Impact of a Concurrent Respiratory Virus Infection on the Clinical Presentation and Response to Initial Treatment of Kawasaki Disease: A Single-Center Observational Study
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. FARP Testing
2.3. Statistical Analysis
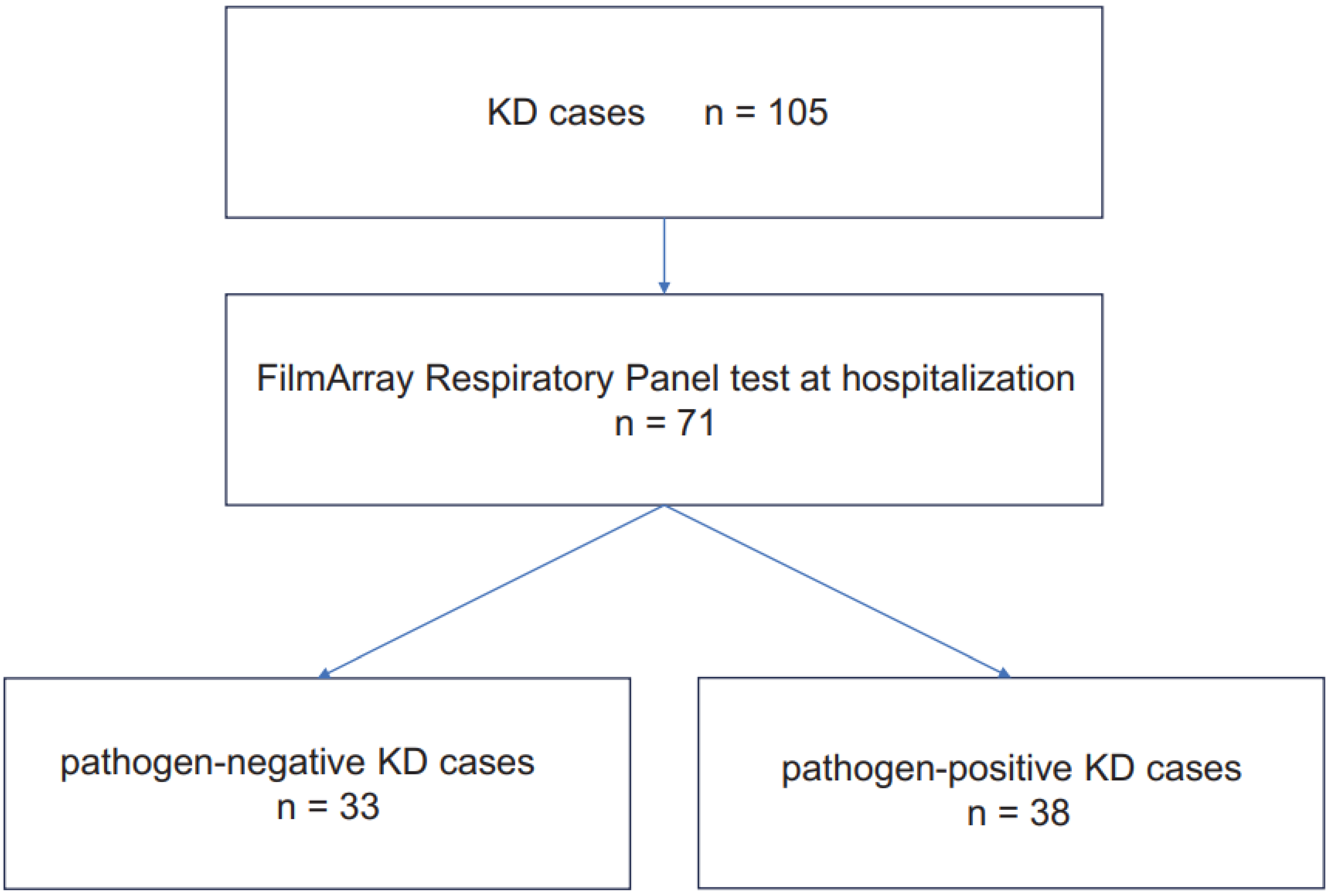
3. Results
4. Discussion
5. Conclusions
6. Key Points
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burns, J.C. Commentary: Translation of Dr. Tomisaku Kawasaki’s original report of fifty patients in 1967. Pediatr. Infect Dis. J. 2002, 21, 993–995. [Google Scholar] [CrossRef] [PubMed]
- Onouchi, Y.; Gunji, T.; Burns, J.C.; Shimizu, C.; Newburger, J.W.; Yashiro, M.; Nakamura, Y.; Yanagawa, H.; Wakui, K.; Fukushima, Y.; et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat. Genet. 2008, 40, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Nakashima, Y.; Sakai, Y.; Nishio, H.; Motomura, Y.; Yamasaki, S. Kawasaki disease: A matter of innate immunity. Clin. Exp. Immunol. 2016, 186, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Huang, P.; Zhang, L.; Xie, X.; Xia, S.; Gong, F.; Yuan, J.; Jin, L. Influenza infection and Kawasaki disease. Rev. Soc. Bras. Med. Trop. 2015, 48, 243–248. [Google Scholar] [CrossRef]
- Nagao, Y.; Urabe, C.; Nakamura, H.; Hatano, N. Predicting the characteristics of the aetiological agent for Kawasaki disease from other paediatric infectious diseases in Japan. Epidemiol. Infect. 2016, 144, 478–492. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, C.Y.; Weng, K.P.; Chien, K.J.; Hung, Y.M.; Hsieh, K.S.; Lin, C.C.; Cheng, M.F.; Lin, C.L.; Wei, J.C. Adenovirus infection and subsequent risk of Kawasaki disease: A population-based cohort study. J. Chin. Med. Assoc. 2020, 83, 302–306. [Google Scholar] [CrossRef]
- Yoshioka, T.; Matsutani, T.; Toyosaki-Maeda, T.; Suzuki, H.; Uemura, S.; Suzuki, R.; Koike, M.; Hinuma, Y. Relation of streptococcal pyrogenic exotoxin C as a causative superantigen for Kawasaki disease. Pediatr. Res. 2003, 53, 403–410. [Google Scholar] [CrossRef]
