoskeletal Conditions? J. Clin. Med. 2025, 14, 1003. https://doi.org/10.3390/jcm14031003
Eboreime KO, Hughes JG, Lee R, Luo J. Can Wearable Device Promote Physical Activity and Reduce Pain in People with Chronic Musculoskeletal Conditions? Journal of Clinical Medicine. 2025; 14(3):1003. https://doi.org/10.3390/jcm14031003
Chicago/Turabian StyleEboreime, Kereaseen Oluwatobiloba, John G. Hughes, Raymond Lee, and Jin Luo. 2025. "Can Wearable Device Promote Physical Activity and Reduce Pain in People with Chronic Musculoskeletal Conditions?" Journal of Clinical Medicine 14, no. 3: 1003. https://doi.org/10.3390/jcm14031003
APA StyleEboreime, K. O., Hughes, J. G., Lee, R., & Luo, J. (2025). Can Wearable Device Promote Physical Activity and Reduce Pain in People with Chronic Musculoskeletal Conditions? Journal of Clinical Medicine, 14(3), 1003. https://doi.org/10.3390/jcm14031003






