Effects of Anticancer Therapy on Osteoporosis in Breast Cancer Patients: A Nationwide Study Using Data from the National Health Insurance Service-National Health Information Database
Abstract
:1. Introduction
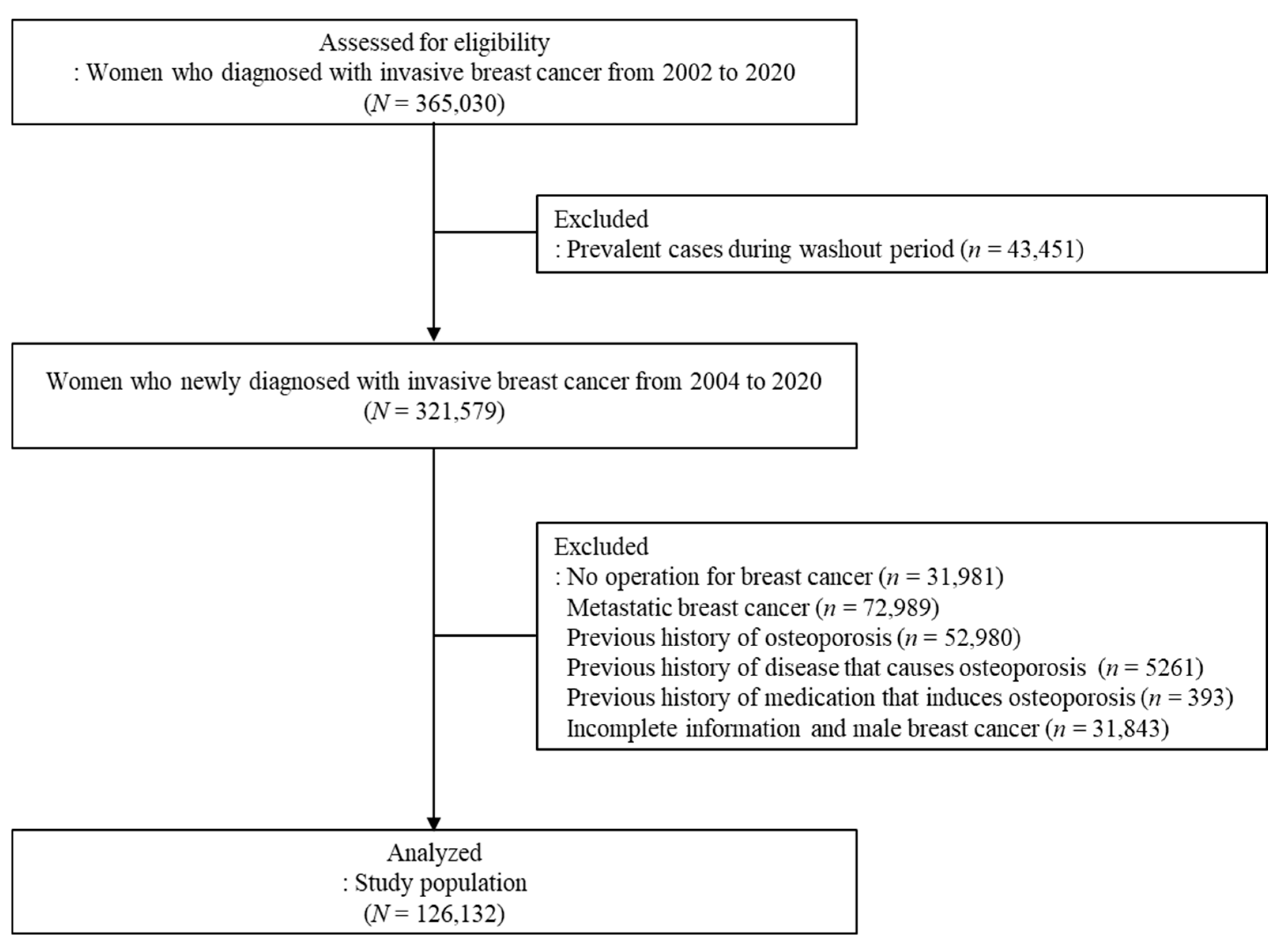
2. Materials and Methods
2.1. Data Source and Study Population
2.2. Operational Definitions
2.3. Statistical Analysis
3. Results
3.1. Participants’ Baseline Characteristics
3.2. Effect of Breast Cancer Treatments on Osteoporosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, M.J.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G.; Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. Cancer Res. Treat. 2022, 54, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S. Late effects of cancer treatment in breast cancer survivors. South Asian J. Cancer 2014, 3, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Aapro, M.S.; Body, J.J.; Gnant, M.; Brandi, M.L.; Reginster, J.Y.; Zillikens, M.C.; Glüer, C.C.; de Villiers, T.; Baber, R.; et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: Joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J. Bone Oncol. 2017, 7, 11–12. [Google Scholar] [CrossRef]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016, 34, 611–635. [Google Scholar] [CrossRef]
- Ramin, C.; May, B.J.; Roden, R.B.S.; Orellana, M.M.; Hogan, B.C.; McCullough, M.S.; Petry, D.; Armstrong, D.K.; Visvanathan, K. Evaluation of osteopenia and osteoporosis in younger breast cancer survivors compared with cancer-free women: A prospective cohort study. Breast Cancer Res. 2018, 20, 134. [Google Scholar] [CrossRef]
- Force USPST. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann. Intern. Med. 2011, 154, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Suskin, J.; Shapiro, C.L. Osteoporosis and musculoskeletal complications related to therapy of breast cancer. Gland Surg. 2018, 7, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Diana, A.; Carlino, F.; Giunta, E.F.; Franzese, E.; Guerrera, L.P.; Di Lauro, V.; Ciardiello, F.; Daniele, B.; Orditura, M. Cancer Treatment-Induced Bone Loss (CTIBL): State of the Art and Proper Management in Breast Cancer Patients on Endocrine Therapy. Curr. Treat. Options Oncol. 2021, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.; Bottomley, C.; Shingler, S.; Giangregorio, L.; de Freitas, H.M.; Patel, C.; Randall, S.; Gold, D.T. The importance of physical function to people with osteoporosis. Osteoporos. Int. 2017, 28, 1597–1607. [Google Scholar] [CrossRef]
