Evaluation and Treatment of Thoracic Insufficiency Syndrome and Early-Onset Scoliosis
Abstract
1. Introduction
2. Diagnosis of EOS and TIS
3. Advancements in Dynamic Imaging
4. The Role of Basic Science
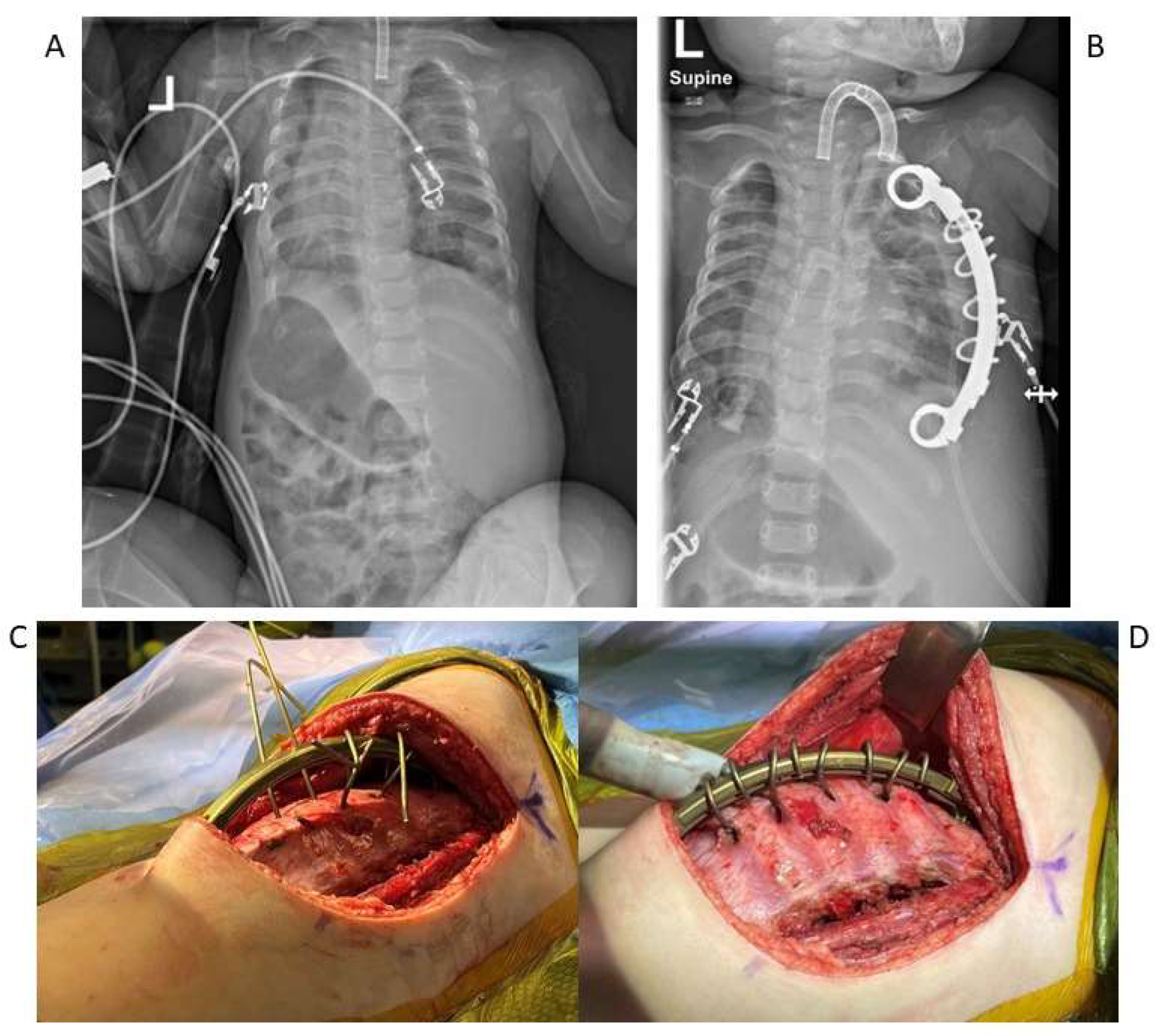
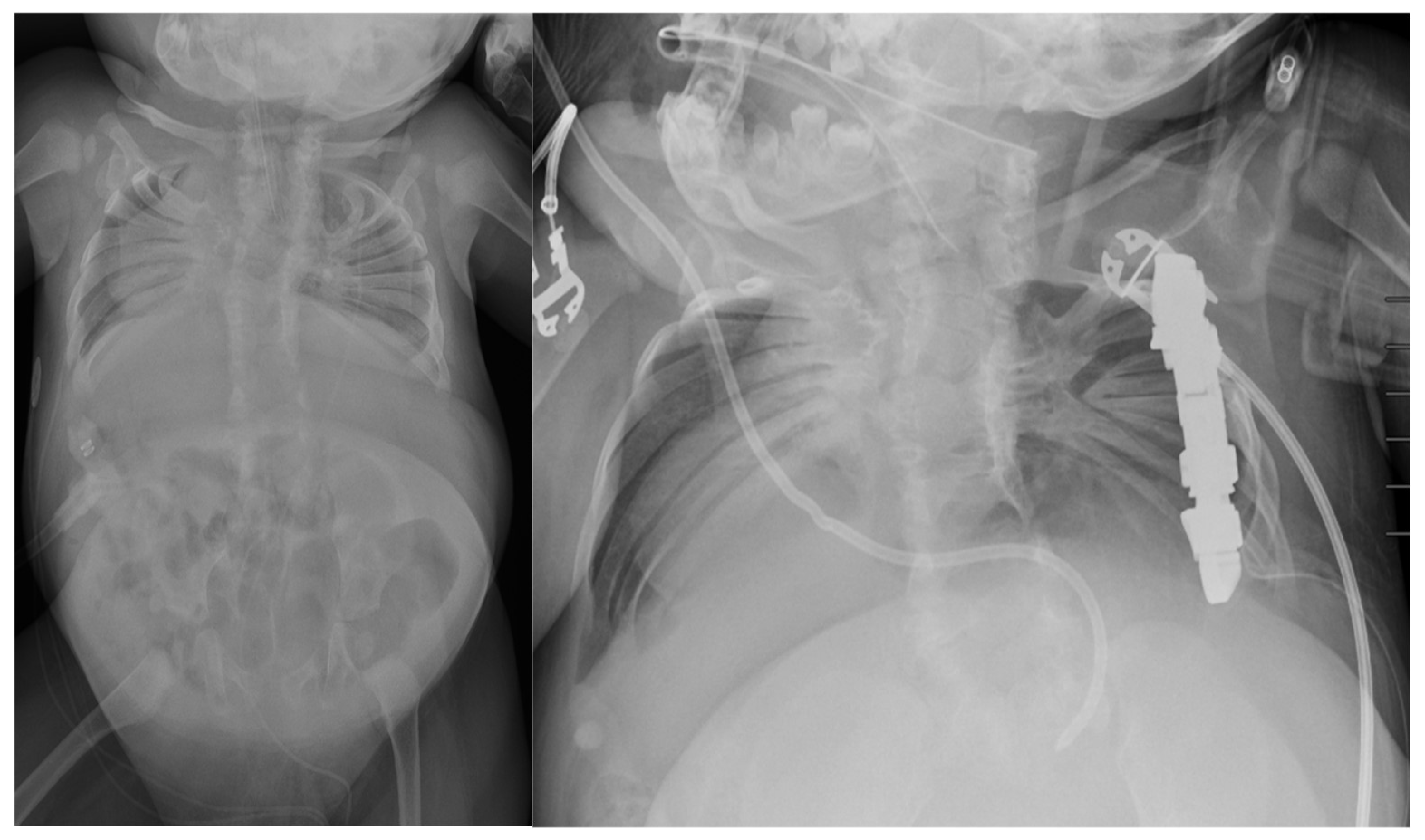
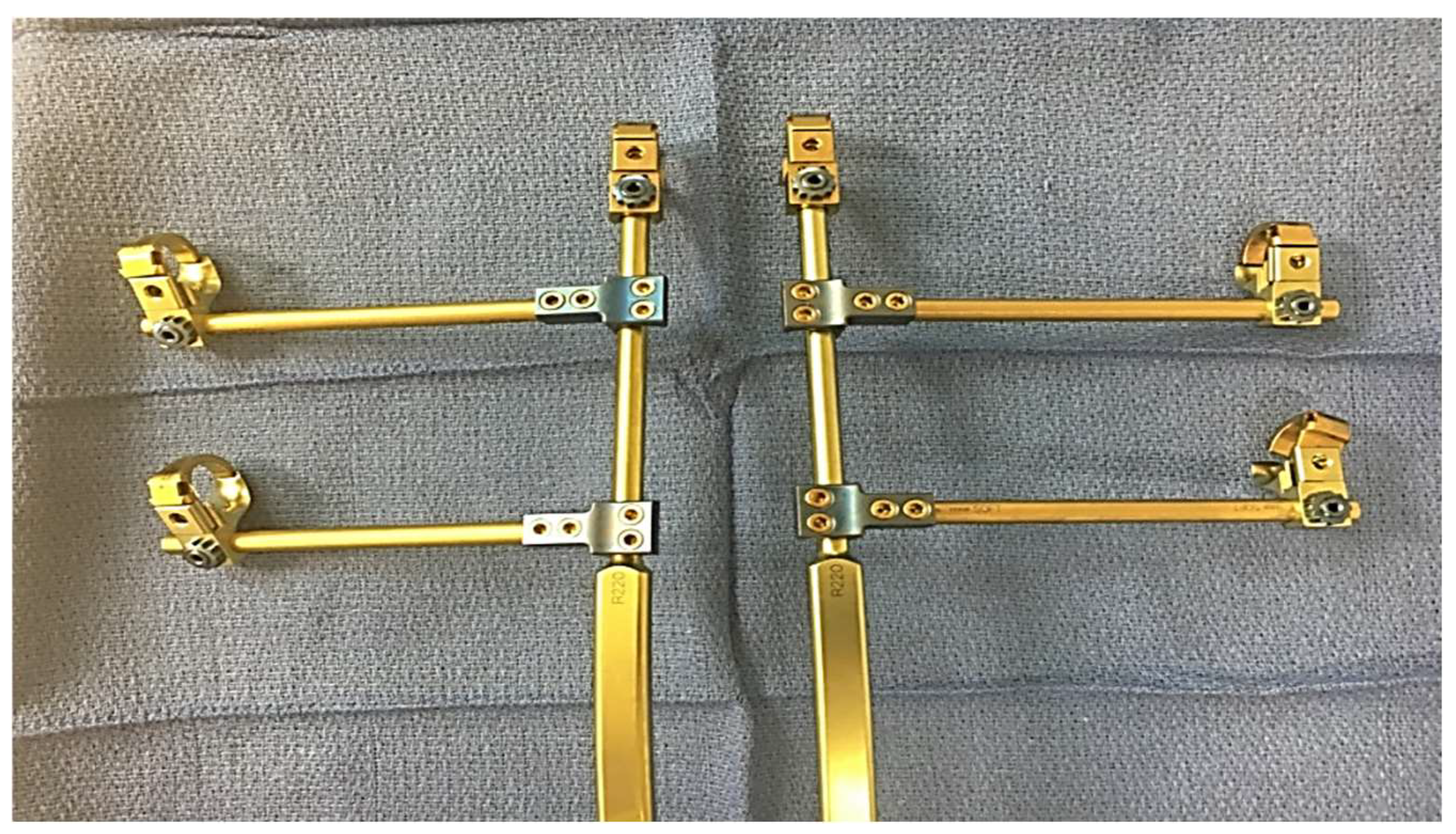
5. Surgical Management
6. Management of Unique Diagnoses
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campbell, R.M.J.; Smith, M.D.; Mayes, T.C.; Mangos, J.A.; Willey-Courand, D.B.; Kose, N. The Characteristics of Thoracic Insufficiency Syndrome Associated with Fused Ribs and Congenital Scoliosis. J. Bone Joint Surg. 2003, 85, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Weinstein, S.L. Natural history of early onset scoliosis. J. Bone Joint Surg. Am. 2007, 89, 21–33. [Google Scholar]
- Pehrsson, K.; Larsson, S.; Oden, A.; Nachemson, A. Long-term follow-up of patients with untreated scoliosis: A study of mortality, causes of death, and symptoms. Spine 1992, 17, 1091–1096. [Google Scholar] [CrossRef]
- Karol, L.A.; Johnston, C.; Mladenov, K.; Schochet, P.; Walters, P.; Browne, R.H. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J. Bone Joint Surg. 2008, 90, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Koumbourlis, A.C. Scoliosis and the respiratory system. Paediatr. Respir. Rev. 2006, 7, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Klyce, W.; Mitchell, S.L.; Pawelek, J.; Skaggs, D.L.; Sanders, J.O.; Shah, S.A. Characterizing Use of Growth-friendly Implants for Early-onset Scoliosis: A 10-Year Update. J. Pediatr. Orthop. 2020, 40, e740–e746. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Matsumoto, H.; McCalla, D.J.; Akbarnia, B.A.; Blakemore, L.C.; Betz, R.R. Development and initial validation of the Classification of Early-Onset Scoliosis (C-EOS). J. Bone Joint Surg. Am. 2014, 96, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Branthwaite, M.A. Cardiorespiratory consequences of unfused idiopathic scoliosis. Br. J. Dis. Chest 1986, 80, 360–369. [Google Scholar] [CrossRef]
- Tsukahara, K.; Mayer, O.H. Thoracic insufficiency syndrome: Approaches to assessment and management. Paediatr. Respir. Rev. 2022, 44, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Swallow, J.; Gagnier, J.; Cahill, P.J.; Sponseller, P.D.; Garg, S. Growth-friendly surgery results in more growth but a higher complication rate and unplanned returns to the operating room compared to single fusion in neuromuscular early-onset scoliosis. Spine Deform. 2021, 9, 851–858. [Google Scholar] [CrossRef]
