Do Lumbar Paravertebral Muscle Properties Show Changes in Mothers with Moderate-Severity Low Back Pain Following a Cesarean Birth? A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Setting
2.1.2. Participants
2.1.3. Sample Size Calculation
2.1.4. Randomization
2.1.5. Data Collection Procedures
2.1.6. Inclusion and Exclusion Criteria
2.2. Outcomes Measure
2.3. Assessment Procedures
2.3.1. MyotonPRO Device
2.3.2. Psychometrics of the MyotonPRO Device
2.4. Measurement Procedures
2.5. Statistical Analysis
3. Results
3.1. Physical Characteristics of Participants
3.2. The Comparison of LPVMs’ Properties (Tone, Stiffness, Elasticity, Stress Relaxation Time, and Creep) Between the Two Groups
3.3. The Binomial Logistic Regression Analysis to Examine the Relationship Between Group Membership and the Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berghella, V.; Baxter, J.K.; Chauhan, S.P. Evidence-based surgery for cesarean delivery. Am. J. Obstet. Gynecol. 2005, 193, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Betrán, A.P.; Ye, J.; Moller, A.B.; Zhang, J.; Gülmezoglu, A.M.; Torloni, M.R. The increasing trend in caesarean section rates: Global, regional and national estimates: 1990–2014. PLoS ONE 2016, 11, e0148343. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, B.; Assar, T.M.; Nucier, A.A.; Raziq, H.E.; Saad, A.S.; Amer, W.M. Analysis of the cesarean section rate using the 10-Group Robson classification at Benha University Hospital, Egypt. Women Birth 2020, 33, e105–e110. [Google Scholar] [CrossRef] [PubMed]
- Egypt’s Central Agency for Public Mobilization and Statistics (CAPMAS). Egypt Family Health Survey: Development in Obstetric Care Between 2014 and 2021. Available online: https://amwalalghad.com/wp-content/uploads/2022/08/%D8%B9%D8%B1%D8%B6-%D9%86%D9%87%D8%A7%D8%A6%D9%89-30-8-2022.pdf (accessed on 30 August 2022).
- Betrán, A.P.; Ye, J.; Moller, A.B.; Souza, J.P.; Zhang, J. Trends and projections of caesarean section rates: Global and regional estimates. BMJ Glob. Health 2021, 6, e005671. [Google Scholar] [CrossRef]
- World Health Organization. Cesarean Section Rates Continue to Rise, Amid Growing Inequalities in Access. Available online: https://www.who.int/news/item/16-06-2021-caesarean-section-rates-continue-to-rise-amid-growing-inequalities-in-access (accessed on 16 June 2021).
- Rifai, A. Trend of cesarean deliveries in Egypt and its associated factors: Evidence from national surveys, 2005–2014. BMC Pregnancy Childbirth 2017, 17, 417. [Google Scholar] [CrossRef] [PubMed]
- Elnakib, S.; Abdel-Tawab, N.; Orbay, D.; Hassanein, N. Medical and non-medical reasons for cesarean section delivery in Egypt: A hospital-based retrospective study. BMC Pregnancy Childbirth 2019, 19, 411. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Co-operation and Development (OECD). Education at a Glance 2010: OECD Indicators; OECD: Paris, France, 2010. [Google Scholar]
- Gibbons, L.; Belizán, J.M.; Lauer, J.A.; Betrán, A.P.; Merialdi, M.; Althabe, F. The global numbers and costs of additionally needed and unnecessary cesarean sections performed per year: Overuse as a barrier to universal coverage. World Health Rep. 2010, 30, 1–31. [Google Scholar]
- Quinlan, J.D.; Murphy, N.J. Cesarean delivery: Counseling issues and complication management. Am. Fam. Physician 2015, 91, 178–184. [Google Scholar] [PubMed]
- Parikh, S.; Suchi, J. Prevalence of low back pain and its impact on quality of life in postpartum women. Int. J. Sci. Res. 2016, 7, 14342–14348. [Google Scholar]
- Australian Commission on Safety and Quality in Health Care (ACSQHC). The Second Australian Atlas of Healthcare Variation; ACSQHC: Sydney, Australia, 2018.
