Multicenter Prospective Pilot Study Identifying Thrombomodulin as a Potential Biomarker for Neurocognitive Outcomes in Immune Thrombotic Thrombocytopenic Purpura
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Eligibility Criteria
2.3. Outcomes Definitions
2.4. Data Collection and Study Assessments
2.5. Statistical Analysis
3. Results
3.1. Enrollment and Study Completion
3.2. Clinical Courses
3.3. Neurocognitive Impairment and Poor Mental Health
3.4. Biomarker Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
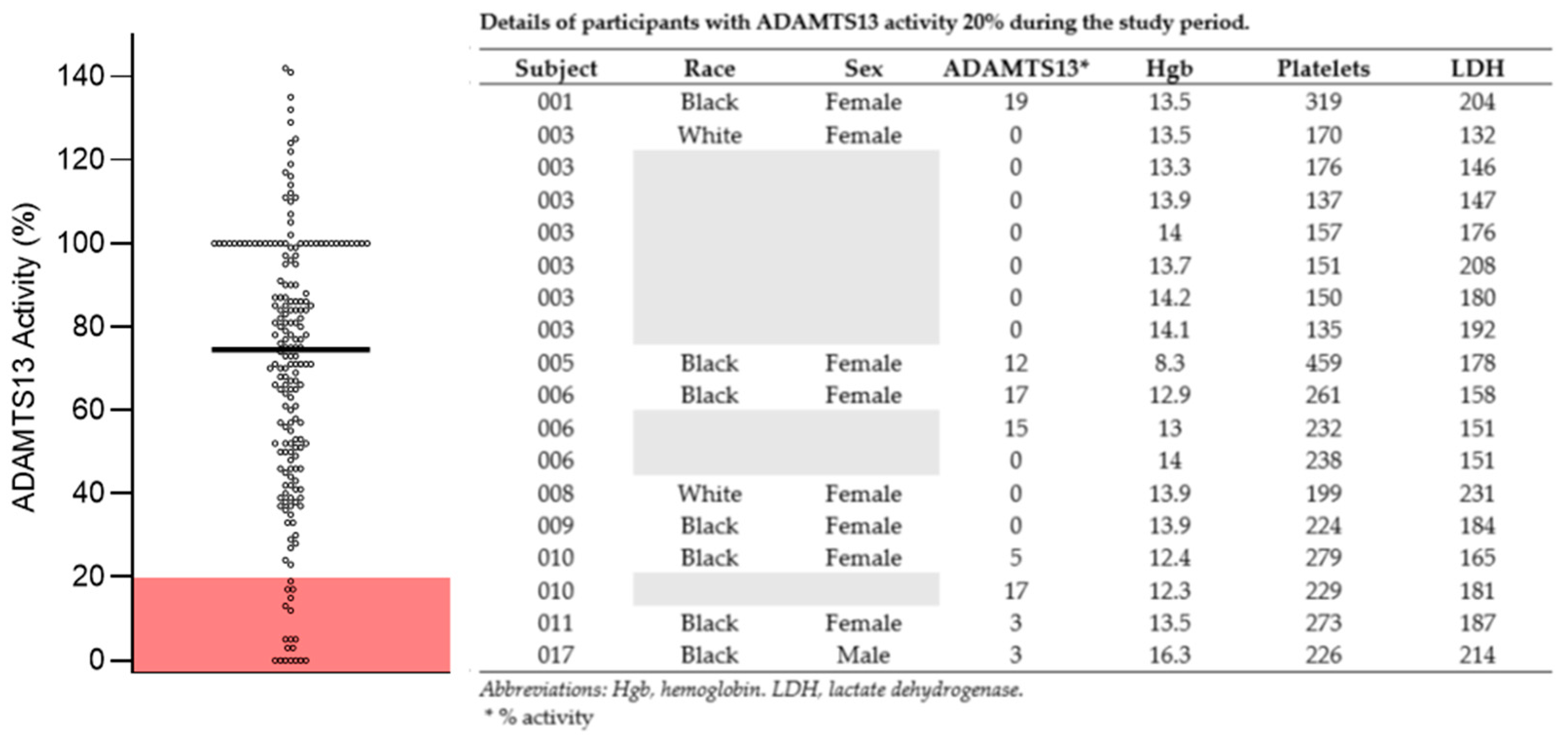
Appendix B
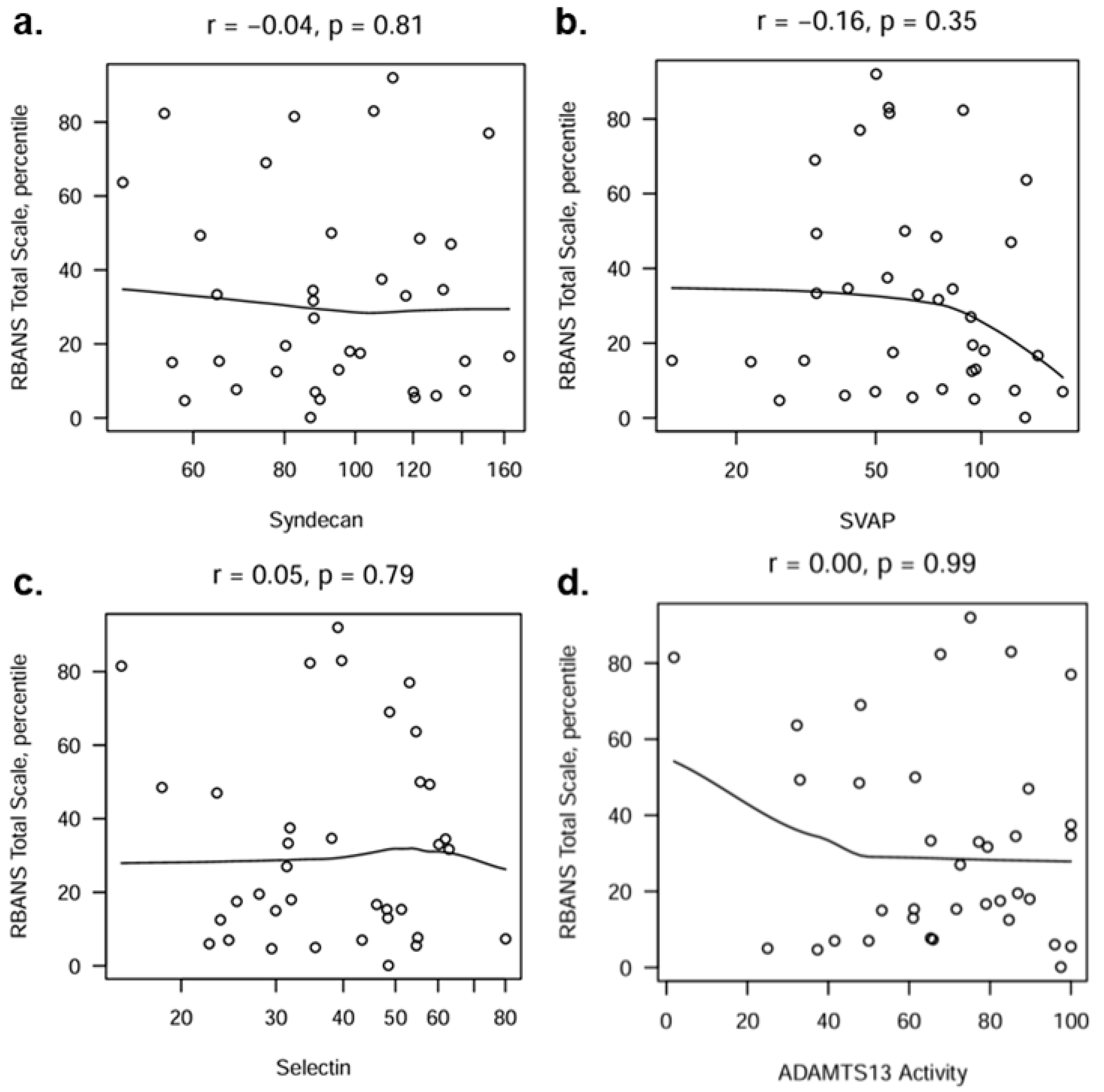
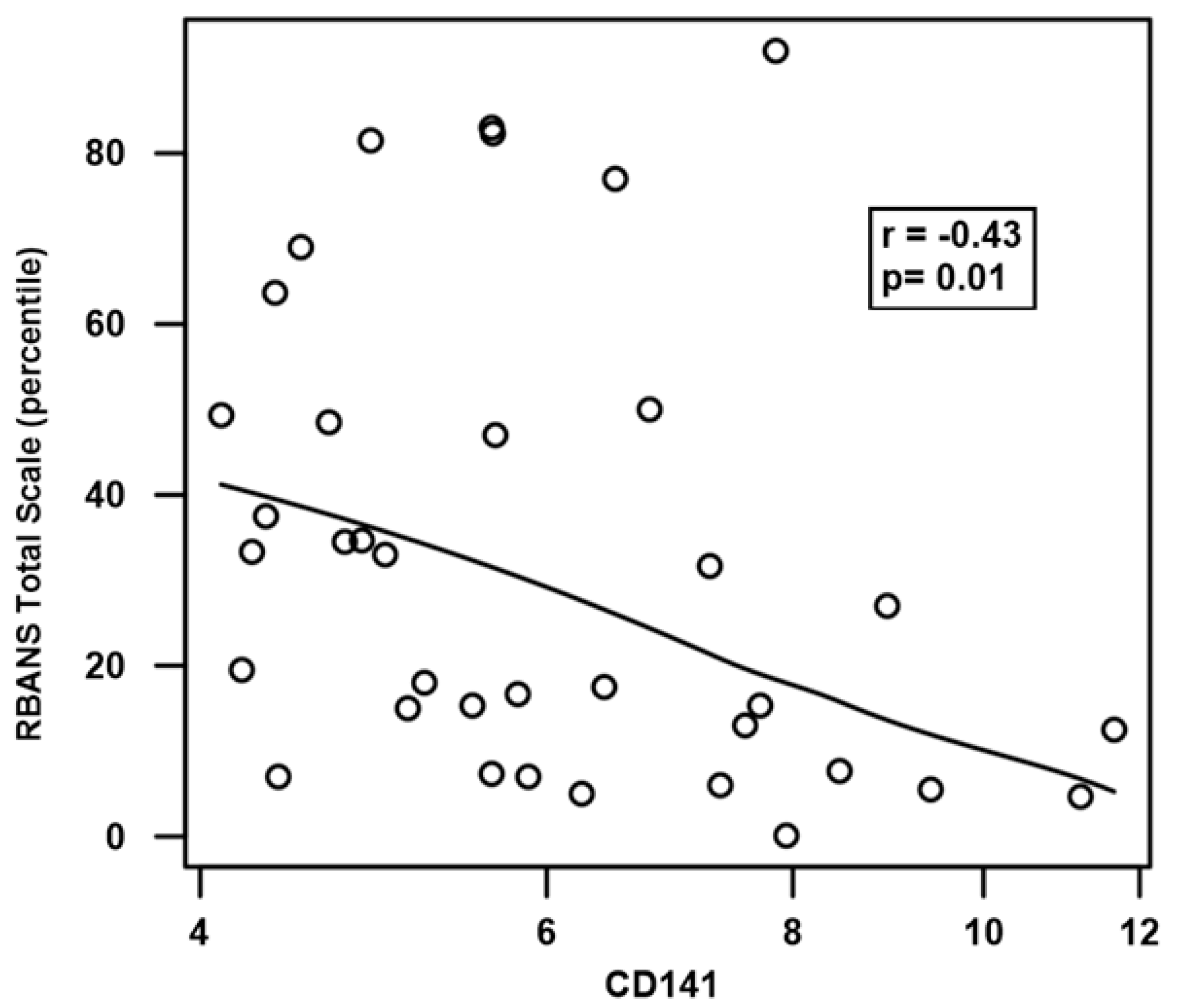
References
- Furlan, M.; Robles, R.; Solenthaler, M.; Lämmle, B. Acquired deficiency of von Willebrand factor-cleaving protease in a patient with thrombotic thrombocytopenic purpura. Blood 1998, 91, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.M.; Lian, E.C. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N. Engl. J. Med. 1998, 339, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Hunt, B.J.; Benjamin, S.; Liesner, R.; Rose, P.; Peyvandi, F.; Cheung, B.; Machin, S.J.; on behalf of British Committee for Standards in Haematology. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br. J. Haematol. 2012, 158, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Amorosi, E.L.; Ultmann, J.E. Thrombotic Thrombocytopenic Pupura: Report of 16 cases and review of the literature. Medicine 1966, 45, 139–159. [Google Scholar] [CrossRef]
- Kremer Hovinga, J.A.; Vesely, S.K.; Terrell, D.R.; Lämmle, B.; George, J.N. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood 2010, 115, 1500–1511; quiz 1662. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.N.; Coe, T.L.; Zhou, H.; Fernando, L.; Holland, P.V.; Wun, T. Improved survival with plasma exchange in patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Am. J. Med. 1999, 107, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Rock, G.A.; Shumak, K.H.; Buskard, N.A.; Blanchette, V.S.; Kelton, J.G.; Nair, R.C.; Spasoff, R.A.; Canadian Apheresis Study Group. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N. Engl. J. Med. 1991, 325, 393–397. [Google Scholar] [CrossRef]
- Peyvandi, F.; Cataland, S.; Scully, M.; Coppo, P.; Knoebl, P.; Hovinga, J.A.K.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Edou, J.M.M.; et al. Caplacizumab prevents refractoriness and mortality in acquired thrombotic thrombocytopenic purpura: Integrated analysis. Blood Adv. 2021, 5, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Cataland, S.R.; Scully, M.A.; Paskavitz, J.; Maruff, P.; Witkoff, L.; Jin, M.; Uva, N.; Gilbert, J.C.; Wu, H.M. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am. J. Hematol. 2011, 86, 87–89. [Google Scholar] [CrossRef]
- Han, B.; Page, E.E.; Stewart, L.M.; Deford, C.C.; Scott, J.G.; Schwartz, L.H.; Perdue, J.J.; Terrell, D.R.; Vesely, S.K.; George, J.N. Depression and cognitive impairment following recovery from thrombotic thrombocytopenic purpura. Am. J. Hematol. 2015, 90, 709–714. [Google Scholar] [CrossRef]
- Riva, S.; Mancini, I.; Maino, A.; Ferrari, B.; Artoni, A.; Agosti, P.; Peyvandi, F. Long-term neuropsychological sequelae, emotional wellbeing and quality of life in patients with acquired thrombotic thrombocytopenic purpura. Haematologica 2020, 105, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Falter, T.; Schmitt, V.; Herold, S.; Weyer, V.; von Auer, C.; Wagner, S.; Hefner, G.; Beutel, M.; Lackner, K.; Lämmle, B.; et al. Depression and cognitive deficits as long-term consequences of thrombotic thrombocytopenic purpura. Transfusion 2017, 57, 1152–1162. [Google Scholar] [CrossRef]