- Campbell, R.M.; Smith, M.D.; Mayes, T.C.; Mangos, J.A.; Willey-Courand, D.B.; Kose, N. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome. J. Bone Joint. Surg. Am. 2004, 86, 1659–1674. [Google Scholar] [CrossRef]
- Tong, Y.; Udupa, J.K.; McDonough, J.M.; Wu, C.; Sun, C.; Xie, L. Assessment of Regional Functional Effects of Surgical Treatment in Thoracic Insufficiency Syndrome via Dynamic MRI. J. Bone Joint Surg. Am. 2023, 105, 53–62. [Google Scholar] [CrossRef]
- Jagadale, B.N.; Udupa, J.K.; Tong, Y.; Wu, C.; McDonough, J.; Torigian, D.A. Lung Parenchymal Analysis on Dynamic MRI in Thoracic Insufficiency Syndrome. Proc. SPIE Int. Soc. Opt. Eng. 2018, 10575, 105753M. [Google Scholar]
- Hosseini, M.; Udupa, J.K.; Hao, Y.; Tong, Y.; Wu, C.; Akhtar, Y. Assessment of 3D hemi-diaphragmatic motion via free-breathing dynamic MRI. medRxiv 2024. [Google Scholar] [CrossRef]
- Hao, Y.; Udupa, J.K.; Tong, Y.; Wu, C.; McDonough, J.M.; Gogel, S. Quantifying Normal Diaphragmatic Motion and Shape. medRxiv 2024. [Google Scholar] [CrossRef]
- Tong, Y.; Udupa, J.K.; McDonough, J.M.; Wu, C.; Xie, L.; Rajapakse, C.S. Characterizing Lung Parenchymal Aeration via Dynamic MRI. Radiol. Cardiothorac. Imaging 2024, 6, e230262. [Google Scholar] [CrossRef]
- Tong, Y.; Udupa, J.K.; McDonough, J.M.; Wileyto, E.P.; Capraro, A.; Wu, C. Quantitative Dynamic Thoracic MRI: Application to TIS. Radiology 2019, 292, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, Y.; Udupa, J.K.; Tong, Y.; Wu, C.; Liu, T.; Tong, L. Auto-segmentation of hemi-diaphragms in MRI. medRxiv 2024. [Google Scholar] [CrossRef]
- Akhtar, Y.; Udupa, J.K.; Tong, Y.; Liu, T.; Wu, C.; Kogan, R. Auto-segmentation of thoraco-abdominal organs in pediatric dynamic MRI. medRxiv 2024. [Google Scholar] [CrossRef]
- Olson, J.C.; Takahashi, A.; Glotzbecker, M.P.; Snyder, B.D. Extent of Spine Deformity Predicts Lung Growth and Function. PLoS ONE 2015, 10, e0136941. [Google Scholar] [CrossRef]
- Strong, A.; Behr, M.; Lott, C.; Clark, A.J.; Mentch, F.; Da Silva, R.P.; Benson, L.; Rice, S.; Johnson, K.; Puri, A.; et al. Molecular diagnosis and novel genes and phenotypes in a pediatric thoracic insufficiency cohort. Sci. Rep. 2023, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; McElroy, M.J.; Akbarnia, B.A.; Salari, P.; Oliveira, D.; Thompson, G.H.; Sponseller, P.D.; Newton, P.O.; Shah, S.A.; Vitale, M.G. Growing rods for spinal deformity: Characterizing consensus and variation in current use. J. Pediatr. Orthop. 2010, 30, 264–270. [Google Scholar] [CrossRef]