- Reyes, B.J.; Mendelson, D.A.; Mujahid, N.; Mears, S.C.; Gleason, L.; Mangione, K.K.; Nana, A.; Mijares, M.; Ouslander, J.G. Postacute Management of Older Adults Suffering an Osteo-porotic Hip Fracture: A Consensus Statement from the International Geriatric Fracture Society. Geriatr. Orthop. Surg. Rehabil. 2020, 11, 2151459320935100. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, S.Y.; Nam, K.; Cha, Y. Analysis of Long-Term Medical Expenses in Vertebral Fracture Patients. Clin. Orthop. Surg. 2023, 15, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Weycker, D.; Li, X.; Barron, R.; Bornheimer, R.; Chandler, D. Hospitalizations for osteoporosis-related fractures: Economic costs and clinical outcomes. Bone Rep. 2016, 5, 186–191. [Google Scholar] [CrossRef] [PubMed]
- El-Setouhy, M.; Khired, Z.; Darraj, H.; Zogel, B.; Alhazmi, M.H.; Maghrabi, R.E.; Sayegh, M.; Akkur, A.A.; Bakri, N.; Alhazmi, A.; et al. The Relation Between Osteoporosis and Bone Fractures and Health-Related Quality of Life in Post-menopausal Saudi Women in the Jazan Region: A Cross-Sectional Study. Cureus 2024, 16, e54412. [Google Scholar] [CrossRef]
- Mayer, E.L. Early and Late Long-Term Effects of Adjuvant Chemotherapy. Am. Soc. Clin. Oncol. Educ. Book. 2013, 33, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Kiesel, L. Chemotherapy-Induced Amenorrhea—An Update. Geburtshilfe Frauenheilkd. 2012, 72, 809–818. [Google Scholar] [PubMed]
- Pan, H.; Gray, R.; Braybrooke, J.; Davies, C.; Taylor, C.; McGale, P.; Peto, R.; Pritchard, K.I.; Bergh, J.; Dowsett, M.; et al. 20-Year Risks of Breast-Cancer Recurrence after Stop-ping Endocrine Therapy at 5 Years. N. Engl. J. Med. 2017, 377, 1836–1846. [Google Scholar] [CrossRef]
- Hadji, P. Cancer Treatment-Induced Bone Loss in women with breast cancer. Bonekey Rep. 2015, 4, 692. [Google Scholar] [CrossRef]
- Love, R.R.; Barden, H.S.; Mazess, R.B.; Epstein, S.; Chappell, R.J. Effect of tamoxifen on lumbar spine bone mineral density in postmenopausal women after 5 years. Arch. Intern. Med. 1994, 154, 2585–2588. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, B.; Ejlertsen, B.; Dalgaard, P.; Larsen, L.; Holmegaard, S.N.; Transbøl, I.; Mouridsen, H.T. Tamoxifen and bone metabolism in postmenopausal low-risk breast cancer patients: A randomized study. J. Clin. Oncol. 1994, 12, 992–997. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.J.; Hickish, T.; Kanis, J.A.; Tidy, A.; Ashley, S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J. Clin. Oncol. 1996, 14, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Ad-vances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Musolino, A.; Gradishar, W.J.; Rugo, H.S.; Nordstrom, J.L.; Rock, E.P.; Arnaldez, F.; Pegram, M.D. Role of Fcgamma receptors in HER2-targeted breast cancer therapy. J. Immunother. Cancer 2022, 10, e003171. [Google Scholar] [CrossRef]
