Evolution and New Perspectives of Balloon Pulmonary Angioplasty in CTEPH
Abstract
1. Introduction
2. Patient Evaluation and Pre-BPA Planning
3. Catheter-Based Pulmonary Angiogram
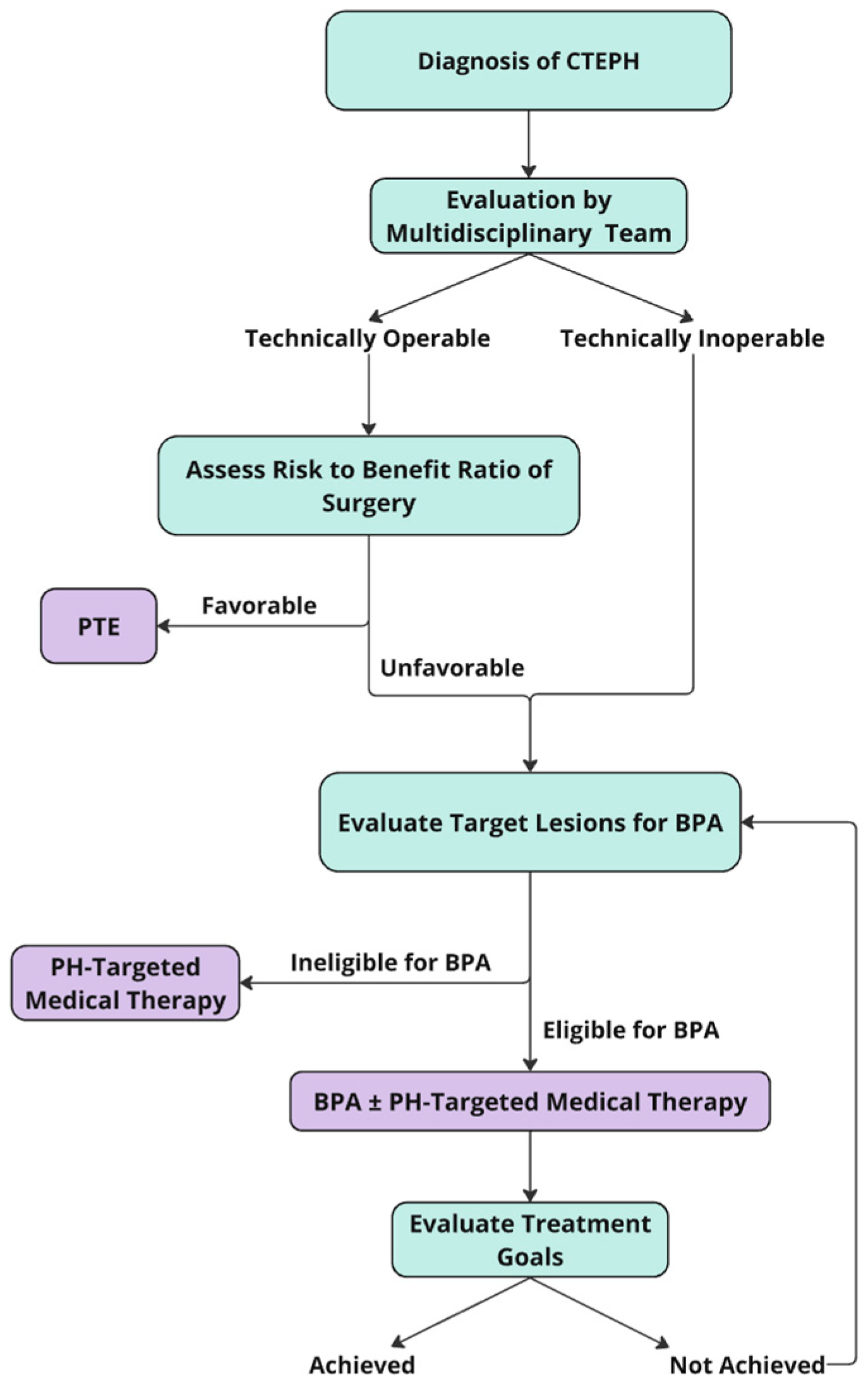
4. Indications and Patient Selection for BPA
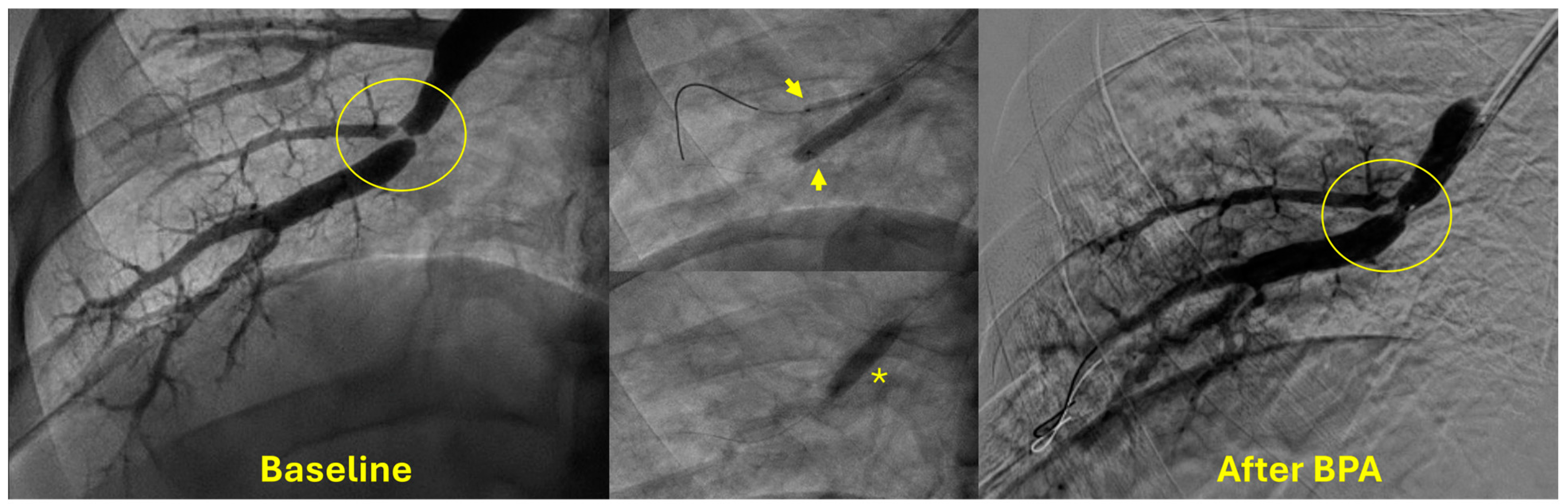
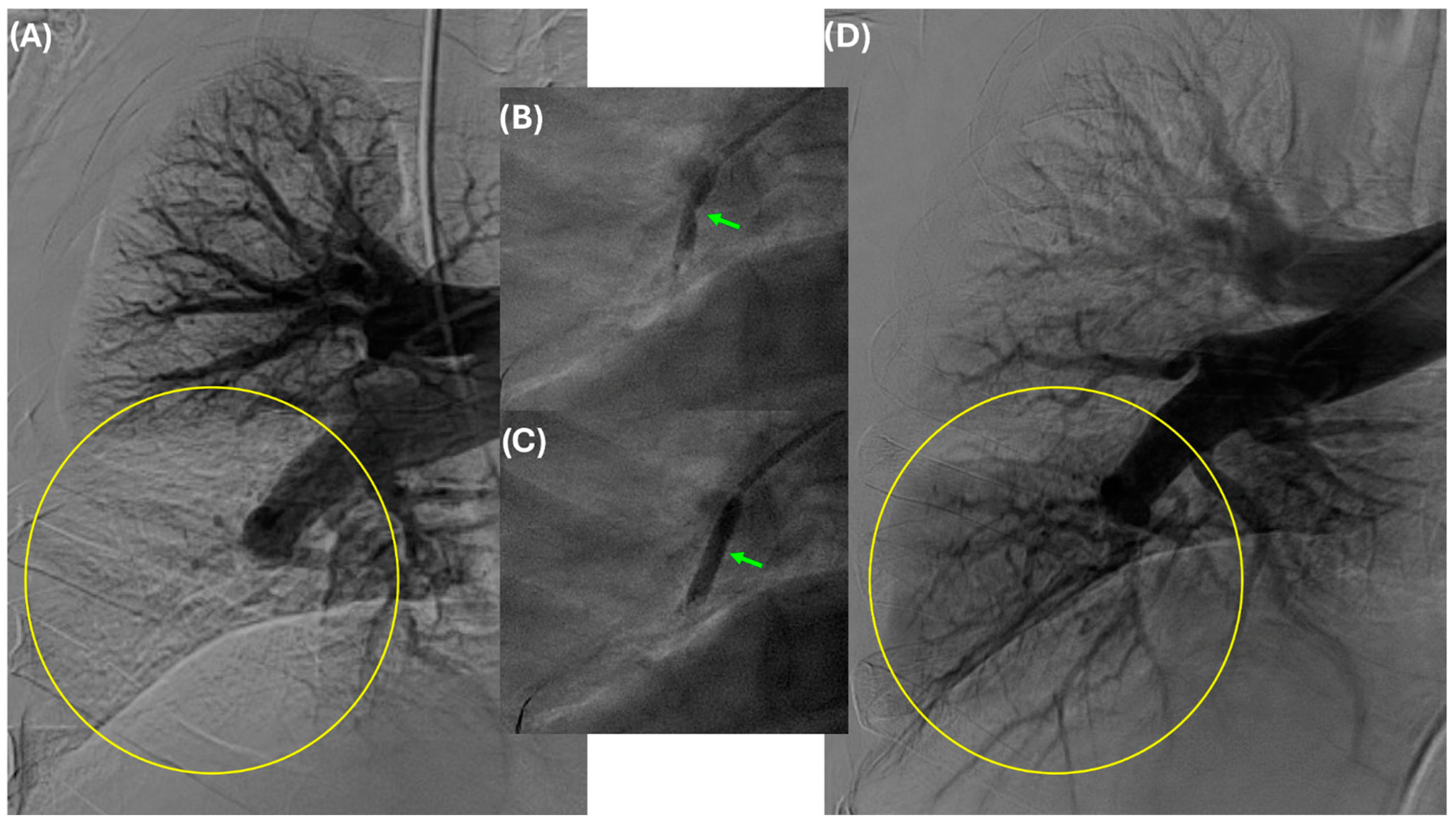
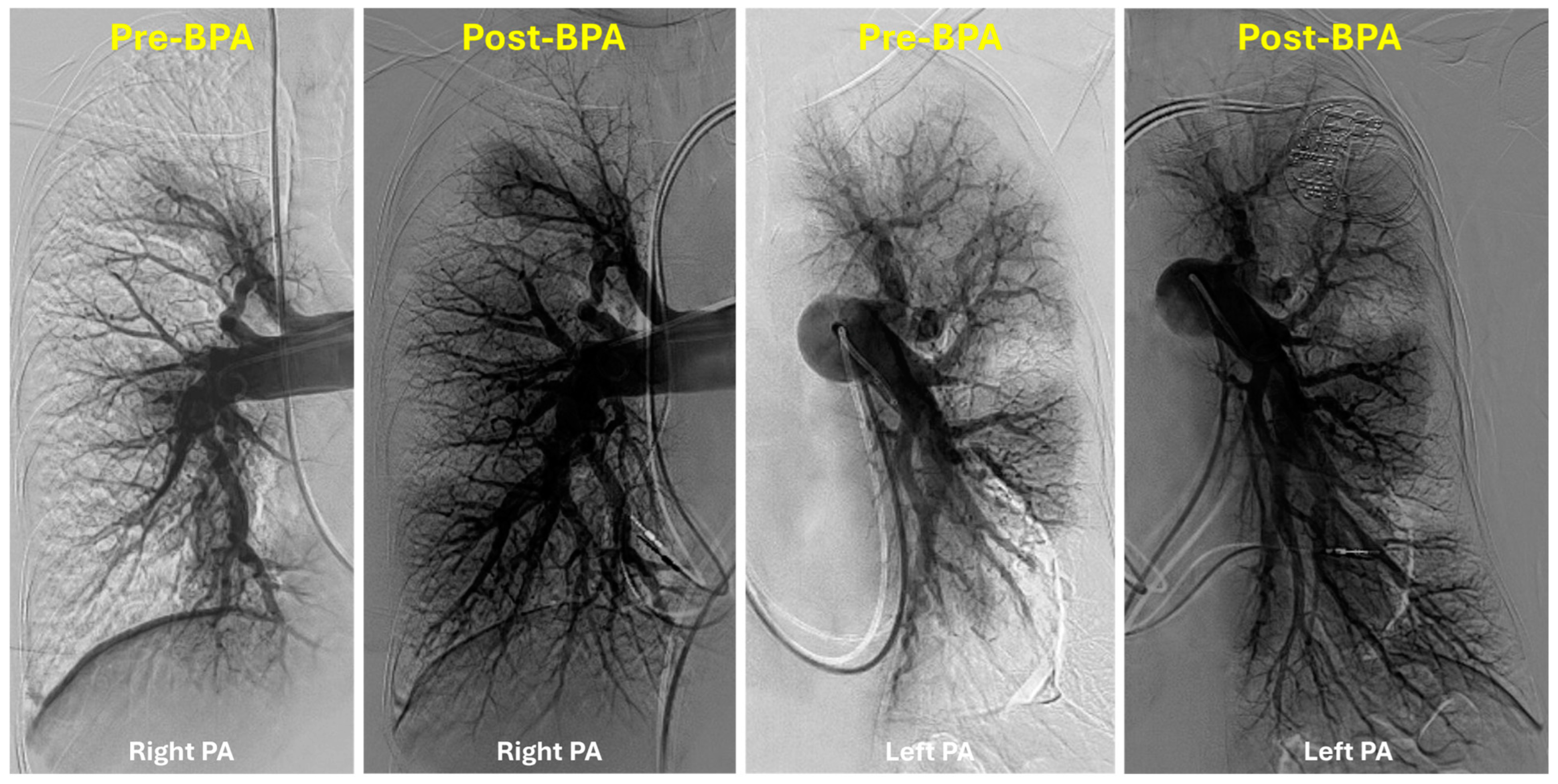
5. Technical Considerations
6. Outcomes
7. Global Heterogeneity of Patient Selection for BPA
8. Findings from Randomized Controlled Trials of BPA
9. Complications
10. Knowledge Gaps
11. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Bashir, R.; Auger, W.R.; Rosenfield, K. “Balloon Pulmonary Angioplasty in CTEPH and CTEPD: Where Is This All Going?” Endovascular Today, Bryn Mawr Communications, 24 January 2024. Available online: https://evtoday.com/articles/2024-jan/balloon-pulmonary-angioplasty-in-cteph-and-ctepd-where-is-this-all-going (accessed on 12 February 2024).
- Mahmud, E.; Madani, M.M.; Kim, N.H.; Poch, D.; Ang, L.; Behnamfar, O.; Patel, M.P.; Auger, W.R. Chronic Thromboembolic Pulmonary Hypertension: Evolving Therapeutic Approaches for Operable and Inoperable Disease. J. Am. Coll. Cardiol. 2018, 71, 2468–2486. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Tao, X.; Xie, W.; Wang, S.; Zhang, S.; Zhang, Z.; Fu, Z.; Li, H.; Zhang, Y.; et al. Safety and efficacy of balloon pulmonary angioplasty for technically operable chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2023, 14, e12327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Connell, C.; Montani, D.; Savale, L.; Sitbon, O.; Parent, F.; Seferian, A.; Bulifon, S.; Fadel, E.; Mercier, O.; Mussot, S.; et al. Chronic thromboembolic pulmonary hypertension. Presse Med. 2015, 44 Pt 2, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.; Korsholm, K.; Mellemkjær, S.; Nielsen-Kudsk, J.E. Switching from sildenafil to riociguat for the treatment of PAH and inoperable CTEPH: Real-life experiences. Respir. Med. Case Rep. 2017, 22, 39–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghofrani, H.A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.M.; Jansa, P.; Kim, N.H.; Mayer, E. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef]
- Drugs.com. Drug Price Information. Available online: https://www.drugs.com/price-guide (accessed on 24 January 2024).