- Murphy, R.F.; Neel, G.B.; Barfield, W.R.; Anari, J.B.; St. Hilaire, T.; Thompson, G.; Cahill, P.J.; Flynn, J.M.; Samdani, A.F.; Vitale, M.G. Trends in the utilization of implants in index procedures for early-onset scoliosis from the Pediatric Spine Study Group. J. Pediatr. Orthop. 2022, 42, e912–e916. [Google Scholar] [CrossRef] [PubMed]
- Ashebo, L.; Anari, J.B.; Cahill, P.J.; Vitale, M.G.; Smith, J.T. Update on the diagnosis and management of early-onset scoliosis. Curr. Rev. Musculoskelet. Med. 2023, 16, 447–456. [Google Scholar] [CrossRef]
- Harrington, P.R. Treatment of scoliosis: Correction and internal fixation by spine instrumentation. J. Bone Joint Surg. 1962, 44, 591–610. [Google Scholar] [CrossRef]
- Wattenbarger, J.M.; Richards, B.S.; Herring, J.A. A Comparison of Single-Rod Instrumentation With Double-Rod Instrumentation in Adolescent Idiopathic Scoliosis. Spine 2000, 25, 1680. [Google Scholar] [CrossRef]
- Akbarnia, B.A.; Marks, D.S.; Boachie-Adjei, O.; Thompson, A.G.; Asher, M.A. Dual Growing Rod Technique for the Treatment of Progressive Early-Onset Scoliosis: A Multicenter Study. Spine 2005, 30, S46. [Google Scholar] [CrossRef] [PubMed]
- Akbarnia, B.A.; Breakwell, L.M.; Marks, D.S.; McCarthy, R.E.; Thompson, A.G.; Canale, S.K.; McCarthy, J.J.; Sponseller, P.D. Dual Growing Rod Technique Followed for Three to Eleven Years Until Final Fusion: The Effect of Frequency of Lengthening. Spine 2008, 33, 984–990. [Google Scholar] [CrossRef]
- Thompson, G.H.; Akbarnia, B.A. Single and Dual Traditional Growing Rods. In The Growing Spine: Management of Spinal Disorders in Young Children; Springer: Berlin/Heidelberg, Germany, 2016; pp. 645–668. [Google Scholar] [CrossRef]
- Matsumoto, H.; Fano, A.N.; Quan, T.; Akbarnia, B.A.; Blakemore, L.C.; Flynn, J.M.; Roye, D.P.; Vitale, M.G. Re-evaluating consensus and uncertainty among treatment options for early onset scoliosis: A 10-year update. Spine Deform. 2023, 11, 11–25. [Google Scholar] [CrossRef]
