Electrophysiological Variations in Auditory Potentials in Chronic Tinnitus Individuals: Treatment Response and Tinnitus Laterality
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Origin, Recruitment Process, and Patient Characteristics
2.2. Electrophysiological Measurements
2.3. Descriptive and Statistical Analyses in the Time Domain and Waveform Categorisation
3. Results
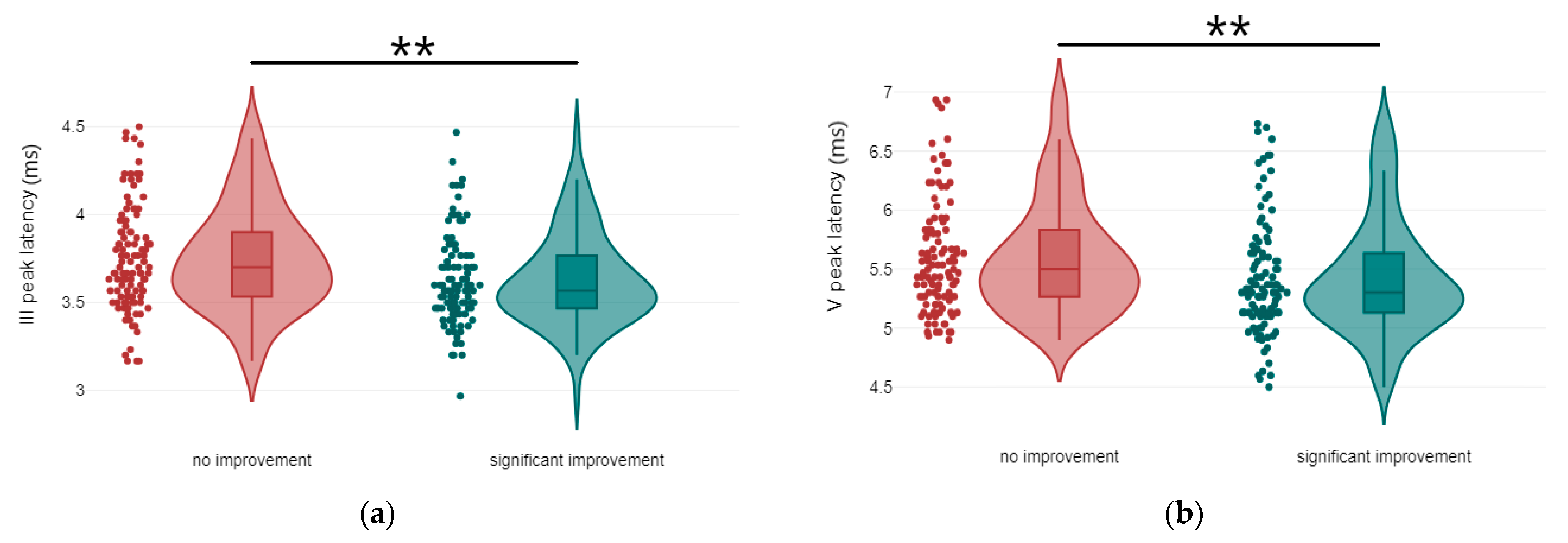
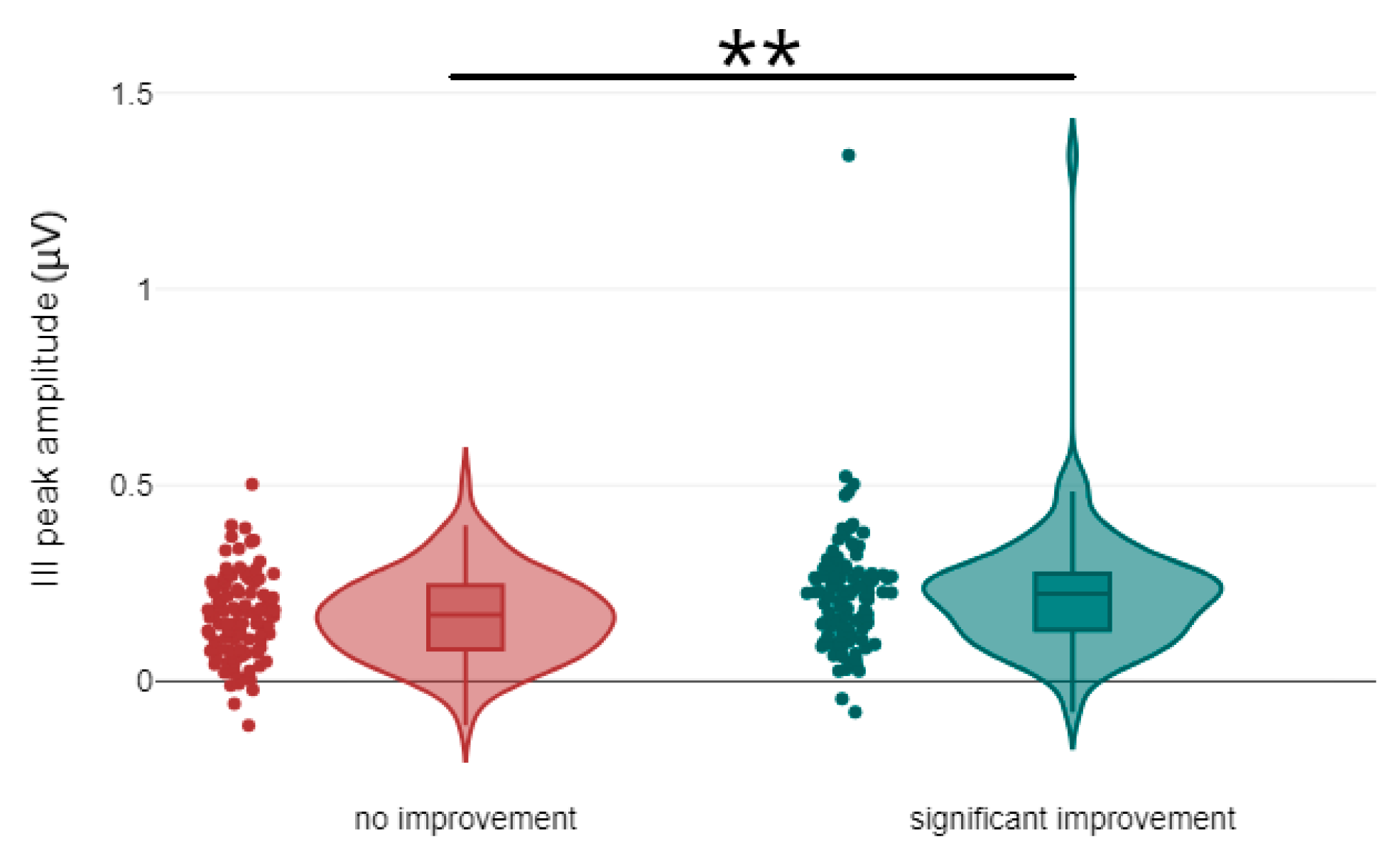
3.1. Correlation Between Treatment Response and the Latency and Amplitude Components of ABR and AMLR Waveforms
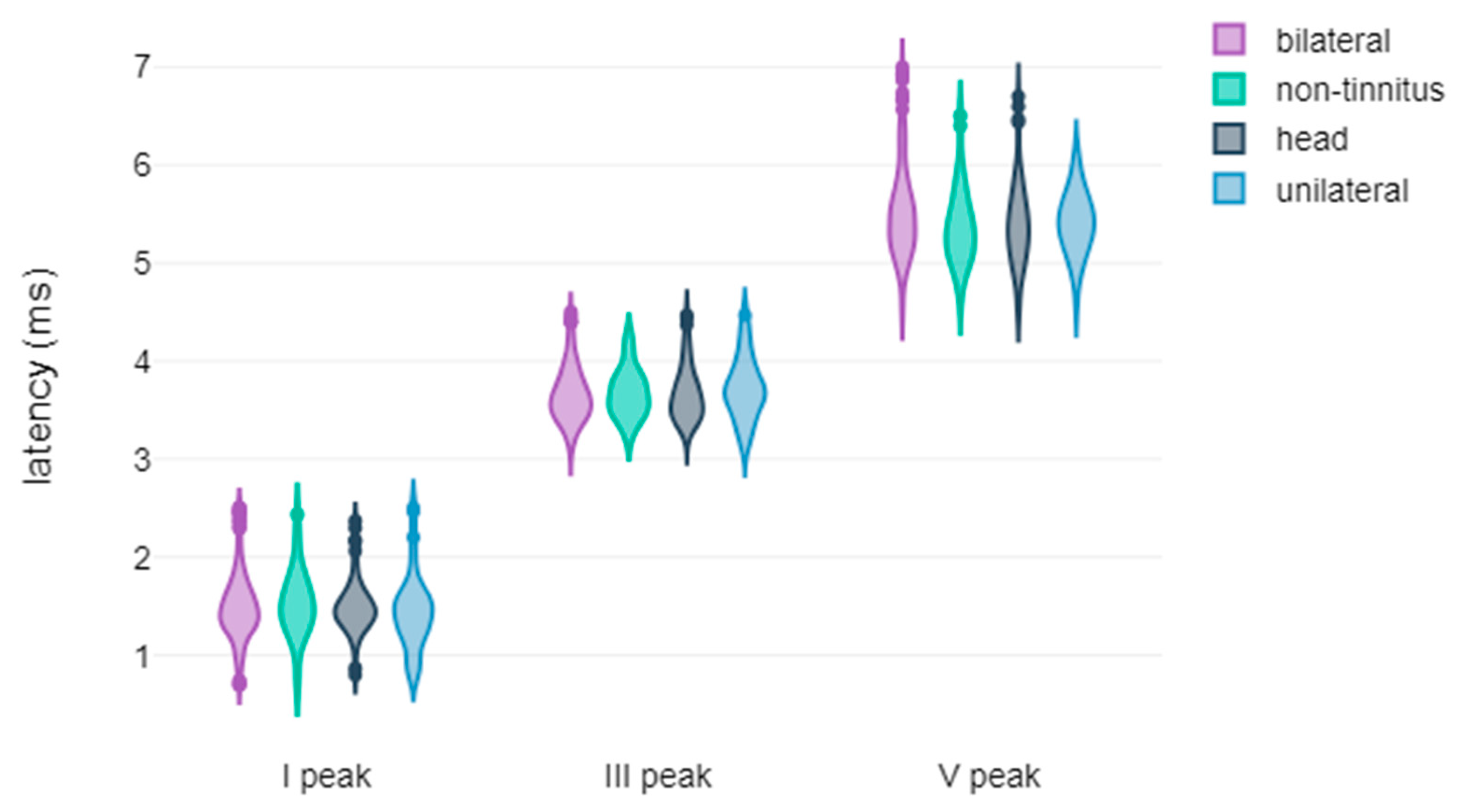
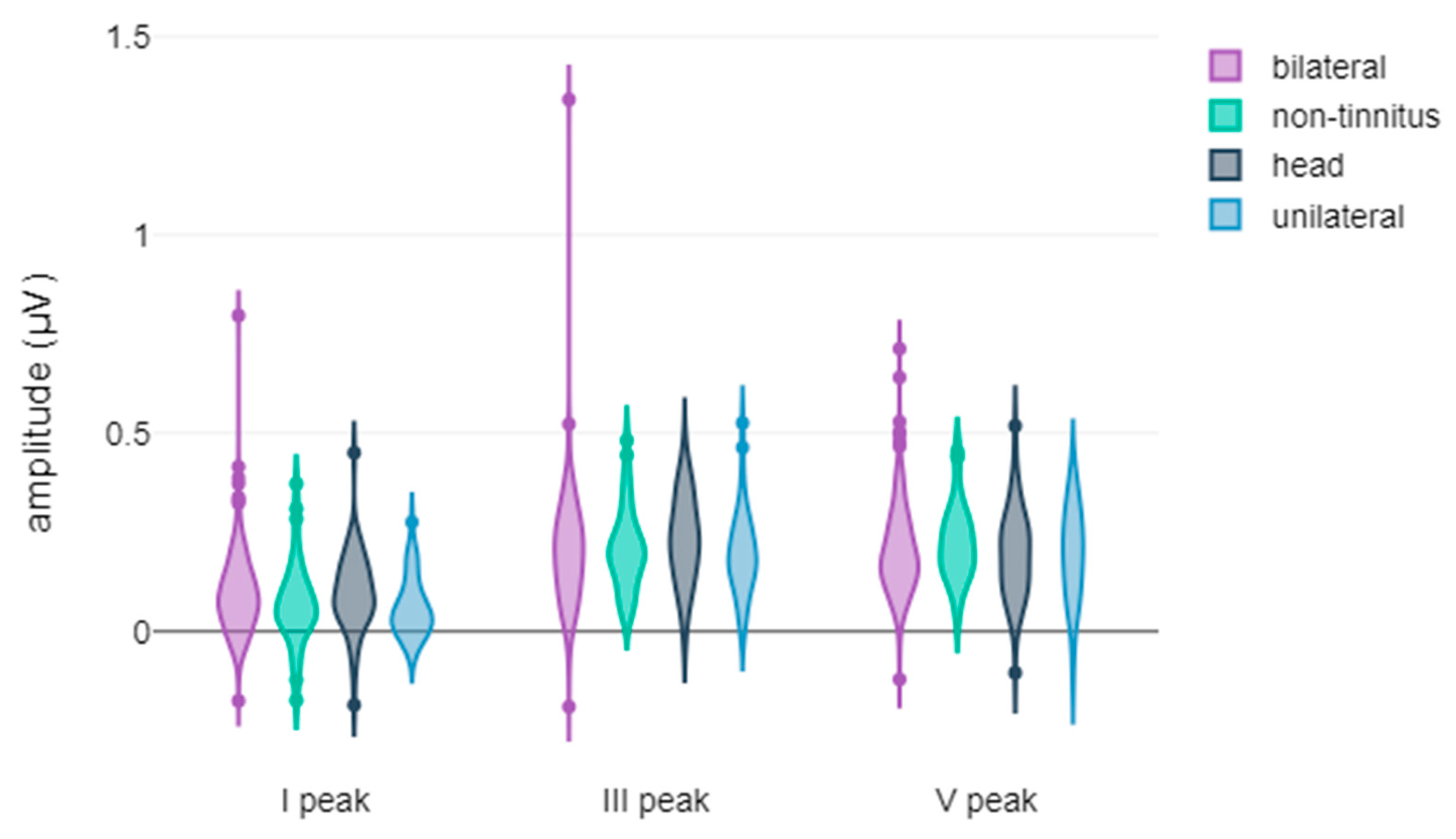
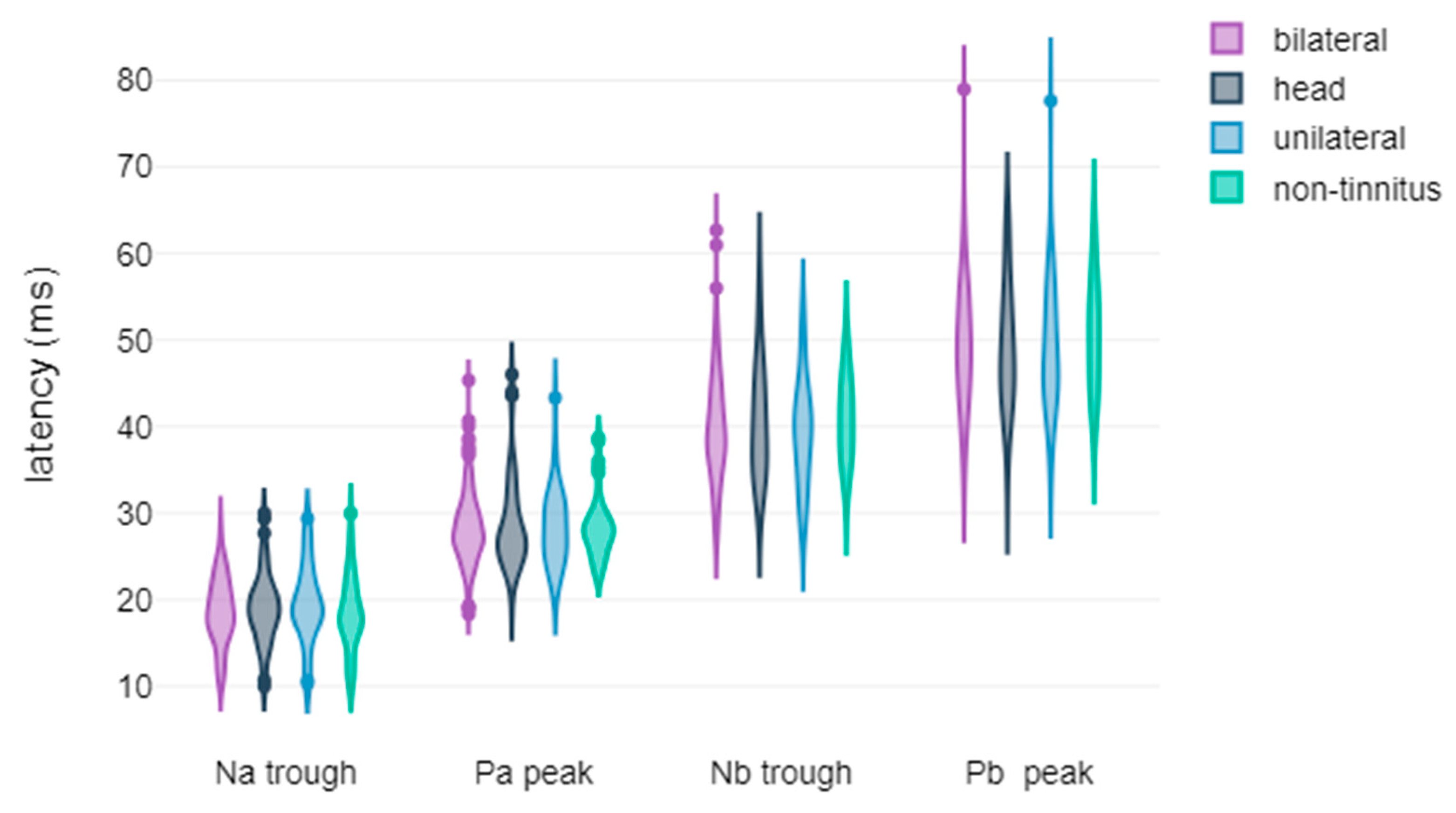
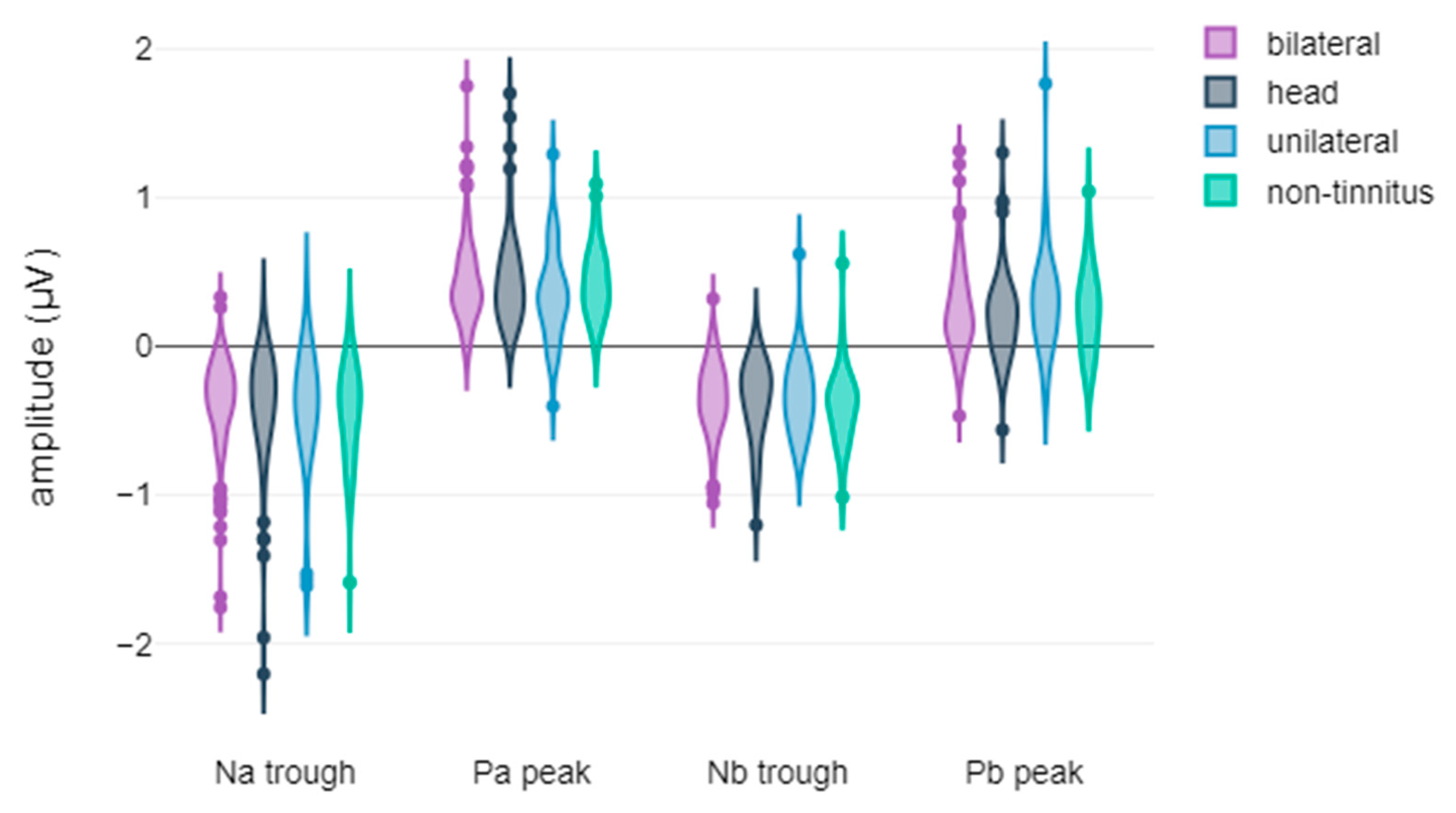