- Barbieri, M.A.; Sorbara, E.E.; Cicala, G.; Santoro, V.; Cutroneo, P.M.; Franchina, T.; Spina, E. Adverse Drug Reactions with HER2-Positive Breast Cancer Treatment: An Analysis from the Italian Pharmacovigilance Database. Drugs-Real World Outcomes 2022, 9, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.L.; Van Poznak, C.; Lacchetti, C.; Kirshner, J.; Eastell, R.; Gagel, R.; Smith, S.; Edwards, B.J.; Frank, E.; Lyman, G.H.; et al. Management of Osteoporosis in Survivors of Adult Cancers with Nonmetastatic Disease: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 2916–2946. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Oh, J.; Lee, H.S.; Jeon, S.; Park, W.C.; Yoon, C.I. Association between tamoxifen and incidence of osteoporosis in Korean patients with ductal carcinoma in situ. Front. Oncol. 2024, 13, 1236188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Alqudaihi, H.M.; Kang, M.S.; Kim, J.; Lee, J.W.; Ko, B.S.; Son, B.H.; Ahn, S.H.; Lee, J.E.; Han, S.W.; et al. Effect of tamoxifen on the risk of osteoporosis and osteoporotic fracture in younger breast cancer survivors: A nationwide study. Front. Oncol. 2020, 10, 366. [Google Scholar] [CrossRef]
- Park, C.; Ha, Y.C.; Jang, S.; Jang, S.; Yoon, H.K.; Lee, Y.K. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J. Bone Miner. Metab. 2011, 29, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Park, S.M.; Park, S.Y.; Yoo, J.I.; Jung, H.S.; Nho, J.H.; Kim, S.H.; Lee, Y.K.; Ha, Y.C.; Jang, S.; et al. Osteoporosis and Osteoporotic Fracture Fact Sheet in Korea. J. Bone Metab. 2020, 27, 281–290. [Google Scholar] [CrossRef]
- Yu, T.Y.; Cho, H.; Kim, T.Y.; Ha, Y.C.; Jang, S.; Kim, H.Y. Utilization of Osteoporosis-Related Health Services: Use of Data from the Korean National Health Insurance Database 2008–2012. J. Korean Med. Sci. 2018, 33, e20. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Baum, M.; Buzdar, A.; Howell, A.; Dowsett, M.; Forbes, J.F.; ATAC/LATTE investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 2010, 11, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Adams, J.E.; Coleman, R.E.; Howell, A.; Hannon, R.A.; Cuzick, J.; Mackey, J.R.; Beckmann, M.W.; Clack, G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J. Clin. Oncol. 2008, 26, 1051–1057. [Google Scholar] [CrossRef]
- Reid, D.M.; Doughty, J.; Eastell, R.; Heys, S.D.; Howell, A.; McCloskey, E.V.; Powles, T.; Selby, P.; Coleman, R.E. Guidance for the management of breast cancer treatment-induced bone loss: A consensus position statement from a UK Expert Group. Cancer Treat. Rev. 2008, 34, S3–S18. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Jacob, L.; Coleman, R.; Kostev, K.; Hadji, P. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res. Treat. 2016, 155, 151–157. [Google Scholar] [CrossRef]