- Bess, S.; Akbarnia, B.A.; Thompson, G.H.; Sponseller, P.D.; Shah, S.A.; El Sebaie, H.; Boachie-Adjei, O.; Karlin, L.I.; Canale, S.; Poe-Kochert, C.; et al. Complications of growing-rod treatment for early-onset scoliosis: Analysis of one hundred and forty patients. J. Bone Joint Surg. Am. 2010, 92, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.M.; Vocke, A.K. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J. Bone Joint Surg. Am. 2003, 85, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Studer, D.; Hasler, C.C. Long term outcome of vertical expandable prosthetic titanium rib treatment in children with early onset scoliosis. Ann. Transl. Med. 2020, 8, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Anari, J.B.; Flynn, J.M.; Cahill, P.J.; Vitale, M.G.; Smith, J.T.; Gomez, J.A.; Sturm, P.F.; Samdani, A.F.; Pahys, J.M.; El-Hawary, R. Unplanned return to OR (UPROR) for children with early onset scoliosis (EOS): A comprehensive evaluation of all diagnoses and instrumentation strategies. Spine Deform. 2020, 8, 295–302. [Google Scholar] [CrossRef]
- Hasler, C.C.; Mehrkens, A.; Hefti, F. Efficacy and safety of VEPTR instrumentation for progressive spine deformities in young children without rib fusions. Eur. Spine J. 2010, 19, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Lucas, G.; Bollini, G.; Jouve, J.L.; de Gauzy, J.S.; Accadbled, F.; Lascombes, P.; Synthetic and Degenerative Group. Complications in Pediatric Spine Surgery Using the Vertical Expandable Prosthetic Titanium Rib: The French Experience. Spine 2013, 38, E1589–E1599. [Google Scholar] [CrossRef]
- Heflin, J.; Welborn, M.; Ramirez-Lluch, N.; Iriarte, I.; El-Hawary, R.; Fedorak, G.T.; Luhmann, S.J.; Kadhim, M. Parallel Proximal Fixation in Rib-Based Growing Rod System: A Novel Approach to Deal with Proximal Anchor Migration. Spine 2018, 43, E855–E862. [Google Scholar] [CrossRef]
- Waldhausen, J.H.T.; Redding, G.; White, K.; Song, K. Complications in using the vertical expandable prosthetic titanium rib (VEPTR) in children. J. Pediatr. Surg. 2016, 51, 1747–1750. [Google Scholar] [CrossRef]
- Ramirez, N.; Olivella, G.; Rodriguez, O.; Marrero, P.; Smith, J.; Garg, S.; Flynn, J.M.; Samdani, A. Incidence of complications in the management of non-ambulatory neuromuscular early-onset scoliosis with a rib-based growing system: High- versus low-tone patients. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 621–627. [Google Scholar] [CrossRef]
- Nossov, S.B.; Curatolo, E.; Campbell, R.M.; Mayer, O.H.; Garg, S.; Cahill, A.P.J.; Flynn, J.M.; Dilella, L.; Smith, J.T. VEPTR: Are We Reducing Respiratory Assistance Requirements? J. Pediatr. Orthop. 2019, 39, 28–32. [Google Scholar] [CrossRef]