- MacArthur, C.; Lewis, M.; Knox, E.G.; Crawford, J.S.; Marx, G.F. Epidural anesthesia and long-term backache after childbirth. Obstet. Anesth. Dig. 1991, 10, 207. [Google Scholar] [CrossRef]
- Fan, C.; Guidolin, D.; Ragazzo, S.; Figueiredo, A.; Souza, D.; Lima, A.; Oliveira, S.; Andrade, S.; Gama, L.; Santos, L.; et al. Effects of Cesarean Section and Vaginal Delivery on Abdominal Muscles and Fasciae. Medicina 2020, 56, 260. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.G.; Yousef, A.M.; Okeel, F.M.; Sarhan, M.A.M.; Alwhaibi, R.; Zakaria, H.M.; Mohammed, A.A.; Mamoon, R.S.; Auais, M. Exploring Mechanical Changes in the Transversus Abdominis Muscle Following Cesarean Delivery in Postpartum Women. In Proceedings of the 2nd Edition of the Global Conference on Gynecology & Women’s Health, Baltimore, MD, USA, 17–19 October 2024; Magnus Group: Chicago, IL, USA, 2024; pp. 57–58. Available online: https://gynecology.magnusconferences.com/program/scientific-program/2024/exploring-mechanical-changes-in-the-transversus-abdominis-muscle-following-cesarean-delivery-in-postpartum-women (accessed on 6 October 2024).
- Van Dieën, J.H.; Selen, L.P.; Cholewicki, J. Trunk muscle activation in low-back pain patients: An analysis of the literature. J. Electromyogr. Kinesiol. 2003, 13, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, S.; Masi, T.; White, A.; Devos, A.; Henderson, J.; Nair, K. Quantified biomechanical properties of lower lumbar myofascia in younger adults with chronic idiopathic low back pain and matched healthy controls. Clin. Biomech. 2020, 73, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kamaya, A.; Wong-You-Cheong, J. Diagnostic Ultrasound: Abdomen and Pelvis, 2nd ed.; Elsevier-Health Sciences Division: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Darby, S.A.; Frysztak, R.J. Neuroanatomy of the spinal cord. In Clinical Anatomy of the Spine, Spinal Cord, and ANS; Elsevier: Amsterdam, The Netherlands, 2014; pp. 341–412. [Google Scholar]
- Van Deun, B.; Hobbelen, J.S.M.; Cagnie, B.; Van Eetvelde, B.; Van Den Noortgate, N.; Cambier, D. Reproducible measurements of muscle characteristics using the MyotonPRO device: Comparison between individuals with and without paratonia. J. Geriatr. Phys. Ther. 2018, 41, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Struzik, A.; Karamanidis, K.; Lorimer, A.; Keogh, J.W.L.; Gajewski, J. Application of leg, vertical, and joint stiffness in running performance: A literature overview. Appl. Bionics Biomech. 2021, 2021, 9914278. [Google Scholar] [CrossRef] [PubMed]
- Myoton. MyotonPRO User Manual, 6th ed.; Myoton: London, UK, 2013. [Google Scholar]
- Abilmona, S.M.; Gorgey, A.S. Associations of the trunk skeletal musculature and dietary intake to biomarkers of cardiometabolic health after spinal cord injury. Clin. Physiol. Funct. Imaging 2018, 38, 949–958. [Google Scholar] [CrossRef]