- Narechania, S.; Torbic, H.; Tonelli, A.R. Treatment discontinuation or interruption in pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. Ther. 2020, 25, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, M.; Inami, T.; Kawakami, T.; Fukuda, K.; Satoh, T. Balloon Pulmonary Angioplasty (Percutaneous Transluminal Pulmonary Angioplasty) for Chronic Thromboembolic Pulmonary Hypertension: A Japanese Perspective. JACC Cardiovasc. Interv. 2019, 12, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Jais, X.; D’Armini, A.M.; Jansa, P.; Torbicki, A.; Delcroix, M.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Mayer, E.; Pepke-Zaba, J.; et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J. Am. Coll. Cardiol. 2008, 52, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Cabrol, S.; Souza, R.; Jais, X.; Fadel, E.; Ali, R.H.; Humbert, M.; Dartevelle, P.; Simonneau, G.; Sitbon, O. Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J. Heart Lung Transpl. 2007, 26, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, H.; Simonneau, G.; Galiè, N.; Higenbottam, T.; Naeije, R.; Rubin, L.J.; Nikkho, S.; Speich, R.; Hoeper, M.M.; Behr, J.; et al. Inhaled iloprost for severe pulmonary hypertension. N. Engl. J. Med. 2002, 347, 322–329. [Google Scholar] [CrossRef]
- Aoki, T.; Sugimura, K.; Tatebe, S.; Miura, M.; Yamamoto, S.; Yaoita, N.; Suzuki, H.; Sato, H.; Kozu, K.; Konno, R.; et al. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo-embolic pulmonary hypertension: Long-term effects and procedure-related complications. Eur. Heart J. 2017, 38, 3152–3159. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.; Meyer, B.C.; Ogo, T.; Matsubara, H.; Kurzyna, M.; Ghofrani, H.A.; Mayer, E.; Brenot, P. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160119, Erratum in Eur. Respir. Rev. 2017, 26, 165119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inami, T.; Kataoka, M.; Ando, M.; Fukuda, K.; Yoshino, H.; Satoh, T. A new era of therapeutic strategies for chronic thromboembolic pulmonary hypertension by two different interventional therapies; pulmonary endarterectomy and percutaneous transluminal pulmonary angioplasty. PLoS ONE 2014, 9, e94587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahmud, E.; Patel, M.; Ang, L.; Poch, D. Advances in balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2021, 11, 20458940211007385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jevnikar, M.; Bokan, A.; Gillem, T.; Bertoletti, L.; Lichtblau, M. Balloon pulmonary angioplasty: Are we there yet? Lessons learned and unanswered questions. Breathe 2022, 18, 220217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voorburg, J.A.; Cats, V.M.; Buis, B.; Bruschke, A.V. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest. 1988, 94, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, J.A.; Goldhaber, S.Z.; Lock, J.E.; Fernanders, S.M.; Landzberg, M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001, 103, 10–13. [Google Scholar] [CrossRef]
- Bashir, R.; Noory, A.; Oliveros, E.; Romero, C.; Maruthi, R.; Mirza, A.; Lakhter, V.; Zhao, H.; Brisco-Bacik, M.; Vaidya, A.; et al. Refined Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension: Initial Results of U.S. Regional Program. JACC Adv. 2023, 2, 100291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Inami, T.; Kataoka, M.; Shimura, N.; Ishiguro, H.; Yanagisawa, R.; Fukuda, K.; Yoshino, H.; Satoh, T. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: A breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc. Interv. 