- Emans, J.B.; Caubet, J.F.; Ordonez, C.L.; Lee, E.Y.; Ciarlo, M. The treatment of spine and chest wall deformities with fused ribs by expansion thoracostomy and insertion of vertical expandable prosthetic titanium rib: Growth of thoracic spine and improvement of lung volumes. Spine 2005, 30, S58–S68. [Google Scholar] [CrossRef]
- Nossov, S.B.; Quinonez, A.; SanJuan, J.; Gaughan, J.P.; Pahys, J.; Samdani, A.; Campbell, R.M.; Smith, J.T. Does ventilator use status correlate with quality of life in patients with early-onset scoliosis treated with rib-based growing system implantation? Spine Deform. 2022, 10, 943–950. [Google Scholar] [CrossRef]
- Hell, A.K.; Groenefeld, K.; Tsaknakis, K.; Braunschweig, L.; Lorenz, H.M. Combining Bilateral Magnetically Controlled Implants Inserted Parallel to the Spine With Rib to Pelvis Fixation: Surgical Technique and Early Results. Clin. Spine Surg. 2018, 31, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Heyer, J.H.; Anari, J.B.; Baldwin, K.D.; Mitchell, S.L.; Flynn, J.M.; Sankar, W.N.; Campbell, R.M.; Smith, J.T. Rib-to-spine and rib-to-pelvis magnetically controlled growing rods: Does the law of diminishing returns still apply? Spine Deform. 2023, 11, 1517–1527. [Google Scholar] [CrossRef]
- La Rosa, G.; Oggiano, L.; Ruzzini, L. Magnetically Controlled Growing Rods for the Management of Early-onset Scoliosis: A Preliminary Report. J. Pediatr. Orthop. 2017, 37, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Skaggs, D.L.; Akbarnia, B.A.; Flynn, J.M.; Myung, K.S.; Sponseller, P.D.; Vitale, M.G.; Growing Spine Study Group. A classification of growth friendly spine implants. J. Pediatr. Orthop. 2014, 34, 260–274. [Google Scholar] [CrossRef]
- Choi, E.; Yaszay, B.; Mundis, G.; Hosseini, P.; Pawelek, J.; Alanay, A.; Yazici, M.; Akbarnia, B.A. Implant Complications After Magnetically Controlled Growing Rods for Early Onset Scoliosis: A Multicenter Retrospective Review. J. Pediatr. Orthop. 2017, 37, e588–e592. [Google Scholar] [CrossRef] [PubMed]
- Glowka, P.; Grabala, P.; Gupta, M.C.; Pereira, D.E.; Latalski, M.; Danielewicz, A.; Karski, M.; Jasiewicz, B.; Zarzycka, M.; Karski, T. Complications and Health-Related Quality of Life in Children with Various Etiologies of Early-Onset Scoliosis Treated with Magnetically Controlled Growing Rods—A Multicenter Study. J. Clin. Med. 2024, 13, 4068. [Google Scholar] [CrossRef]
- Hughes, M.S.; Swarup, I.; Makarewich, C.A.; Williams, B.A.; Talwar, D.; Cahill, P.J.; Flynn, J.M.; Glotzbecker, M.P. Expert Consensus for Early Onset Scoliosis Surgery. J. Pediatr. Orthop. 2020, 40, e621–e628. [Google Scholar] [CrossRef]