- Nakagawa, R.; Okada, M.; Hashimoto, S.; Yokoyama, H.; Shimoyama, T.; Udagawa, T.; Oshiba, A.; Nagasawa, M. COVID-19 pandemic-altered epidemiology of pediatric infectious diseases and vasculitis: A single-center observational study. Int. J. Rheum. Dis. 2023, 26, 2592–2595. [Google Scholar] [CrossRef]
- Yang, Y.L.; Kuo, H.C. Public Health Interventions for COVID-19 Reduce Kawasaki Disease in Taiwan. Children 2021, 8, 623. [Google Scholar] [CrossRef]
- Hong, S.J.; Kang, B.; Hwang, J.H.; Kim, Y.B.; Lee, Y.M.; Jang, H.J.; Lee, K.J.; Kim, S.C.; Kang, Y.; Kim, H.J.; et al. The occurrence of infection-related systemic diseases in Korean children and adolescents has decreased after the spread of the COVID-19 pandemic: A multicenter retrospective study. Transl. Pediatr. 2021, 10, 2888–2896. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ayusawa, M.; Suzuki, H.; Abe, J.; Ito, S.; Kato, T.; Kamada, M.; Shiono, J.; Suda, K.; Tsuchiya, K.; et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition). Pediatr. Int. 2020, 62, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Newburger, J.W.; Takahashi, M.; Beiser, A.S.; Burns, J.C.; Bastian, J.; Chung, K.J.; Colan, S.D.; Duffy, C.E.; Fulton, D.R.; Glode, M.P.; et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N. Engl. J. Med. 1991, 324, 1633–1639. [Google Scholar] [CrossRef]
- Furusho, K.; Kamiya, T.; Nakano, H.; Kiyosawa, N.; Shinomiya, K.; Hayashidera, T.; Tamura, T.; Hirose, O.; Manabe, Y.; Yokoyama, T.; et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 1984, 2, 1055–1058. [Google Scholar] [CrossRef]
- Newburger, J.W.; Takahashi, M.; Burns, J.C.; Beiser, A.S.; Chung, K.J.; Duffy, C.E.; Glode, M.P.; Mason, W.H.; Reddy, V.; Sanders, S.P.; et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 1986, 315, 341–347. [Google Scholar] [CrossRef]
- Kobayashi, T.; Inoue, Y.; Takeuchi, K.; Okada, Y.; Tamura, K.; Tomomasa, T.; Kobayashi, T.; Morikawa, A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006, 113, 2606–2612. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef]
- Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Digest Version Circ. J. 2014, 78, 2521–2562.