- Krkoska, P.; Kokosova, V.; Dostal, M.; Vlazna, D.; Kerkovsky, M.; Straka, M.; Gerstberger, R.; Matulova, K.; Ovesna, P.; Adamova, B. Assessment of lumbar paraspinal muscle morphology using mDixon Quant magnetic resonance imaging (MRI): A cross-sectional study in healthy subjects. Quant. Imaging Med. Surg. 2024, 14, 6015. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Danneels, L. Changes in structure and function of the back muscles in low back pain: Different time points, observations, and mechanisms. J. Orthop. Sports Phys. Ther. 2019, 49, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.I.; Garden, C.L.P.; Brown, S.J. The immediate effect of a spinal mobilization intervention on muscle stiffness, tone and elasticity in subjects with lower back pain: A randomized cross-over trial. J. Bodyw. Mov. Ther. 2022, 29, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.L.A.; Yu, Q.; Mao, Y.; Li, W.; Hu, C.; Li, L. Lumbar muscles biomechanical characteristics in young people with chronic spinal pain. BMC Musculoskelet. Disord. 2019, 20, 559. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Cohen, M.; Baron, R.; Gebhart, G.F.; Mico, J.A.; Rice, A.S.; Rief, W.; Sluka, A.K. Do we need a third mechanistic descriptor for chronic pain states? Pain 2016, 157, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Balk, G.A.; Stewart, R.E. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain 2014, 155, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, Y.; Ye, Z.; Guan, Y.; Ye, X.; Chen, Z.; Li, C.; Chen, G.; Zhu, Y.; Du, J.; et al. Effects of age and sex on properties of lumbar erector spinae in healthy people: Preliminary results from a pilot study. Front. Physiol. 2021, 12, 718068. [Google Scholar] [CrossRef] [PubMed]
- Koes, B.W.; van Tulder, M.; Lin, C.W.C.; Macedo, L.G.; McAuley, J.; Maher, C. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur. Spine J. 2010, 19, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Peipsi, A.; Kerpe, R.; Jäger, H.; Soeder, S.; Gordon, C.; Schleip, R. Myoton pro: A novel tool for the assessment of mechanical properties of fascial tissues. J. Bodyw. Mov. Ther. 2012, 16, 527. [Google Scholar] [CrossRef]
- Lohr, C.; Braumann, K.M.; Reer, R.; Schroeder, J.; Schmidt, T. Reliability of tensiomyography and myotonometry in detecting mechanical and contractile characteristics of the lumbar erector spinae in healthy volunteers. Eur. J. Appl. Physiol. 2018, 118, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lei, D.; Li, L.; Leng, Y.; Yu, Q.; Wei, X.; Lo, W.L.A. Quantifying paraspinal muscle tone and stiffness in young adults with chronic low back pain: A reliability study. Sci. Rep. 2018, 8, 14343. [Google Scholar] [CrossRef]