2014, 7, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Tunariu, N.; Gibbs, S.J.; Win, Z.; Gin-Sing, W.; Graham, A.; Gishen, P.; Al-Nahhas, A. Ventilation-perfusion scintigraphy is more sensitive than multidetector CTPA in detecting chronic thromboembolic pulmonary disease as a treatable cause of pulmonary hypertension. J. Nucl. Med. 2007, 48, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Bartnik, A.; Pepke-Zaba, J.; Bunclark, K.; Ruggiero, A.; Jenkins, D.; Taghavi, J.; Tsui, S.; Screaton, N.; D’Errico, L.; Weir-McCall, J. Cardiac MRI in the assessment of chronic thromboembolic pulmonary hypertension and response to treatment. Thorax 2024, 79, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.; McDivit Mizzell, A.; Daniels, L.B.; Ben-Yehuda, O.; Mahmud, E. Optimal Technique for Performing Invasive Pulmonary Angiography for Chronic Thromboembolic Pulmonary Disease. J. Invasive Cardiol. 2019, 31, E211–E219. [Google Scholar] [PubMed]
- Piazza, G.; Goldhaber, S.Z. Chronic thromboembolic pulmonary hypertension. N. Engl. J. Med. 2011, 364, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Giri, J.; Visovatti, S.H.; Mahmud, E.; Matsubara, H.; Madani, M.; Rogers, F.; Gopalan, D.; Rosenfield, K.; McLaughlin, V.V.; et al. Status and Future Directions for Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Disease with and Without Pulmonary Hypertension: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e1090–e1107. [Google Scholar] [CrossRef]
- Lang, I.M.; Andreassen, A.K.; Andersen, A.; Bouvaist, H.; Coghlan, G.; Escribano-Subias, P.; Jansa, P.; Kopec, G.; Kurzyna, M.; Matsubara, H.; et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: A clinical consensus statement of the ESC working group on pulmonary circulation and right ventricular function. Eur. Heart J. 2023, 44, 2659–2671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogawa, A.; Matsubara, H. Balloon Pulmonary Angioplasty: A Treatment Option for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension. Front. Cardiovasc. Med. 2015, 2, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahmud, E.; Behnamfar, O.; Ang, L.; Patel, M.P.; Poch, D.; Kim, N.H. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Interv. Cardiol. Clin. 2018, 7, 103–117. [Google Scholar] [PubMed]
- Daigo, K.; Katsumata, Y.; Esaki, K.; Iwasawa, Y.; Ichihara, G.; Miura, K.; Shirakawa, K.; Sato, Y.; Sato, K.; Fukuda, K. Predictors of Improvement in Exercise Tolerance After Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2023, 12, e8137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Darocha, S.; Banaszkiewicz, M.; Pietrasik, A.; Siennicka, A.; Piorunek, M.; Grochowska, E.; Piłka, M.; Dobosiewicz, A.; Florczyk, M.; Pietura, R. Changes in estimated glomerular filtration after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Cardiorenal Med. 2020, 10, 22–31. [Google Scholar] [CrossRef]
- Kriechbaum, S.D.; Wiedenroth, C.B.; Hesse, M.L.; Ajnwojner, R.; Keller, T.; Wolter, S.J.; Haas, M.; Roller, F.C.; Breithecker, A.; Rieth, A.J. Development of renal function during staged balloon pulmonary angioplasty for inoperable chronic thromboembolic pulmonary hypertension. Scand. J. Clin. Lab. Investig. 2019, 79, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.E.; Su, L.; Kiely, D.G.; Page, K.; Toshner, M.; Swietlik, E.; Treacy, C.; Ponnaberanam, A.; Condliffe, R.; Sheares, K.; et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: Results from the United Kingdom National Cohort. Circulation 2016, 133, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.; Lang, I.; Pepke-Zaba, J.; Jansa, P.; D’Armini, A.M.; Snijder, R.; Bresser, P.; Torbicki, A.; Mellemkjaer, S.; Lewczuk, J.; et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. Circulation 2016, 133, 859–871. [Google Scholar] [CrossRef]