- Coleman, R.E.; Banks, L.M.; Girgis, S.I.; Kilburn, L.S.; Vrdoljak, E.; Fox, J.; Cawthorn, S.J.; Patel, A.; Snowdon, C.F.; Hall, E.; et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): A randomised controlled study. Lancet Oncol. 2007, 8, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocana, A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2011, 103, 1299–1309. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar]
- Bundred, N.J.; Campbell, I.D.; Davidson, N.; DeBoer, R.H.; Eidtmann, H.; Monnier, A.; Neven, P.; von Minckwitz, G.; Miller, J.C.; Schenk, N.L.; et al. Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST Study results. Cancer 2008, 112, 1001–1010. [Google Scholar] [CrossRef]
- Vehmanen, L.; Elomaa, I.; Blomqvist, C.; Saarto, T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J. Clin. Oncol. 2006, 24, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Sverrisdottir, A.; Fornander, T.; Jacobsson, H.; von Schoultz, E.; Rutqvist, L.E. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J. Clin. Oncol. 2004, 22, 3694–3699. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, U.; Kostev, K.; Kyvernitakis, J.; Bocker, W.; Hadji, P. Incidence of fractures in young women with breast cancer—A retrospective cohort study. J. Bone Oncol. 2019, 18, 100254. [Google Scholar] [CrossRef] [PubMed]
- Ellmen, J.; Hakulinen, P.; Partanen, A.; Hayes, D.F. Estrogenic effects of toremifene and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. Treat. 2003, 82, 103–111. [Google Scholar] [CrossRef]
- Tzeng, H.E.; Muo, C.H.; Chen, H.T.; Hwang, W.L.; Hsu, H.C.; Tsai, C.H. Tamoxifen use reduces the risk of osteoporotic fractures in women with breast cancer in Asia: A nationwide population-based cohort study. BMC Musculoskelet. Disord. 2015, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.A.; Regan, M.M.; Fleming, G.F.; Láng, I.; Ciruelos, E.; Bellet, M.; Bonnefoi, H.R.; Climent, M.A.; Da Prada, G.A.; Burstein, H.J.; et al. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 2015, 372, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Ramchand, S.K.; Cheung, Y.M.; Yeo, B.; Grossmann, M. The effects of adjuvant endocrine therapy on bone health in women with breast cancer. J. Endocrinol. 2019, 241, R111–R124. [Google Scholar] [CrossRef] [PubMed]