- Mannion, A.F.; Dumas, G.A.; Cooper, R.G.; Espinosa, F.J.; Faris, M.W.; Stevenson, J.M. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: Normal values and sex differences. J. Anat. 1997, 190, 505–513. [Google Scholar] [CrossRef]
- Devantéry, K.; Morin, M.; Grimard, J.; Gaudreault, N. Effects of a Myofascial Technique on the Stiffness and Thickness of the Thoracolumbar Fascia and Lumbar Erector Spinae Muscles in Adults with Chronic Low Back Pain: A Randomized Before-and-After Experimental Study. Bioengineering 2023, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz-Clariana, S.; García-Luque, L.; Garrido-Castro, J.L.; Aranda-Valera, I.C.; Ladehesa-Pineda, L.; Puche-Larrubia, M.Á.; Carmona-Pérez, C.; Rodrigues-de-Souza, D.P.; Alburquerque-Sendín, F. Paravertebral muscle mechanical properties in patients with axial spondyloarthritis or low back pain: A case-control study. Diagnostics 2021, 11, 1898. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.; Zhang, J.; Zhang, Z.; Wang, X. Quantifying the stiffness of lumbar erector spinae during different positions among participants with chronic low back pain. PLoS ONE 2022, 17, e0270286. [Google Scholar] [CrossRef] [PubMed]
- Magee, D.J.; Zachazewski, J.E.; Quillen, W.S.; Manske, R.C. Pathology and Intervention in Musculoskeletal Rehabilitation, 2nd ed.; Saunders: Philadelphia, PA, USA, 2016. [Google Scholar]
- Abboud, J.; Nougarou, F.; Descarreaux, M. Muscle activity adaptations to spinal tissue creep in the presence of muscle fatigue. PLoS ONE 2016, 11, e0149076. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rasheed, N.; Khan, M.H.; Rasheed, N. Comparison of incidence of low back pain in women with normal vaginal delivery and cesarean section. J. Pak. Orthop. Assoc. 2017, 29, 152–156. [Google Scholar]
- Wiezer, M.; Hage-Fransen, M.A.H.; Otto, A.; Wieffer-Platvoet, M.S.; Slotman, M.H.; Nijhuis-Van der Sanden, M.W.G.; Pool-Goudzwaard, A.L. Risk factors for pelvic girdle pain postpartum and pregnancy-related low back pain postpartum: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2020, 48, 102154. [Google Scholar] [CrossRef]

| Groups | Mean ± SD | p-Value | |
|---|---|---|---|
| Age | Control group | 23.3 ± 1.8 | 0.225 Z |
| Case group | 25.2 ± 4.9 | ||
| BMI | Control group | 23.4 ± 4.1 | 0.060 Z |
| Case group | 25.6 ± 2.5 | ||
| PPD | Control group | 0.00 ± 0.000 | 0.000 Z |
| Case group | 7.9 ± 1.9 | ||
| Parity | Control group | 0.00 ± 0.000 | 0.000 Z |
| Case group | 2.1 ± 1.1 | ||
| VAS | Control group | 1.8 ± 0.8 | 0.000 Z |
| Case group | 5.2 ± 1.1 |
| Right LPVMs | Left LPVMs | ||||
|---|---|---|---|---|---|
| Groups | Mean ± SD | p | Mean ± SD | p | |
| Tone | Control group | 13.7 ± 2.0 | 0.002 * Z | 13.5 ± 1.8 | 0.015 * Z |
| Case group | 12.2 ± 1.3 | 12.5 ± 1.2 | |||
| Stiffness | Control group | 162.6 ± 36.3 | 0.055 Z | 186.9 ± 55.3 | 0.367 Z |
| Case group | 162.6 ± 36.3 | 169.9 ± 40.0 | |||