- Upreti, H.; Kasmani, J.; Dane, K.; Braunstein, E.M.; Streiff, M.B.; Shanbhag, S.; Moliterno, A.R.; Sperati, C.J.; Gottesman, R.F.; Brodsky, R.A.; et al. Reduced ADAMTS13 activity during TTP remission is associated with stroke in TTP survivors. Blood 2019, 134, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Yu, J.; Brown, J.; Wei, A.; Selvakumar, S.; Gerber, G.F.; Moliterno, A.R.; Streiff, M.B.; Kraus, P.; Logue, C.M.; et al. Silent cerebral infarction during immune TTP remission: Prevalence, predictors, and impact on cognition. Blood 2023, 142, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Saultz, J.N.; Wu, H.M.; Cataland, S. Headache prevalence following recovery from TTP and aHUS. Ann. Hematol. 2015, 94, 1473–1476. [Google Scholar] [CrossRef]
- Deford, C.C.; Reese, J.A.; Schwartz, L.H.; Perdue, J.J.; Hovinga, J.A.K.; Lämmle, B.; Terrell, D.R.; Vesely, S.K.; George, J.N. Multiple major morbidities and increased mortality during long-term follow-up after recovery from thrombotic thrombocytopenic purpura. Blood 2013, 122, 2023–2029; quiz 2142. [Google Scholar] [CrossRef]
- Elias, M.F.; Wolf, P.A.; D’Agostino, R.B.; Cobb, J.; White, L.R. Untreated blood pressure level is inversely related to cognitive functioning: The Framingham Study. Am. J. Epidemiol. 1993, 138, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Sahu, T.; Pande, B.; Sinha, M.; Sinha, R.; Verma, H.K. Neurocognitive Changes in Sickle Cell Disease: A Comprehensive Review. Ann. Neurosci. 2022, 29, 255–268. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Erkinjuntti, T.; Reisberg, B.; Roman, G.; Sawada, T.; Pantoni, L.; Bowler, J.V.; Ballard, C.; DeCarli, C.; Gorelick, P.B.; et al. Vascular cognitive impairment. Lancet Neurol. 2003, 2, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Akwaa, F.; Antun, A.; Cataland, S.R. How I treat immune-mediated thrombotic thrombocytopenic purpura after hospital discharge. Blood 2022, 140, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Sui, J.; Zheng, X.L. Elevated plasma levels of syndecan-1 and soluble thrombomodulin predict adverse outcomes in thrombotic thrombocytopenic purpura. Blood Adv. 2020, 4, 5378–5388. [Google Scholar] [CrossRef] [PubMed]