- Ashiarai, M.; Shinbo, A.K.M.; Nakatani, H.; Onda, K.; Okada, M.; Imai, M.; Suzuki, N.; Oshiba, A.; Nagasawa, M. Evaluation of Kasasaki Disease Risk Scoring System in a Single Center Experience from Japan. Clin. Res. Pediatrics 2018, 1, 1. [Google Scholar]
- Noval Rivas, M.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020, 16, 391–405. [Google Scholar] [CrossRef]
- Dong, C. Diversification of T-helper-cell lineages: Finding the family root of IL-17-producing cells. Nat. Rev. Immunol. 2006, 6, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, B.J.; Craft, J.E.; Kaech, S.M. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat. Rev. Immunol. 2016, 16, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Udagawa, T.; Kato, T.; Tanaka, I.; Yamamoto, R.; Sakaguchi, H.; Sekikawa, Y. Observational Study on the Clinical Reality of Community-Acquired Respiratory Virus Infections in Adults and Older Individuals. Pathogens 2024, 13, 983. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wu, M.; Zhou, J.; Wang, Y.; Hao, C.; Ji, W.; Zhang, X.; Gu, W.; Shao, X. Etiologic spectrum and occurrence of coinfections in children hospitalized with community-acquired pneumonia. BMC Infect. Dis. 2017, 17, 787. [Google Scholar] [CrossRef]
- Marutani, K.; Murata, K.; Mizuno, Y.; Onoyama, S.; Hoshina, T.; Yamamura, K.; Furuno, K.; Sakai, Y.; Kishimoto, J.; Kusuhura, K.; et al. Respiratory viral infections and Kawasaki disease: A molecular epidemiological analysis. J. Microbiol. Immunol. Infect. 2024, 57, 691–699. [Google Scholar] [CrossRef]
- Stephens, L.M.; Varga, S.M. Function and Modulation of Type I Interferons during Respiratory Syncytial Virus Infection. Vaccines 2020, 8, 177. [Google Scholar] [CrossRef]
- Ajay, M.; Mantan, M.; Dabas, A.; Asraf, A.; Yadav, S.; Chakravarti, A. Seroprotection for Diphtheria, Pertussis, Tetanus and Measles in Children With Nephrotic Syndrome. Indian. Pediatr. 2021, 58, 233–236. [Google Scholar]
- Lin, C.Y.; Hsu, H.C. Histopathological and immunological studies in spontaneous remission of nephrotic syndrome after intercurrent measles infection. Nephron 1986, 42, 110–115. [Google Scholar] [CrossRef]
- Tamura, H.; Kuraoka, S.; Hidaka, Y.; Nagata, H.; Furuie, K.; Nakazato, H. A Case of Nephrotic Syndrome that Resolved with Influenza B Infection. Case Rep. Nephrol. Dial. 2021, 11, 103–109. [Google Scholar] [CrossRef]
- Edwards, M.R.; Strong, K.; Cameron, A.; Walton, R.P.; Jackson, D.J.; Johnston, S.L. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J. Allergy Clin. Immunol. 2017, 140, 909–920. [Google Scholar] [CrossRef]