3.2. Correlation Between Tinnitus Laterality and the Latency and Amplitude Components of ABR and AMLR Waveforms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cima, R.F.F.; Mazurek, B.; Haider, H.; Kikidis, D.; Lapira, A.; Noreña, A.; Hoare, D.J. A Multidisciplinary European Guideline for Tinnitus: Diagnostics, Assessment, and Treatment. HNO 2019, 67, 10–42. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, A.A.; Renton, J. A Review of Tinnitus. Aust. J. Gen. Pract. 2018, 47, 205–208. [Google Scholar] [CrossRef]
- Gopinath, B.; McMahon, C.M.; Rochtchina, E.; Karpa, M.J.; Mitchell, P. Incidence, Persistence, and Progression of Tinnitus Symptoms in Older Adults: The Blue Mountains Hearing Study. Ear Hear. 2010, 31, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Wallenhorst, C.; McFerran, D.; Hall, D.A. Incidence Rates of Clinically Significant Tinnitus: 10-Year Trend from a Cohort Study in England. Ear Hear. 2015, 36, e69–e75. [Google Scholar] [CrossRef]
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Kullar, P.; Malhotra, C.; Stankovic, K.M. Current and Emerging Therapies for Chronic Subjective Tinnitus. J. Clin. Med. 2023, 12, 6555. [Google Scholar] [CrossRef]
- Person, O.C.; Veiga, F.; Junior, A.; Altoé, J.; Portes, L.M.; Lopes, P.R.; Dos, M.E.; Puga, S.; Clayton, O.; Brasileiros De Ciências Da, A.; et al. O Que Revisões Sistemáticas Cochrane Dizem Sobre Terapêutica Para Zumbido? ABCS Health Sci. 2022, 47, e022301. [Google Scholar] [CrossRef]
- Lewkowski, K.; Heyworth, J.; Ytterstad, E.; Williams, W.; Goulios, H.; Fritschi, L. The Prevalence of Tinnitus in the Australian Working Population. Med. J. Aust. 2022, 216, 189–193. [Google Scholar] [CrossRef]
- Nondahl, D.M.; Cruickshanks, K.J.; Huang, G.H.; Klein, B.E.K.; Klein, R.; Tweed, T.S.; Zhan, W. Generational Differences in the Reporting of Tinnitus. Ear Hear. 2012, 33, 640. [Google Scholar] [CrossRef]