| Elasticity | Control group | 0.8 ± 0.1 | 0.115 Z | 0.8 ± 0.2 | 0.231 Z |
| Case group | 0.9 ± 0.4 | 0.9 ± 0.3 | |||
| Relaxation time | Control group | 21.5 ± 4.1 | 0.002 * | 21.9 ± 4.1 | 0.022 * |
| Case group | 24.9 ± 4.0 | 24.2 ± 3.3 | |||
| Creep | Control group | 1.1 ± 0.2 | 0.006 * | 1.1 ± 0.2 | 0.013 * Z |
| Case group | 1.3 ± 0.2 | 1.3 ± 0.2 | |||
| Model | Model Fitting Criteria | Likelihood Ratio Tests | Pseudo R-Square | |||
| −2 Log Likelihood | Chi-Square | df | p-value | Cox and Snell | 0.75 | |
| Null | 40.20 | Nagelkerke | 1.00 | |||
| McFadden | 1.00 | |||||
| Final | 0.00 | 40.20 | 15 | 0.000 | ||
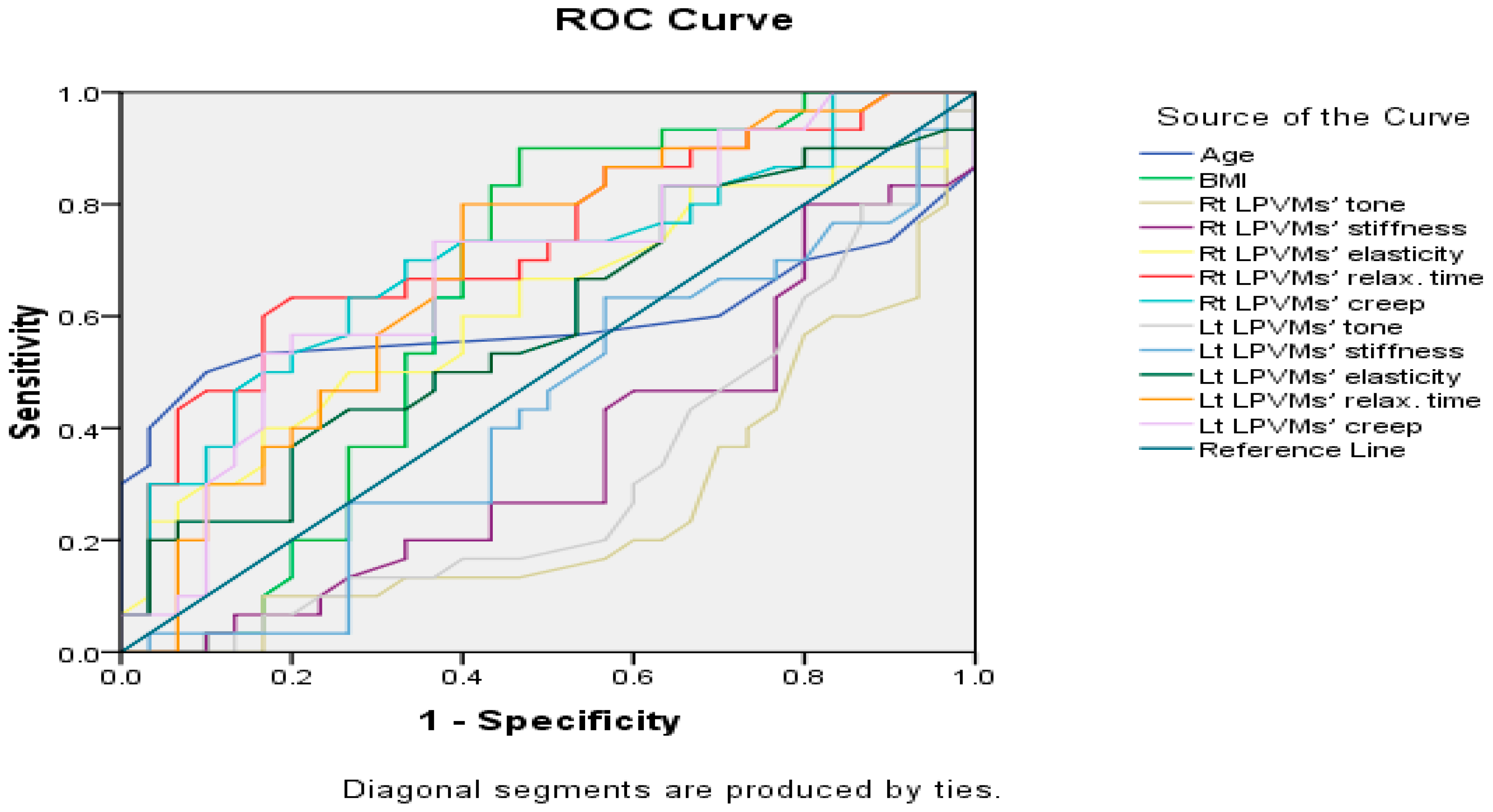
| Variables | AUC | Standard Error | p-Value | 95% Confidence Interval |
|---|---|---|---|---|
| Age | 0.59 | 0.08 | 0.228 | 0.43–0.75 |
| BMI | 0.64 | 0.08 | 0.060 | 0.49–0.79 |
| Rt LPVMs’ tone | 0.26 | 0.07 | 0.002 * | 0.13–0.39 |
| Rt LPVMs’ stiffness | 0.36 | 0.07 | 0.055 | 0.22–0.50 |
| Rt LPVMs’ elasticity | 0.62 | 0.07 | 0.115 | 0.47–0.76 |
| Rt LPVMs’ relax. time | 0.73 | 0.07 | 0.002 * | 0.60–0.86 |
| Rt LPVMs’ creep | 0.70 | 0.07 | 0.008 * | 0.57–0.83 |
| Lt LPVMs’ tone | 0.32 | 0.07 | 0.015 * | 0.18–0.45 |
| Lt LPVMs’ stiffness | 0.43 | 0.08 | 0.367 | 0.29–0.58 |
| Lt LPVMs’ elasticity | 0.59 | 0.07 | 0.231 | 0.45–0.73 |
| Lt LPVMs’ relax. time | 0.69 | 0.07 | 0.012 * | 0.55–0.82 |
| Lt LPVMs’ creep | 0.69 | 0.07 | 0.013 * | 0.55–0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.G.; Mohammed, A.A.; Ragab, W.M.; Zakaria, H.M.; Alwhaibi, R.M.; Ibrahim, Z.M.; Mamoon, R.S. Do Lumbar Paravertebral Muscle Properties Show Changes in Mothers with Moderate-Severity Low Back Pain Following a Cesarean Birth? A Case–Control Study. J. Clin. Med. 2025, 14, 719. https://doi.org/10.3390/jcm14030719
Ali MG, Mohammed AA, Ragab WM, Zakaria HM, Alwhaibi RM, Ibrahim ZM, Mamoon RS. Do Lumbar Paravertebral Muscle Properties Show Changes in Mothers with Moderate-Severity Low Back Pain Following a Cesarean Birth? A Case–Control Study. Journal of Clinical Medicine. 2025; 14(3):719. https://doi.org/10.3390/jcm14030719
Chicago/Turabian StyleAli, Mohamed G., Abeer A. Mohammed, Walaa M. Ragab, Hoda M. Zakaria, Reem M. Alwhaibi, Zizi M. Ibrahim, and Rehab S. Mamoon. 2025. "Do Lumbar Paravertebral Muscle Properties Show Changes in Mothers with Moderate-Severity Low Back Pain Following a Cesarean Birth? A Case–Control Study" Journal of Clinical Medicine 14, no. 3: 719. https://doi.org/10.3390/jcm14030719
APA StyleAli, M. G., Mohammed, A. A., Ragab, W. M., Zakaria, H. M., Alwhaibi, R. M., Ibrahim, Z. M., & Mamoon, R. S. (2025). Do Lumbar Paravertebral Muscle Properties Show Changes in Mothers with Moderate-Severity Low Back Pain Following a Cesarean Birth? A Case–Control Study. Journal of Clinical Medicine, 14(3), 719. https://doi.org/10.3390/jcm14030719