- Pagani, O.; Regan, M.M.; Walley, B.A.; Fleming, G.F.; Colleoni, M.; Láng, I.; Gomez, H.L.; Tondini, C.; Burstein, H.J.; Perez, E.A.; et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 2014, 371, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Jakesz, R.; Jonat, W.; Gnant, M.; Mittlboeck, M.; Greil, R.; Tausch, C.; Hilfrich, J.; Kwasny, W.; Menzel, C.; Samonigg, H.; et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005, 366, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Body, J.J.; Brandi, M.L.; Cannata-Andia, J.; Chappard, D.; El Maghraoui, A.; Glüer, C.C.; Kendler, D.; Napoli, N.; Papaioannou, A.; et al. Cancer-associated bone disease. Osteoporos. Int. 2013, 24, 2929–2953. [Google Scholar] [CrossRef]
- Cameron, D.A.; Douglas, S.; Brown, J.E.; Anderson, R.A. Bone mineral density loss during adjuvant chemotherapy in pre-menopausal women with early breast cancer: Is it dependent on oestrogen deficiency? Breast Cancer Res. Treat. 2010, 123, 805–814. [Google Scholar] [CrossRef]
- Taxel, P.; Faircloth, E.; Idrees, S.; Van Poznak, C. Cancer Treatment-Induced Bone Loss in Women with Breast Cancer and Men with Prostate Cancer. J. Endocr. Soc. 2018, 2, 574–588. [Google Scholar] [CrossRef]
- Saarto, T.; Blomqvist, C.; Valimaki, M.; Makela, P.; Sarna, S.; Elomaa, I. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: A randomized study in premenopausal breast cancer patients. J. Clin. Oncol. 1997, 15, 1341–1347. [Google Scholar] [CrossRef]
- Banfi, A.; Podesta, M.; Fazzuoli, L.; Sertoli, M.R.; Venturini, M.; Santini, G.; Cancedda, R.; Quarto, R. High-dose chemotherapy shows a dose-dependent toxicity to bone marrow osteoprogenitors: A mechanism for post-bone marrow transplantation osteopenia. Cancer 2001, 92, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Greep, N.C.; Giuliano, A.E.; Hansen, N.M.; Taketani, T.; Wang, H.J.; Singer, F.R. The effects of adjuvant chemotherapy on bone density in postmenopausal women with early breast cancer. Am. J. Med. 2003, 114, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, L.M.; Rodríguez-Rodríguez, E.M.; Oramas-Rodríguez, J.M.; Santolaria-Fernandez, F.; Llanos, M.; Cruz, J.; Martínez, A.; González-Reimers, E.; Gómez, A.; Batista, N. Changes on bone mineral density after adjuvant treatment in women with non-metastatic breast cancer. Breast Cancer Res. Treat. 2005, 93, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Moreno, A.; Palomeras, S.; Pedersen, K.; Morancho, B.; Pascual, T.; Galván, P.; Benítez, S.; Gomez-Miragaya, J.; Ciscar, M.; Jimenez, M.; et al. RANK signaling increases after anti-HER2 therapy contributing to the emergence of resistance in HER2-positive breast cancer. Breast Cancer Res. 2021, 23, 42. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.H.; Kong, Y.Y.; Penninger, J.M. Role of RANKL and RANK in bone loss and arthritis. Ann. Rheum. Dis. 2002, 61, ii32–ii39. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Muo, C.H.; Tzeng, H.E.; Tang, C.H.; Hsu, H.C.; Sung, F.C. Fracture in asian women with breast cancer occurs at younger age. PLoS ONE 2013, 8, e75109. [Google Scholar] [CrossRef]