- Hasson, D.; Theorell, T.; Westerlund, H.; Canlon, B. Prevalence and Characteristics of Hearing Problems in a Working and Non-Working Swedish Population. J. Epidemiol. Community Health 2010, 64, 453–460. [Google Scholar] [CrossRef]
- Wu, N.; Xu, M.; Liu, C.; Chen, Q.; Gao, J.H.; Wang, Z.; Lv, H. Treatment Outcomes in Tinnitus Patients Are Associated with Brain Functional Network: Evidence from Connectome Gradient and Gene Expression Analysis. Neuroscience 2024, 553, 89–97. [Google Scholar] [CrossRef]
- Manta, O.; Sarafidis, M.; Schlee, W.; Mazurek, B.; Matsopoulos, G.K.; Koutsouris, D.D. Development of Machine-Learning Models for Tinnitus-Related Distress Classification Using Wavelet-Transformed Auditory Evoked Potential Signals and Clinical Data. J. Clin. Med. 2023, 12, 3843. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, M.P.; Subramaniam, K.; Bin Yaccob, S.; Bin Adom, A.H.; Hema, C.R. Auditory Evoked Potential Response and Hearing Loss: A Review. Open Biomed. Eng. J. 2015, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.W. (Ed.) Handbook of Auditory Evoked Responses; Pearson Education, Inc.: New York, NY, USA, 2015; ISBN 0205135668. [Google Scholar]
- Sörnmo, L.; Laguna, P. Evoked Potentials. In Bioelectrical Signal Processing in Cardiac and Neurological Applications; Academic Press: Cambridge, MA, USA, 2005; pp. 181–336. [Google Scholar]
- Young, A.; Cornejo, J.; Spinner, A. Auditory Brainstem Response; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kim, L.-S.; Ahn, Y.-M.; Yoo, K.-H.; Heo, S.-D.; Park, H.-S. Normative Data of Auditory Middle Latency Responses in Adults. Korean J. Audiol. 1997, 1, 48–56. [Google Scholar]
- McFadden, D.; Champlin, C.A.; Pho, M.H.; Pasanen, E.G.; Malone, M.M.; Leshikar, E.M. Auditory Evoked Potentials: Differences by Sex, Race, and Menstrual Cycle and Correlations with Common Psychoacoustical Tasks. PLoS ONE 2021, 16, e0251363. [Google Scholar] [CrossRef]
- Schlee, W.; Schoisswohl, S.; Staudinger, S.; Schiller, A.; Lehner, A.; Langguth, B.; Schecklmann, M.; Simoes, J.; Neff, P.; Marcrum, S.C.; et al. Towards a unification of treatments and interventions for tinnitus patients: The EU research and innovation action UNITI. In Progress in Brain Research; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 260, pp. 441–451. ISBN 9780128215869. [Google Scholar]
- Home—Unification of Treatments and Interventions for Tinnitus Patients (UNITI). Available online: https://uniti.tinnitusresearch.net/index.php (accessed on 7 January 2025).