- Vitale, M.G.; Riedel, M.D.; Glotzbecker, M.P.; Matsumoto, H.; Roye, D.P.; Akbarnia, B.A.; Anderson, R.C.; Brockmeyer, D.L.; Emans, J.B.; Erickson, M.; et al. Building consensus: Development of a Best Practice Guideline (BPG) for surgical site infection (SSI) prevention in high-risk pediatric spine surgery. J. Pediatr. Orthop. 2013, 33, 471–478. [Google Scholar] [CrossRef]
- El-Hawary, R.; Akbarnia, B.A. Early Onset Scoliosis-Time for Consensus. Spine Deform. 2015, 3, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Striano, B.M.; Refakis, C.; Garg, S.; El-Hawary, R.; Pahys, J.M.; Vitale, M.; Braun, J.T.; Campbell, R.M.; Flynn, J.M.; Smith, J.T.; et al. How Often Do You Lengthen? A Physician Survey on Lengthening Practice for Prosthetic Rib Devices. Spine Deform. 2018, 6, 473–477. [Google Scholar] [CrossRef]
- Feinberg, N.; Matsumoto, H.; Hung, C.W.; St Hilaire, T.; Pawelek, J.; Sawyer, J.R.; Sturm, P.F.; El-Hawary, R.; Chang, T.N.; Flynn, J.M.; et al. Expert Consensus and Equipoise: Planning a Randomized Controlled Trial of Magnetically Controlled Growing Rods. Spine Deform. 2018, 6, 303–307. [Google Scholar] [CrossRef]
- Williams, B.A.; Asghar, J.; Matsumoto, H.; Flynn, J.M.; Roye, D.P.; Vitale, M.G. More experienced surgeons less likely to fuse: A focus group review of 315 hypothetical EOS cases. J. Pediatr. Orthop. 2013, 33, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Schroth, M.K.; Deans, J.; Bharucha Goebel, D.X.; Burnette, W.B.; Darras, B.T.; Elsheikh, B.H.; Davis, R.; Finkel, R.S.; Lopes, P. Spinal Muscular Atrophy Update in Best Practices: Recommendations for Treatment Considerations. Neurol. Clin. Pract. 2025, 15, e200374. [Google Scholar] [CrossRef]
- Phillips, J.H.; Knapp, D.R.; Herrera-Soto, J. Mortality and morbidity in early-onset scoliosis surgery. Spine 2013, 38, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Tis, J.E.; Karlin, L.I.; Akbarnia, B.A.; Blakemore, L.C.; Thompson, G.H.; McCarthy, R.E. Early onset scoliosis: Modern treatment and results. J. Pediatr. Orthop. 2012, 32, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.M.; Budac, S. Jeune Syndrome (Asphyxiating Thoracic Dystrophy). In Essence of Anesthesia Practice; WB Saunders: Philadelphia, PA, USA, 2011; p. 222. [Google Scholar]
- Phillips, J.D.; van Aalst, J.A. Jeune’s syndrome: Congenital and acquired. Semin. Pediatr. Surg. 2008, 17, 167–172. [Google Scholar] [CrossRef]