- Cuker, A.; Cataland, S.R.; Coppo, P.; de la Rubia, J.; Friedman, K.D.; George, J.N.; Knoebl, P.N.; Kremer Hovinga, J.A.; Lӓmmle, B.; Matsumoto, M.; et al. Redefining outcomes in immune TTP: An international working group consensus report. Blood 2021, 137, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Fydrich, T.; Dowdall, D.; Chambless, D.L. Reliability and validity of the beck anxiety inventory. J. Anxiety Disord. 1992, 6, 55–61. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, M.; Bayliss, M.; Bjorner, J.; Ware, J.E., Jr.; Garber, W.; Batenhorst, A.; Cady, R.; Dahlöf, C.; Dowson, A.; Tepper, S. A six-item short-form survey for measuring headache impact: The HIT-6. Qual. Life Res. 2003, 12, 963–974. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Berger, I.; Slobodin, O.; Cassuto, H. Usefulness and Validity of Continuous Performance Tests in the Diagnosis of Attention-Deficit Hyperactivity Disorder Children. Arch. Clin. Neuropsychol. 2017, 32, 81–93. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Scully, M.; de la Rubia, J.; Pavenski, K.; Metjian, A.; Knöbl, P.; Peyvandi, F.; Cataland, S.; Coppo, P.; Hovinga, J.A.K.; Edou, J.M.M.; et al. Long-term follow-up of patients treated with caplacizumab and safety and efficacy of repeat caplacizumab use: Post-HERCULES study. J. Thromb. Haemost. 2022, 20, 2810–2822. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Antun, A.G.; Farland, A.M.; Woods, R.; Metjian, A.; Park, Y.A.; de Ridder, G.; Gibson, B.; Kasthuri, R.S.; Liles, D.K.; et al. Race rituximab relapse in TTP. Blood 2022, 140, 1335–1344. [Google Scholar] [CrossRef]
- Giri, H.; Biswas, I.; Rezaie, A.R. Thrombomodulin: A multifunctional receptor modulating the endothelial quiescence. J. Thromb. Haemost. 2024, 22, 905–914. [Google Scholar] [CrossRef]