- Interacoustics Eclipse EP25 Manuals|ManualsLib. Available online: https://www.manualslib.com/products/Interacoustics-Eclipse-Ep25-11647463.html (accessed on 18 October 2022).
- Manta, O.; Sarafidis, M.; Vasileiou, N.; Schlee, W.; Consoulas, C.; Kikidis, D.; Vassou, E.; Matsopoulos, G.K.; Koutsouris, D.D. Development and Evaluation of Automated Tools for Auditory-Brainstem and Middle-Auditory Evoked Potentials Waves Detection and Annotation. Brain Sci. 2022, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, Z.; Marouf, A.; Zenke, J.; Leung, A.W.S.; Gomaa, N.A. Functional Brain Regions Linked to Tinnitus Pathology and Compensation During Task Performance: A Systematic Review. Otolaryngol. Head. Neck Surg. 2023, 169, 1409–1423. [Google Scholar] [CrossRef]
- Jacxsens, L.; De Pauw, J.; Cardon, E.; van der Wal, A.; Jacquemin, L.; Gilles, A.; Michiels, S.; Van Rompaey, V.; Lammers, M.J.W.; De Hertogh, W. Brainstem Evoked Auditory Potentials in Tinnitus: A Best-Evidence Synthesis and Meta-Analysis. Front. Neurol. 2022, 13, 941876. [Google Scholar] [CrossRef] [PubMed]
- Tinnitus in Temporomandibular Disorders: Electrophysiological Aspects|Request PDF. Available online: https://www.researchgate.net/publication/286324809_Tinnitus_in_Temporomandibular_Disorders_Electrophysiological_Aspects (accessed on 31 August 2023).
- Edvall, N.K.; Mehraei, G.; Claeson, M.; Lazar, A.; Bulla, J.; Leineweber, C.; Uhlén, I.; Canlon, B.; Cederroth, C.R. Alterations in Auditory Brain Stem Response Distinguish Occasional and Constant Tinnitus. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Lelic, D.; Caporali, S.; Parker, D.; Nielsen, J.; Balling, L.W. Impact of a Combination Sound Therapy on Tinnitus Distress: An Exploratory One-Year Longitudinal Study. Front. Audiol. Otol. 2024, 2, 1322596. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, L.; Zhang, H.; Kuang, Z.; Yu, H.; Li, T.; Zhu, Z.; Guo, Y. A Knowledge Graph Based Intelligent Auxiliary Diagnosis and Treatment System for Primary Tinnitus Using Traditional Chinese Medicine. Egypt. Inform. J. 2024, 27, 100525. [Google Scholar] [CrossRef]
- Theodoroff, S.M.; Kaltenbach, J.A. The Role of the Brainstem in Generating and Modulating Tinnitus. Am. J. Audiol. 2019, 28, 225–238. [Google Scholar] [CrossRef]
- AlSamhori, J.F.; AlSamhori, A.R.F.; Amourah, R.M.; AlQadi, Y.; Koro, Z.W.; Haddad, T.R.A.; AlSamhori, A.F.; Kakish, D.; Kawwa, M.J.; Zuriekat, M.; et al. Artificial Intelligence for Hearing Loss Prevention, Diagnosis, and Management. J. Med. Surg. Public Health 2024, 3, 100133. [Google Scholar] [CrossRef]
- Simoes, J.P.; Daoud, E.; Shabbir, M.; Amanat, S.; Assouly, K.; Biswas, R.; Casolani, C.; Dode, A.; Enzler, F.; Jacquemin, L.; et al. Multidisciplinary Tinnitus Research: Challenges and Future Directions From the Perspective of Early Stage Researchers. Front. Aging Neurosci. 2021, 13, 647285. [Google Scholar] [CrossRef]

| Treatment Response | Mean | SD | # | Mann–Whitney U-Test | Effect Size (r) | |
|---|---|---|---|---|---|---|
| I peak latency | no improvement | 1.53 | 0.42 | 109 | U = 5384, p = 0.98, r = 0 | too small |
| significant improvement | 1.54 | 0.38 | 99 | |||
| III peak latency | no improvement | 3.75 | 0.3 | 109 | U = 4113, p = 0.001, r = 0.22 | medium |
| significant improvement | 3.63 | 0.26 | 102 | |||
| V peak latency | no improvement | 5.6 | 0.48 | 113 | U = 4746, p = 0.004, r = 0.19 | small to medium |
| significant improvement | 5.42 | 0.49 | 108 | |||