- Rizzoli, R.; Body, J.J.; DeCensi, A.; Reginster, J.Y.; Piscitelli, P.; Brandi, M.L.; European Society for Clinical and Economical aspects of Osteoporosis and Osteoarthritis (ESCEO). Guidance for the prevention of bone loss and fractures in postmenopausal women treated with aromatase inhibitors for breast cancer: An ESCEO position paper. Osteoporos. Int. 2012, 23, 2567–2576. [Google Scholar] [CrossRef] [PubMed]


| Total (N = 126,132) | Event (n = 28,603) | Incidence Rate (95% CI) | |
|---|---|---|---|
| Age at diagnosis | |||
| <30 | 1081 (0.86) | 74 (0.26) | 11.72 (9.20–14.72) |
| 30–39 | 12,809 (10.16) | 1235 (4.32) | 16.28 (15.38–17.21) |
| 40–49 | 51,438 (40.78) | 8769 (30.66) | 31.31 (30.66–31.97) |
| 50–59 | 43,419 (34.42) | 12,267 (42.89) | 56.46 (55.46–57.47) |
| 60–69 | 14,034 (11.13) | 5140 (17.97) | 90.20 (87.75–92.70) |
| 70–79 | 2896 (2.30) | 1013 (3.54) | 85.34 (80.16–90.76) |
| 80– | 455 (0.36) | 105 (0.37) | 59.94 (49.02–72.56) |
| Type of Insurance | |||
| National Health Insurance | 122,350 (97.00) | 27,432 (95.91) | 43.59 (43.08–44.11) |
| Medical Aid | 3729 (2.96) | 1158 (4.05) | 56.57 (53.36–59.92) |
| Others (Unknown) | 53 (0.04) | 13 (0.05) | 40.68 (21.66–69.57) |
| Charlson Comorbidity Index | |||
| 0 | 17,466 (13.85) | 4183 (14.62) | 35.75 (34.67–36.85) |
| 1 | 31,904 (25.29) | 7047 (24.64) | 38.83 (37.93–39.75) |
| 2 | 32,644 (25.88) | 7212 (25.21) | 44.49 (43.47–45.53) |
| 3 | 21,080 (16.71) | 4746 (16.59) | 50.00 (48.59–51.44) |
| 4 | 10,818 (8.58) | 2533 (8.86) | 55.91 (53.75–58.13) |
| 5+ | 12,220 (9.69) | 2882 (10.08) | 58.46 (56.35–60.64) |
| Treatment | |||
| No treatment | 11,403 (9.04) | 1848 (6.44) | 33.66 (32.14–35.24) |
| TMX | 29,050 (23.03) | 4640 (16.22) | 30.37 (29.51–31.26) |
| AIs | 9248 (7.33) | 2989 (10.45) | 87.10 (84.01–90.28) |
| CTx | 20,400 (16.17) | 4372 (15.29) | 41.14 (39.93–42.37) |
| CTx+TMX | 28,912 (22.92) | 6556 (22.92) | 37.12 (36.23–38.03) |
| CTx+AIs | 11,203 (8.88) | 4583 (16.02) | 86.23 (83.75–88.76) |
| CTx+Anti-HER2 Tx | 6961 (5.52) | 1547 (22.22) | 50.33 (47.86–52.90) |
| CTx+Anti-HER2 Tx+TMX | 6239 (4.95) | 1105 (3.86) | 35.60 (33.53–37.76) |
| CTx+Anti-HER2 Tx+AIs | 2716 (2.15) | 968 (3.38) | 92.53 (86.79–98.55) |
| Total (N = 103,532) n (%) | Event (n = 24,022) n (%) | Crude HR (95% CI) | Adjusted HR (95% CI) * | IPW HR (95% CI) | |