- Beale, J.; Jayaraman, A.; Jackson, D.J.; Macintyre, J.D.R.; Edwards, M.R.; Walton, R.P.; Zhu, J.; Man Ching, Y.; Shamji, B.; Edwards, M.; et al. Rhinovirus-induced IL-25 in asthma exacerbation drives type 2 immunity and allergic pulmonary inflammation. Sci. Transl. Med. 2014, 6, 256ra134. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Makrinioti, H.; Rana, B.M.; Shamji, B.W.; Trujillo-Torralbo, M.B.; Footitt, J.; Jerico, D.-R.; Telcian, A.G.; Nikonova, A.; Zhu, J.; et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am. J. Respir. Crit. Care Med. 2014, 190, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.L.; Peel, S.; Fox, J.; Panova, V.; Hardman, C.S.; Camelo, A.; Bucks, C.; Wu, X.; Kane, C.M.; Neill, D.R.; et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 2013, 132, 933–941. [Google Scholar] [CrossRef]
- Steinke, J.W.; Liu, L.; Turner, R.B.; Braciale, T.J.; Borish, L. Immune surveillance by rhinovirus-specific circulating CD4+ and CD8+ T lymphocytes. PLoS ONE 2015, 10, e0115271. [Google Scholar] [CrossRef]
- Ko, Y.K.; Zhang, Y.L.; Wee, J.H.; Han, D.H.; Kim, H.J.; Rhee, C.S. Human Rhinovirus Infection Enhances the Th2 Environment in Allergic and Non-allergic Patients with Chronic Rhinosinusitis. Clin. Exp. Otorhinolaryngol. 2021, 14, 217–224. [Google Scholar] [CrossRef]
- Jansen, K.; Wirz, O.F.; van de Veen, W.; Tan, G.; Mirer, D.; Sokolowska, M.; Satitsuksanoa, P.; Message, S.D.; Kebadze, T.; Glanville, N.; et al. Loss of regulatory capacity in Treg cells following rhinovirus infection. J. Allergy Clin. Immunol. 2021, 148, 1016–1029.e16. [Google Scholar] [CrossRef]
- Davies, S.; Sutton, N.; Blackstock, S.; Gormley, S.; Hoggart, C.J.; Levin, M.; Herberg, J.A. Predicting IVIG resistance in UK Kawasaki disease. Arch. Dis. Child. 2015, 100, 366–368. [Google Scholar] [CrossRef]
- Sleeper, L.A.; Minich, L.L.; McCrindle, B.M.; Li, J.S.; Mason, W.; Colan, S.D.; Atz, A.M.; Printz, B.F.; Baker, A.; Vetter, V.L.; et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J. Pediatr. 2011, 158, 831–835.e3. [Google Scholar] [CrossRef]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef]
- Li, Y.W.; Wan, Q.; Cheng, Y.; Hu, H.B. Possible Involvement of Infection with Human Rhinoviruses in Children with Kawasaki Disease. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023049. [Google Scholar]
- Turnier, J.L.; Anderson, M.S.; Heizer, H.R.; Jone, P.N.; Glodé, M.P.; Dominguez, S.R. Concurrent Respiratory Viruses and Kawasaki Disease. Pediatrics 2015, 136, e609–e614. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, J.J.; Lee, J.; Kim, M.N.; Ko, H.K.; Choi, H.S.; Kim, Y.H.; Ko, J.K. Detection rate and clinical impact of respiratory viruses in children with Kawasaki disease. Korean J. Pediatr. 