- Watanabe-Kusunoki, K.; Nakazawa, D.; Ishizu, A.; Atsumi, T. Thrombomodulin as a Physiological Modulator of Intravascular Injury. Front. Immunol. 2020, 11, 575890. [Google Scholar] [CrossRef]
- Vincent, J.L.; Francois, B.; Zabolotskikh, I.; Daga, M.K.; Lascarrou, J.B.; Kirov, M.Y.; Pettilä, V.; Wittebole, X.; Meziani, F.; Mercier, E.; et al. Effect of a Recombinant Human Soluble Thrombomodulin on Mortality in Patients With Sepsis-Associated Coagulopathy: The SCARLET Randomized Clinical Trial. JAMA 2019, 321, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Boron, M.; Hauzer-Martin, T.; Keil, J.; Sun, X.L. Circulating Thrombomodulin: Release Mechanisms, Measurements, and Levels in Diseases and Medical Procedures. TH Open 2022, 6, e194–e212. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | n = 38 |
|---|---|
| Female | 32 (84.2%) |
| Black race | 16 (42.1%) |
| ADAMTS13 activity (IQR) | 65.7 (42–97.5) |
| Years since first iTTP episode (IQR) | 13.9 (1.5–18.5) |
| Years since last iTTP episode (IQR) | 6.3 (1–12.5) |
| Relapses and Treatment Agents | |
|---|---|
| ADAMTS13 activity measures < 20% | 18/194 |
| Clinical relapse * | 4 |
| Corticosteroid | 5 |
| Rituximab (clinical relapse *) | 3 |
| Rituximab (ADAMTS13 relapse *) | 7 |
| Cyclosporine | 7 |
| Other immune suppressive agents | 2 |
| Caplacizumab | 1 |
| Beck Index | Minimal | Mild | Moderate | Severe |
|---|---|---|---|---|
| Anxiety | 29% (11/38) | 24% (9/38) | 29% (11/38) | 18% (7/38) |
| Depression | 37% (14/38) | 18% (7/38) | 29% (11/38) | 16% (6/38) |
| HIT-6 | Grade 1 (normal) | Grade 2 | Grade 3 | Grade 4 |
| 29% (11/38) | 16% (6/38) | 8% (3/38) | 47% (18/38) | |
| CPT-3 | 1–2 SD slower | |||
| Reaction time | 26% (9/35) | |||
| RBANS * | ||||
| Immed. memory | Delayed memory | Visuospatial | Language | Attention |