- Banaszkiewicz, M.; Kurzyna, P.; Kubikowska, N.; Mucha, M.; Rudnik, A.; Gąsecka, A.; Pietrasik, A.; Grabowski, M.; Jaguszewski, M.J.; Kasprzyk, P.; et al. Emerging Role of Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Disease-Insights from the 2022 ESC Guidelines. J. Clin. Med. 2023, 12, 5336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiedenroth, C.B.; Liebetrau, C.; Breithecker, A.; Guth, S.; Lautze, H.J.; Ortmann, E.; Arlt, M.; Krombach, G.A.; Bandorski, D.; Hamm, C.W.; et al. Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J. Heart. Lung Transpl. 2016, 35, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.P.; Wei, Y.P.; Yang, Y.J.; Xu, X.Q.; Wu, T.; Liu, C.; Mei, K.Y.; Peng, F.H.; Wang, H.P.; Sun, K.; et al. Percutaneous Pulmonary Angioplasty for Patients with Takayasu Arteritis and Pulmonary Hypertension. J. Am. Coll. Cardiol. 2022, 79, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, A.; Chamarthy, M. Pulmonary vascular anatomy & anatomical variants. Cardiovasc. Diagn. Ther. 2018, 8, 201–207. [Google Scholar] [CrossRef]
- Witowski, J.; Darocha, S.; Kownacki, L.; Pietrasik, A.; Pietura, R.; Banaszkiewicz, M.; Kaminski, J.; Biederman, A.; Torbicki, A.; Kurzyna, M. Augmented reality and three-dimensional printing in percutaneous interventions on pulmonary arteries. Quant. Imaging Med. Surg. 2019, 9, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Jin, Q.; Luo, Q.; Zhao, Q.; Yang, T.; Zeng, Q.; Yan, L.; Duan, A.; Huang, Z.; et al. Predictors of early response to balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Ther. Adv. Respir. Dis. 2022, 16, 17534666221138001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kennedy, M.K.; Kennedy, S.A.; Tan, K.T.; de Perrot, M.; Bassett, P.; McInnis, M.C.; Thenganatt, J.; Donahoe, L.; Granton, J.; Mafeld, S. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: A systematic review and meta-analysis. Cardiovasc. Intervent. Radiol. 2023, 46, 5–18. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Fukuda, T.; Sugimura, K.; Fukumoto, Y.; Emoto, N.; Yamada, N.; Yao, A.; Ando, M.; Ogino, H.; et al. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: Results of a multicenter registry. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e004029. [Google Scholar] [CrossRef]
- Brenot, P.; Jaïs, X.; Taniguchi, Y.; Garcia Alonso, C.; Gerardin, B.; Mussot, S.; Mercier, O.; Fabre, D.; Parent, F.; Jevnikar, M.; et al. French experience of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1802095. [Google Scholar] [CrossRef] [PubMed]
- Poch, D.S.; Mahmud, E.; Patel, M.; Papamatheakis, D.; Fernandes, T.; Kerr, K.; Yang, J.; Pretorius, V.; Madani, M.M.; Kim, N.H. Patient selection for balloon pulmonary angioplasty: Six-year results from a high volume PTE surgical center. Pulm. Circ. 2022, 12, e12148. [Google Scholar] [CrossRef]
- Carlozzi, L.N.; Lee, J.; Barros, L.M.; Buber, Y.; Chen, D.L.; Mulligan, M.; Ordovas, K.; Ralph, D.D.; Rayner, S.G.; Leary, P.J.; et al. Establishing a balloon pulmonary angioplasty program for chronic thromboembolic pulmonary hypertension: A United States single-center experience. Respir. Med. 2023, 211, 107215. [Google Scholar] [CrossRef] [PubMed]
- Anand, V.; Frantz, R.P.; DuBrock, H.; Kane, G.C.; Krowka, M.; Yanagisawa, R.; Sandhu, G.S. Balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension: Initial single-center experience. Mayo Clin. Proc. Innov. Qual. Outcomes. 2019, 3, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.; Patel, N.; Hyder, S.N.; Gurm, H.; Moles, V.; Agarwal, P.P.; Visovatti, S.; Haft, J.; Cascino, T.; Mclaughlin, V.V.; et al. Safe and effective balloon pulmonary angioplasty in the outpatient setting: The Michigan Medicine experience. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100589. [Google Scholar] [CrossRef]