2012, 55, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Higuchi, Y.; Shimizu, J. Respiratory pathogen trends in patients with Kawasaki disease during the COVID-19 pandemic and respiratory syncytial virus epidemic in Japan. Pediatr. Neonatol. 2023, 64, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Lehtinen, P.; Vuorinen, T.; Koskenvuo, M.; Ruuskanen, O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J. Med. Virol. 2004, 72, 695–699. [Google Scholar] [CrossRef]
| Pathogen | Number of Cases in KD (%) | Number of Cases in Non-KD (%) |
|---|---|---|
| RV/EV | 27 (38.0) | 398 (39.1) |
| RSV | 7 (9.9) | 188 (18.5) |
| PIV3 | 1 (1.4) | 101 (9.9) |
| PIV4 | 1 (1.4) | 6 (0.6) |
| AdV | 2 (2.8) | 82 (8.1) |
| HKU1 | 1 (1.4) | 4 (0.4) |
| OC43 | 2 (2.8) | 12 (1.2) |
| NL63 | 2 (2.8) | 7 (0.7) |
| hMPV | 1 (1.4) | 57 (5.6) |
| PIV1 | 0 | 16 (1.6) |
| FluAH3 | 0 | 10 (1.0) |
| FluH1N1pdm | 0 | 1 (0.1) |
| SARS-CoV-2 | 3 (4.2) | 57 (5.6) |
| At least one or more positive | 38 (53.5) | 677 (66.5) |
| Double positive | 6 (8.5) | 165 (16.2) |
| Triple positive | 2 (2.8) | 34 (3.3) |
| Quadruple positive | 0 | 7 (0.7) |
| Quintuple positive | 0 | 2 (0.2) |
| Multiple positive | 8 (11.3) | 208 (20.4) |
| FARP | Negative n = 33 | Positive n = 38 | p-Value | RV/EV (+) n = 27 | p-Value | RSV (+) n = 7 | p-Value |
|---|---|---|---|---|---|---|---|
| Sex (male/female) | 20/13 | 27/11 | 0.45 | 20/7 | 0.41 | 5/2 | 0.63 |
| Age (month, IQR) | 33 (10–47) | 26.5 (19.5–43.3) | 0.96 | 28 (22–43.5) | 0.95 | 15 (9–20.5) | 0.29 |
| Days since illness onset (day, IQR) | 4 (3–5) | 5 (4–6.8) | 0.16 | 5 (4–6) | 0.33 | 4 (3–6.5) | 0.96 |
| K-score ≥ 5 | 7 (21.2%) | 16 (42.1%) | 0.23 | 9 (33.3%) | 0.26 | 5 (71.4%) | 0.008 a |
| K-score (IQR) | 3 (2–4) | 3 (1–5) | 0.90 | 3 (1–5) | 0.49 | 6 (4–6.5) | 0.07 |
| Resistance to initial treatment | 7 (21.2%) | 3 (7.9%) | 0.17 | 2 (7.4%) | 0.17 | 0 (0%) | 0.18 |
| WBC (/μL, IQR) | 14,600 (11,500–15,500) | 13,150 (11,550–15,885) | 0.51 | 12,900 (11,000–15,150) | 0.28 | 13,200 (1650–15,950) | 0.75 |
| Platelet (×104/μL, IQR) | 35.4 (26.9–42.5) | 33.5 (27.8–42.1) | 0.77 | 33.9 (27.5–42.1) | 0.85 | 29.3 (25.9–35.5) | 0.38 |
| Na (mEq/L, IQR) | 135 (133–136) | 135.5 (133.3–136.8) | 0.25 | 136 (135–136) | 0.07 | 133 (131–135) | 0.14 |
| AST (IU/L, IQR) | 35 (27–41) | 41.5 (31.5–73.3) | 0.04 b | 40 (33.5–72.5) | 0.09 | 59 (43.5–150.5) | 0.004 b |
| ALT (IU/L, IQR) | 18 (14–27) | 24.5 (15–105.5) | 0.12 | 22 (16–89.5) | 0.15 | 40 (28.5–244.5) | 0.02 b |
| CRP (mg/dL, IQR) | 6.5 (4.1–8.5) | 6.6 (4.2–10.3) | 0.55 | 5.0 (4.0–8.3) | 0.66 | 12.0 (7.3–14.2) | 0.03 b |
| CAA | 1 (3.0%) | 0 | 0.28 | 0 | 0.36 | 0 | 0.64 |
| FARP | Negative n = 33 | RV/EV (+) n = 19 | p-Value | RSV (+) n = 4 | p-Value |