| 13th (7–50) | 42nd (18–58) | 10th (2–39) | 30th (25–53) | 46th (27–67.5) |
| SF-36 | ||||
| Social function | Fatigue | Wellbeing | Pain | General health |
| 62.5 (40.63–83.75) | 30 (20–50) | 64 (48–76) | 57.5 (45–86.88) | 35 (21.25–60) |
| Biomarker | The Intraclass Correlation Coefficient |
|---|---|
| ADAMTS13 | 0.60 |
| SVAP | 0.80 |
| Selectin | 0.74 |
| Syndecan | 0.84 |
| Thrombomodulin (CD141) | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boothby, A.B.; Evans, M.D.; Yang, S.; Sukumar, S.; Scott, J.G.; Terrell, D.R.; Cataland, S.; Mazepa, M. Multicenter Prospective Pilot Study Identifying Thrombomodulin as a Potential Biomarker for Neurocognitive Outcomes in Immune Thrombotic Thrombocytopenic Purpura. J. Clin. Med. 2025, 14, 694. https://doi.org/10.3390/jcm14030694
Boothby AB, Evans MD, Yang S, Sukumar S, Scott JG, Terrell DR, Cataland S, Mazepa M. Multicenter Prospective Pilot Study Identifying Thrombomodulin as a Potential Biomarker for Neurocognitive Outcomes in Immune Thrombotic Thrombocytopenic Purpura. Journal of Clinical Medicine. 2025; 14(3):694. https://doi.org/10.3390/jcm14030694
Chicago/Turabian StyleBoothby, Aaron B., Michael D. Evans, Shangbin Yang, Senthil Sukumar, James G. Scott, Deirdra R. Terrell, Spero Cataland, and Marshall Mazepa. 2025. "Multicenter Prospective Pilot Study Identifying Thrombomodulin as a Potential Biomarker for Neurocognitive Outcomes in Immune Thrombotic Thrombocytopenic Purpura" Journal of Clinical Medicine 14, no. 3: 694. https://doi.org/10.3390/jcm14030694
APA StyleBoothby, A. B., Evans, M. D., Yang, S., Sukumar, S., Scott, J. G., Terrell, D. R., Cataland, S., & Mazepa, M. (2025). Multicenter Prospective Pilot Study Identifying Thrombomodulin as a Potential Biomarker for Neurocognitive Outcomes in Immune Thrombotic Thrombocytopenic Purpura. Journal of Clinical Medicine, 14(3), 694. https://doi.org/10.3390/jcm14030694