| I peak amplitude | no improvement | 0.07 | 0.09 | 109 | U = 4547.5, p = 0.051, r = 0.14 | small to medium |
| significant improvement | 0.11 | 0.12 | 99 | |||
| III peak amplitude | no improvement | 0.16 | 0.11 | 109 | U = 4183, p = 0.002, r = 0.21 | medium |
| significant improvement | 0.22 | 0.16 | 102 | |||
| V peak amplitude | no improvement | 0.18 | 0.11 | 113 | U = 5371, p = 0.125, r = 0.1 | small |
| significant improvement | 0.2 | 0.14 | 108 |
| AEP Component | Treatment Response | Mean | SD | # | Mann–Whitney U-Test or t-Test for Independent Samples | Effect Size (r or d) |
|---|---|---|---|---|---|---|
| Na trough latency | no improvement | 18.85 | 4.4 | 102 | U = 4719.5, p = 0.852, r = 0.01 | too small |
| significant improvement | 19.17 | 3.73 | 94 | |||
| Pa peak latency | no improvement | 28.65 | 4.89 | 103 | U = 4701, p = 0.551, r = 0.04 | too small |
| significant improvement | 28.15 | 3.93 | 96 | |||
| Nb trough latency | no improvement | 40.3 | 6.78 | 103 | U = 4824, p = 0.769, r = 0.02 | too small |
| significant improvement | 40.13 | 6.28 | 96 | |||
| Pb peak latency | no improvement | 49.51 | 8.27 | 103 | t(197) = 0.26, p = 0.797, 95% CI [−1.87, 2.42] Cohen’s d = 0.04 variance equality | too small |
| significant improvement | 49.23 | 6.88 | 96 | |||
| Na trough amplitude | no improvement | −0.33 | 0.31 | 102 | U = 3871.5, p = 0.02, r = 0.17 | small to medium |
| significant improvement | −0.43 | 0.34 | 94 | |||
| Pa peak amplitude | no improvement | 0.39 | 0.26 | 103 | U = 4340, p = 0.138, r = 0.11 | small to medium |
| significant improvement | 0.44 | 0.29 | 96 | |||
| Nb trough amplitude | no improvement | −0.33 | 0.24 | 103 | U = 4108.5, p = 0.04, r = 0.15 | small to medium |
| significant improvement | −0.39 | 0.25 | 96 | |||
| Pb peak amplitude | no improvement | 0.21 | 0.24 | 103 | U = 4309, p = 0.118, r = 0.11 | small to medium |
| significant improvement | 0.27 | 0.35 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manta, O.; Kikidis, D.; Schlee, W.; Langguth, B.; Mazurek, B.; Lopez-Escamez, J.A.; Martin-Lagos, J.; Cima, R.; Bromis, K.; Vellidou, E.; et al. Electrophysiological Variations in Auditory Potentials in Chronic Tinnitus Individuals: Treatment Response and Tinnitus Laterality. J. Clin. Med. 2025, 14, 760. https://doi.org/10.3390/jcm14030760
Manta O, Kikidis D, Schlee W, Langguth B, Mazurek B, Lopez-Escamez JA, Martin-Lagos J, Cima R, Bromis K, Vellidou E, et al. Electrophysiological Variations in Auditory Potentials in Chronic Tinnitus Individuals: Treatment Response and Tinnitus Laterality. Journal of Clinical Medicine. 2025; 14(3):760. https://doi.org/10.3390/jcm14030760
Chicago/Turabian StyleManta, Ourania, Dimitris Kikidis, Winfried Schlee, Berthold Langguth, Birgit Mazurek, Jose A. Lopez-Escamez, Juan Martin-Lagos, Rilana Cima, Konstantinos Bromis, Eleftheria Vellidou, and et al. 2025. "Electrophysiological Variations in Auditory Potentials in Chronic Tinnitus Individuals: Treatment Response and Tinnitus Laterality" Journal of Clinical Medicine 14, no. 3: 760. https://doi.org/10.3390/jcm14030760
APA StyleManta, O., Kikidis, D., Schlee, W., Langguth, B., Mazurek, B., Lopez-Escamez, J. A., Martin-Lagos, J., Cima, R., Bromis, K., Vellidou, E., Zachou, Z., Markatos, N., Vassou, E., Kouris, I., Matsopoulos, G. K., & Koutsouris, D. D. (2025). Electrophysiological Variations in Auditory Potentials in Chronic Tinnitus Individuals: Treatment Response and Tinnitus Laterality. Journal of Clinical Medicine, 14(3), 760. https://doi.org/10.3390/jcm14030760