- Guth, S.; D’Armini, A.M.; Delcroix, M.; Nakayama, K.; Fadel, E.; Hoole, S.P.; Jenkins, D.P.; Kiely, D.G.; Kim, N.H.; Lang, I.M.; et al. Current strategies for managing chronic thromboembolic pulmonary hypertension: Results of the worldwide prospective CTEPH registry. ERJ Open Res. 2021, 7, 00850–02020. [Google Scholar] [CrossRef]
- Kerr, K.M.; Elliott, C.G.; Chin, K.; Benza, R.L.; Channick, R.N.; Davis, R.D.; He, F.; LaCroix, A.; Madani, M.M.; McLaughlin, V.V.; et al. Results from the United States chronic thromboembolic pulmonary hyperten-sion registry: Enrollment characteristics and 1-year follow-up. Chest 2021, 160, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Jaïs, X.; Brenot, P.; Bouvaist, H.; Jevnikar, M.; Canuet, M.; Chabanne, C.; Chaouat, A.; Cottin, V.; De Groote, P.; Favrolt, N.; et al. Balloon pulmonary angioplasty versus riociguat for the treatment of inoperable chronic thromboembolic pulmonary hypertension (RACE): A multicentre, phase 3, open-label, randomized controlled trial and ancillary follow-up study. Lancet Respir. Med. 2022, 10, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Matsubara, H.; Shinke, T.; Abe, K.; Kohsaka, S.; Hosokawa, K.; Taniguchi, Y.; Shimokawahara, H.; Yamada, Y.; Kataoka, M.; et al. Balloon pulmonary angioplasty versus riociguat in inoperable chronic thromboembolic pulmonary hypertension (MR BPA): An open-label, randomised controlled trial. Lancet Respir. Med. 2022, 10, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Inami, T.; Kataoka, M.; Shimura, N.; Ishiguro, H.; Yanagisawa, R.; Taguchi, H.; Fukuda, K.; Yoshino, H.; Satoh, T. Pulmonary Edema Predictive Scoring Index (PEPSI), a New Index to Predict Risk of Reperfusion Pulmonary Edema and Improvement of Hemodynamics in Percutaneous Transluminal Pulmonary Angioplasty. J. Am. Coll. Cardiol. Interv. 2013, 6, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.; Valencia Villeda, G.; Takeda, K.; Rosenzweig, E.B. Chronic Thromboembolic Pulmonary Hypertension in a Child with Sickle Cell Disease. Front. Pediatr. 2020, 8, 363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Outcomes at United States Centers | University of California [46] | Temple University [21] | University of Washington [47] | Mayo Clinic [48] | University of Michigan [49] |
|---|---|---|---|---|---|
| 00Total no. of patients | 97 | 77 | 30 | 31 | 18 |
| Hemoptysis rate/sessions | 9.2 | 4.7 | 17.8 | 4 | 3.8 |
| Death (% of patients) | 0 | 1.3 | 6.6 | 3.2 | 0 |
| Change in mean PAP (mmHg) | −5.6 | −6.4 | −6 | −11 | −8.3 |
| Change in PVR (Wood Units) | −1.2 | −1.7 | −1.9 | −2.2 | −1.7 |
| Change in 6-min walk distance (m) | 36.8 | 71.7 | 40 | 37 | 67.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsen, J.; Lakhter, V.; Nasri, A.; Bashir, R. Evolution and New Perspectives of Balloon Pulmonary Angioplasty in CTEPH. J. Clin. Med. 2025, 14, 699. https://doi.org/10.3390/jcm14030699
Larsen J, Lakhter V, Nasri A, Bashir R. Evolution and New Perspectives of Balloon Pulmonary Angioplasty in CTEPH. Journal of Clinical Medicine. 2025; 14(3):699. https://doi.org/10.3390/jcm14030699
Chicago/Turabian StyleLarsen, Julia, Vladimir Lakhter, Amine Nasri, and Riyaz Bashir. 2025. "Evolution and New Perspectives of Balloon Pulmonary Angioplasty in CTEPH" Journal of Clinical Medicine 14, no. 3: 699. https://doi.org/10.3390/jcm14030699
APA StyleLarsen, J., Lakhter, V., Nasri, A., & Bashir, R. (2025). Evolution and New Perspectives of Balloon Pulmonary Angioplasty in CTEPH. Journal of Clinical Medicine, 14(3), 699. https://doi.org/10.3390/jcm14030699