|---|---|---|---|---|---|
| Treatment | |||||
| No treatment | 8476 (8.19) | 1841 (7.66) | Reference | Reference | Reference |
| TMX | 24,009 (23.19) | 3959 (16.48) | 0.94 (0.89–0.99) | 1.06 (1.00–1.12) | 1.14 (1.08–1.21) |
| AIs | 6418 (6.2) | 2174 (9.05) | 2.66 (2.50–2.83) | 1.84 (1.73–1.96) | 2.53 (2.37–2.70) |
| CTx | 15,419 (14.89) | 3618 (15.06) | 1.22 (1.15–1.29) | 1.27 (1.20–1.34) | 1.30 (1.23–1.38) |
| CTx+TMX | 26,358 (25.46) | 5775 (24.04) | 1.12 (1.07–1.19) | 1.35 (1.28–1.42) | 1.39 (1.32–1.47) |
| CTx+AIs | 9101 (8.79) | 3595 (14.97) | 2.49 (2.35–2.63) | 1.92 (1.81–2.03) | 2.15 (2.03–2.28) |
| CTx+Anti-HER2 Tx | 5802 (5.6) | 1254 (5.22) | 1.51 (1.40–1.62) | 1.39 (1.30–1.50) | 1.41 (1.31–1.52) |
| CTx+Anti-HER2 Tx+TMX | 5673 (5.48) | 998 (4.15) | 1.13 (1.05–1.22) | 1.38 (1.28–1.49) | 1.42 (1.32–1.53) |
| CTx+Anti-HER2 Tx+AIs | 2276 (2.2) | 808 (3.36) | 3.01 (2.77–3.28) | 2.26 (2.08–2.46) | 2.63 (2.41–2.86) |
| Total N (%) | Event n (%) | Time (Years) * | Crude HR (95% CI) | Adjusted HR (95% CI) ** | IPW HR (95% CI) | |
|---|---|---|---|---|---|---|
| AGE < 50 | 54,833 | 8796 | 5.00 ± 3.02 | |||
| Treatment | ||||||
| No treatment | 3839 (7.00) | 477 (5.42) | Reference | Reference | Reference | |
| TMX | 16,142 (29.44) | 2111 (24.00) | 1.46 (1.32–1.62) | 1.45 (1.31–1.6) | 1.32 (1.20–1.45) | |
| AIs | 155 (0.28) | 45 (0.51) | 3.97 (2.92–5.39) | 3.84 (2.83–5.22) | 4.12 (3.72–4.56) | |
| CTx | 7962 (14.52) | 1246 (14.17) | 1.48 (1.33–1.64) | 1.46 (1.31–1.62) | 1.49 (1.35–1.64) | |
| CTx+TMX | 19,340 (35.27) | 3632 (41.29) | 1.89 (1.72–2.08) | 1.88 (1.71–2.06) | 1.75 (1.60–1.91) | |
| CTx+AIs | 890 (1.62) | 315 (3.58) | 3.24 (2.81–3.74) | 3.19 (2.76–3.67) | 3.03 (2.76–3.33) | |
| CTx+Anti-HER2 Tx | 2132 (3.89) | 284 (3.23) | 1.74 (1.50–2.01) | 1.71 (1.47–1.98) | 1.62 (1.43–1.84) | |
| CTx+Anti-HER2 Tx+TMX | 4240 (7.73) | 644 (7.32) | 1.98 (1.76–2.23) | 1.94 (1.73–2.19) | 1.80 (1.60–2.04) | |
| CTx+Anti-HER2 Tx+AIs | 133 (0.24) | 42 (0.48) | 4.61 (3.36–6.32) | 4.39 (3.20–6.02) | 3.82 (3.35–4.37) | |
| AGE 50–59 | 35,562 | 10,293 | 4.51 ± 2.72 | |||
| Treatment | ||||||
| No treatment | 3060 (8.60) | 802 (7.79) | Reference | Reference | Reference | |
| TMX | 6995 (19.67) | 1554 (15.10) | 1.04 (0.95–1.13) | 1.04 (0.95–1.13) | 1.04 (0.95–1.13) | |
| AIs | 2957 (8.32) | 937 (9.10) | 1.95 (1.77–2.14) | 1.93 (1.75–2.12) | 1.94 (1.75–2.15) | |
| CTx | 5296 (14.89) | 1594 (15.49) | 1.33 (1.22–1.44) | 1.32 (1.21–1.44) | 1.31 (1.20–1.43) | |
| CTx+TMX | 6429 (18.08) | 1879 (18.26) | 1.23 (1.14–1.34) | 1.23 (1.13–1.34) | 1.23 (1.13–1.33) | |