- O’Brien, A.; Roth, M.K.; Athreya, H.; Reinker, K.; Koeck, W.; Patil, V. Management of TIS in Jeune Syndrome. J. Pediatr. Orthop. 2015, 35, 783–797. [Google Scholar] [CrossRef]
- Hennekam, R.C.; Beemer, F.A.; Gerards, L.J.; Cats, B.P. Thoracic pelvic phalangeal dystrophy (Jeune syndrome). Tijdschr. Kindergeneeskd. 1983, 51, 95–100. [Google Scholar] [PubMed]
- Cornier, A.S.; Staehling-Hampton, K.; Delventhal, K.M.; Katcherian, A.; Tsin, A.; Couzon, D.; Young, C.; Mitchell, R.; Gordon, T. Mutations in the MESP2 gene cause spondylothoracic dysostosis/Jarcho-Levin syndrome. Am. J. Hum. Genet. 2008, 82, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Berdon, W.E.; Lampl, B.S.; Cornier, A.S.; Greenberg, L.; Greenberg, J.; Johnston, J. Clinical and radiological distinction between spondylothoracic dysostosis (Lavy-Moseley syndrome) and spondylocostal dysostosis (Jarcho-Levin syndrome). Pediatr. Radiol. 2011, 41, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.M., Jr. Spine deformities in rare congenital syndromes: Clinical issues. Spine 2009, 34, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N.; Cornier, A.S.; Campbell, R.M., Jr. Natural history of thoracic insufficiency syndrome: A spondylothoracic dysplasia perspective. J. Bone Joint Surg. Am. 2007, 89, 2663–2675. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Galasko, C.S.; Delaney, C.; Williamson, J.B.; Barrie, J.L. Scoliosis and lung function in spinal muscular atrophy. Eur. Spine J. 1995, 4, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.J.; Kissel, J.T. Spinal muscular atrophy: A timely review. Arch. Neurol. 2011, 68, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.J.; Coffey, C.S.; Yankey, J.W.; Krosschell, K.J.; Arnold, W.D.; Lowes, L.P.; Chakraverty, D.; Kaspar, B.K.; Kissel, J.T. Natural history of infantile-onset spinal muscular atrophy. Ann. Neurol. 2017, 82, 883–891. [Google Scholar] [CrossRef]
- Livingston, K.; Zurakowski, D.; Snyder, B.; Growing Spine Study Group; Children’s Spine Study Group. Parasol rib deformity in hypotonic neuromuscular scoliosis: A new radiographical definition and a comparison of short-term treatment outcomes with VEPTR and growing rods. Spine 2015, 40, E780–E786. [Google Scholar] [CrossRef]
- Swarup, I.; MacAlpine, E.M.; Mayer, O.H.; Lark, R.K.; Smith, J.T.; Vitale, M.G.; Cahill, P.J. Impact of growth-friendly interventions on spine and pulmonary outcomes of patients with spinal muscular atrophy. Eur. Spine J. 2021, 30, 768–774. [Google Scholar] [CrossRef]