|---|---|---|---|---|---|
| Sex (male/female) | 20/13 | 5/14 | 0.01 a | 3/1 | 0.58 |
| Age (month) | 33 (10–47) | 32 (23.5–47.5) | 0.58 | 20.5(16.3–27.5) | 0.70 |
| Days since illness onset (day) | 4 (3–5) | 5 (4–6) | 0.3 | 5 (3.8–6.3) | 0.63 |
| K-score ≥ 5 | 7 (21.2%) | 6 (31.5%) | 0.45 | 4 (100%) | 0.001 a |
| K-score | 3 (2–4) | 3 (1–5) | 0.3 | 6 (5.8–6.8) | 0.02 b |
| Resistance to initial treatment | 7 (21.2%) | 1 (5.2%) | 0.09 | 0 (0%) | 0.57 |
| WBC (/μL) | 14,600 (11,500–15,500) | 13,100 (11,950–14,700) | 0.4 | 14,250 (12,725–16,100) | 0.85 |
| Platelet (×104) | 35.4 (26.9–42.5) | 37 (28.0–39.7) | 1.0 | 29.2 (26–33.8) | 0.261 |
| Na (mEq/L) | 135 (133.0–136.0) | 136 (134.5–136) | 0.07 | 131 (129.5–132.3) | 0.02 b |
| AST (IU/L) | 35 (27.0–41.0) | 37 (31–64) | 0.31 | 51.5 (43.5–101.8) | 0.003 b |
| ALT (IU/L) | 18 (14–27) | 21 (14.5–89.5) | 0.45 | 118.5 (22.5–228.3) | 0.17 |
| CRP (mg/dL) | 6.5 (4.1–8.5) | 4.96 (3.7–7.1) | 0.26 | 13 (11.0–14.1) | 0.01 b |
| CAA | 1 (3.0%) | 0 | 0.45 | 0 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koyanagi, T.; Nakagawa, R.; Okada, M.; Yokoyama, H.; Amano, S.; Shimoyama, T.; Udagawa, T.; Suzuki, N.; Hosokawa, S.; Nagasawa, M. Impact of a Concurrent Respiratory Virus Infection on the Clinical Presentation and Response to Initial Treatment of Kawasaki Disease: A Single-Center Observational Study. J. Clin. Med. 2025, 14, 775. https://doi.org/10.3390/jcm14030775
Koyanagi T, Nakagawa R, Okada M, Yokoyama H, Amano S, Shimoyama T, Udagawa T, Suzuki N, Hosokawa S, Nagasawa M. Impact of a Concurrent Respiratory Virus Infection on the Clinical Presentation and Response to Initial Treatment of Kawasaki Disease: A Single-Center Observational Study. Journal of Clinical Medicine. 2025; 14(3):775. https://doi.org/10.3390/jcm14030775
Chicago/Turabian StyleKoyanagi, Taichi, Ryuichi Nakagawa, Mari Okada, Haruna Yokoyama, Saori Amano, Teruyoshi Shimoyama, Tomohiro Udagawa, Natsuko Suzuki, Susumu Hosokawa, and Masayuki Nagasawa. 2025. "Impact of a Concurrent Respiratory Virus Infection on the Clinical Presentation and Response to Initial Treatment of Kawasaki Disease: A Single-Center Observational Study" Journal of Clinical Medicine 14, no. 3: 775. https://doi.org/10.3390/jcm14030775
APA StyleKoyanagi, T., Nakagawa, R., Okada, M., Yokoyama, H., Amano, S., Shimoyama, T., Udagawa, T., Suzuki, N., Hosokawa, S., & Nagasawa, M. (2025). Impact of a Concurrent Respiratory Virus Infection on the Clinical Presentation and Response to Initial Treatment of Kawasaki Disease: A Single-Center Observational Study. Journal of Clinical Medicine, 14(3), 775. https://doi.org/10.3390/jcm14030775