| CTx+AIs | 5412 (15.22) | 2061 (20.02) | 1.93 (1.78–2.09) | 1.91 (1.76–2.08) | 1.92 (1.75–2.10) | |
| CTx+Anti-HER2 Tx | 2605 (7.33) | 644 (6.26) | 1.44 (1.30–1.60) | 1.42 (1.28–1.58) | 1.44 (1.29–1.61) | |
| CTx+Anti-HER2 Tx+TMX | 1326 (3.73) | 311 (3.02) | 1.24 (1.09–1.42) | 1.23 (1.08–1.41) | 1.24 (1.10–1.39) | |
| CTx+Anti-HER2 Tx+AIs | 1482 (4.17) | 511 (4.96) | 2.35 (2.10–2.63) | 2.32 (2.07–2.59) | 2.35 (2.05–2.70) | |
| AGE ≥ 60 | 13,137 | 4933 | 3.89 ± 2.33 | |||
| Treatment | ||||||
| No treatment | 1577 (12.00) | 562 (11.39) | Reference | Reference | Reference | |
| TMX | 872 (6.64) | 294 (5.96) | 0.94 (0.82–1.09) | 0.94 (0.82–1.09) | 0.93 (0.83–1.04) | |
| AIs | 3306 (25.17) | 1192 (24.16) | 1.37 (1.24–1.51) | 1.37 (1.24–1.51) | 1.35 (1.16–1.57) | |
| CTx | 2161 (16.45) | 778 (15.77) | 1.06 (0.95–1.18) | 1.06 (0.95–1.18) | 1.01 (0.89–1.14) | |
| CTx+TMX | 589 (4.48) | 264 (5.35) | 1.12 (0.97–1.30) | 1.11 (0.96–1.29) | 1.04 (0.93–1.16) | |
| CTx+AIs | 2799 (21.31) | 1219 (24.71) | 1.48 (1.34–1.63) | 1.47 (1.33–1.62) | 1.42 (1.24–1.62) | |
| CTx+Anti-HER2 Tx | 1065 (8.11) | 326 (6.61) | 1.10 (0.96–1.26) | 1.09 (0.95–1.25) | 1.06 (0.90–1.25) | |
| CTx+Anti-HER2 Tx+TMX | 107 (0.81) | 43 (0.87) | 1.16 (0.85–1.58) | 1.15 (0.84–1.56) | 1.07 (0.92–1.25) | |
| CTx+Anti-HER2 Tx+AIs | 661 (5.03) | 255 (5.17) | 1.60 (1.38–1.86) | 1.59 (1.37–1.84) | 1.51 (1.22–1.86) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.; Kim, B.-H.; Min, S.Y.; Chae, S. Effects of Anticancer Therapy on Osteoporosis in Breast Cancer Patients: A Nationwide Study Using Data from the National Health Insurance Service-National Health Information Database. J. Clin. Med. 2025, 14, 732. https://doi.org/10.3390/jcm14030732
Kwon M, Kim B-H, Min SY, Chae S. Effects of Anticancer Therapy on Osteoporosis in Breast Cancer Patients: A Nationwide Study Using Data from the National Health Insurance Service-National Health Information Database. Journal of Clinical Medicine. 2025; 14(3):732. https://doi.org/10.3390/jcm14030732
Chicago/Turabian StyleKwon, Minji, Bo-Hyung Kim, Sun Young Min, and Sumin Chae. 2025. "Effects of Anticancer Therapy on Osteoporosis in Breast Cancer Patients: A Nationwide Study Using Data from the National Health Insurance Service-National Health Information Database" Journal of Clinical Medicine 14, no. 3: 732. https://doi.org/10.3390/jcm14030732
APA StyleKwon, M., Kim, B.-H., Min, S. Y., & Chae, S. (2025). Effects of Anticancer Therapy on Osteoporosis in Breast Cancer Patients: A Nationwide Study Using Data from the National Health Insurance Service-National Health Information Database. Journal of Clinical Medicine, 14(3), 732. https://doi.org/10.3390/jcm14030732