- Lorenz, H.M.; Badwan, B.; Hecker, M.M.; Tsaknakis, K.; Groenefeld, K.; Braunschweig, L.; Hell, A.K. Magnetically controlled devices parallel to the spine in children with spinal muscular atrophy. JBJS Open Access 2017, 2, e0036. [Google Scholar] [CrossRef] [PubMed]
- Lenhart, R.L.; Youlo, S.; Schroth, M.K.; Noonan, K.J.; McCarthy, J.; Mann, D.; Patel, P.; Flynn, J.M. Radiographic and respiratory effects of growing rods in children with spinal muscular atrophy. J. Pediatr. Orthop. 2017, 37, e500–e505. [Google Scholar] [CrossRef]
- Colombo, L.; Martini, C.; Bersanini, C.; Izzo, F.; Villafañe, J.H.; Berjano, P.; Montanari, C.; Pedretti, A.; Caserta, F. Effects of magnetically controlled growing rods surgery on pulmonary function in young subjects with spinal muscular atrophy type 2 and other neuromuscular scoliosis. J. Neurosurg. Sci. 2020, 64, 253–257. [Google Scholar] [CrossRef]
- Chandran, S.; McCarthy, J.; Noonan, K.; Mann, D.; Nemeth, B.; Guiliani, T.; Flynn, J.M.; Smith, J.T. Early treatment of scoliosis with growing rods in children with severe spinal muscular atrophy: A preliminary report. J. Pediatr. Orthop. 2011, 31, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.M.; Hecker, M.M.; Braunschweig, L.; Badwan, B.; Tsaknakis, K.; Hell, A.K. Continuous lengthening potential after four years of magnetically controlled spinal deformity correction in children with spinal muscular atrophy. Sci. Rep. 2020, 10, 22420. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Nusinersen: First global approval. Drugs 2017, 77, 473–479. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowen, M.; Desai, V.; Anari, J.B.; Cahill, P.J. Evaluation and Treatment of Thoracic Insufficiency Syndrome and Early-Onset Scoliosis. J. Clin. Med. 2025, 14, 753. https://doi.org/10.3390/jcm14030753
Bowen M, Desai V, Anari JB, Cahill PJ. Evaluation and Treatment of Thoracic Insufficiency Syndrome and Early-Onset Scoliosis. Journal of Clinical Medicine. 2025; 14(3):753. https://doi.org/10.3390/jcm14030753
Chicago/Turabian StyleBowen, Margaret, Vineet Desai, Jason B. Anari, and Patrick J. Cahill. 2025. "Evaluation and Treatment of Thoracic Insufficiency Syndrome and Early-Onset Scoliosis" Journal of Clinical Medicine 14, no. 3: 753. https://doi.org/10.3390/jcm14030753
APA StyleBowen, M., Desai, V., Anari, J. B., & Cahill, P. J. (2025). Evaluation and Treatment of Thoracic Insufficiency Syndrome and Early-Onset Scoliosis. Journal of Clinical Medicine, 14(3), 753. https://doi.org/10.3390/jcm14030753




