Machine Learning in Pediatric Healthcare: Current Trends, Challenges, and Future Directions
Abstract
1. Introduction
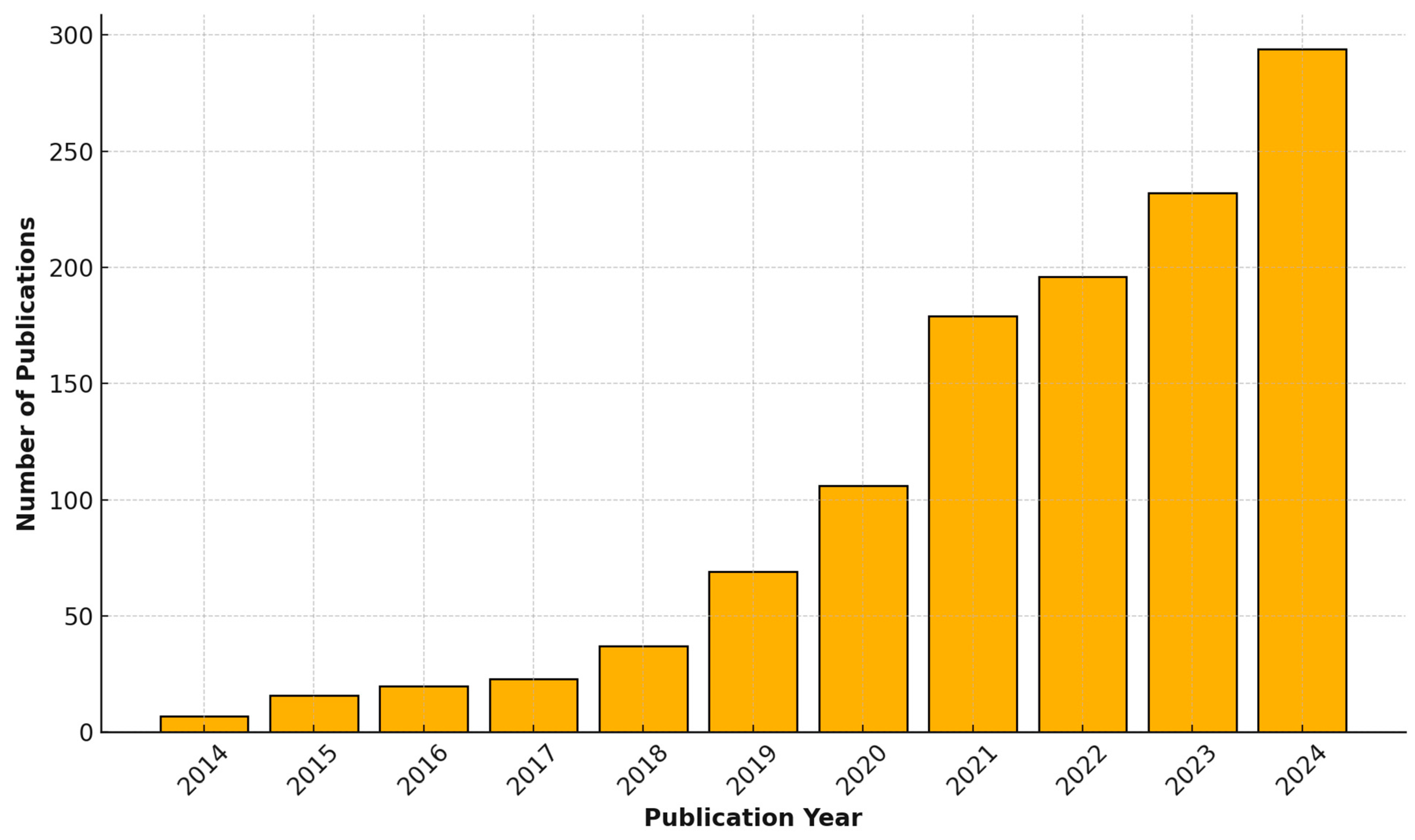
2. Review Methodology
3. Current Applications of Machine Learning in Pediatrics
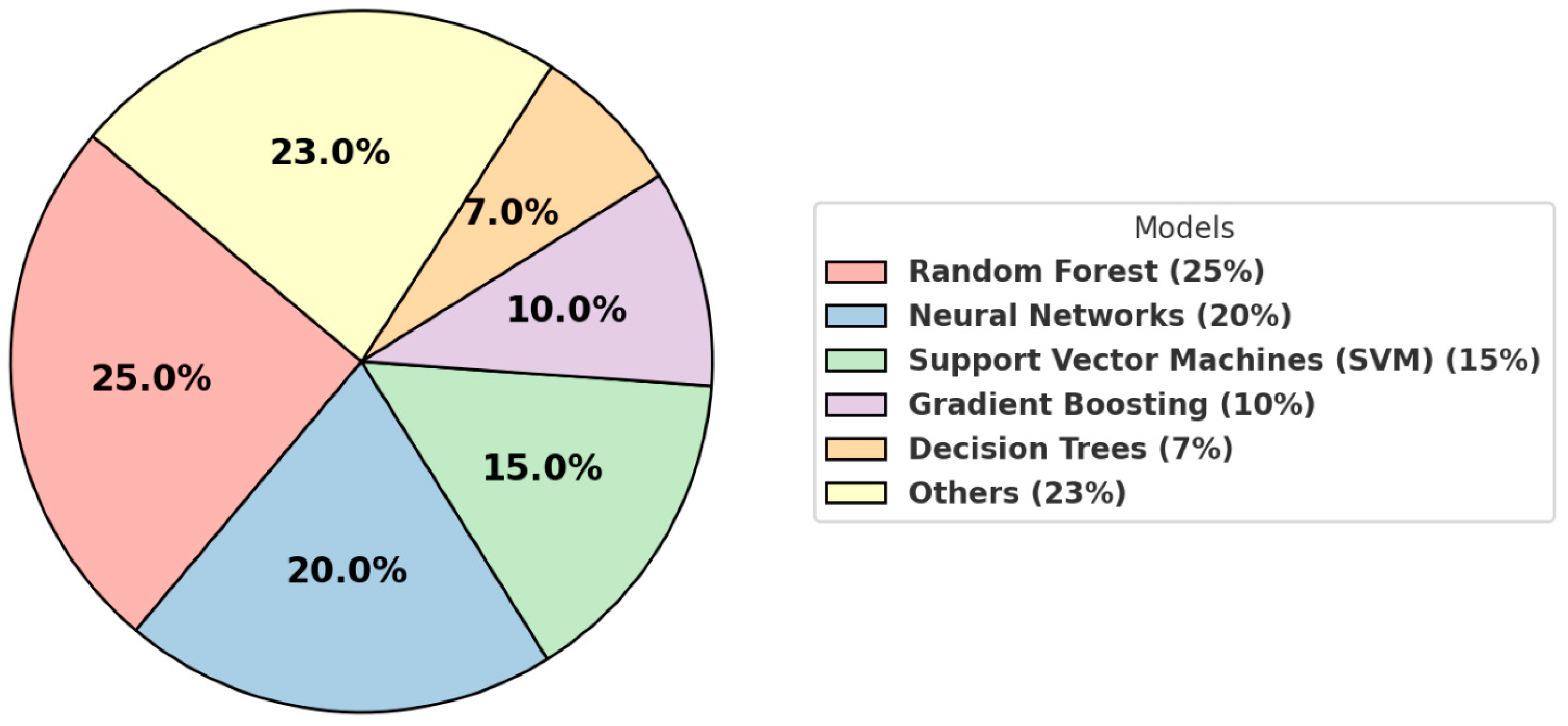
4. Trends and Common Techniques
5. Challenges and Limitations
6. Opportunities and Future Directions
7. Glossary
- Artificial intelligence (AI): A branch of computer science focused on creating systems capable of tasks that typically require human intelligence, such as decision-making and problem-solving.
- Machine learning (ML): A subset of AI that uses algorithms to identify patterns in data and improve performance on specific tasks without explicit programming.
- Convolutional neural network (CNN): A type of deep learning model particularly suited to analyzing visual data, such as medical images.
- Decision tree: A simple, interpretable model that splits data into branches based on feature values for classification or regression tasks.
- Random forest: An ensemble learning technique that uses multiple decision trees to make predictions, improving accuracy and reducing overfitting.
- Gradient boosting: A machine learning method in which models are built sequentially, with each one correcting the errors of the previous, often used for predictive tasks.
- Support vector machine (SVM): A supervised learning algorithm that classifies data by finding the best boundary (or hyperplane) between classes.
- Explainable AI (XAI): A set of tools and techniques to make the predictions and workings of machine learning models interpretable to clinicians and stakeholders.
- Electronic health records (EHRs): Digital records of patients’ medical histories, treatment plans, test results, and other healthcare information.
- Neural networks: A type of machine learning model inspired by the human brain, consisting of layers of interconnected nodes (neurons) that process data.
- Black-box models: Machine learning models, such as deep learning, whose internal processes are not transparent or easily interpretable.
- Bias (in machine learning): Systematic errors in models caused by non-representative or imbalanced datasets, potentially leading to unfair outcomes.
- Federated learning: A technique allowing multiple institutions to train machine learning models collaboratively without sharing sensitive raw data.
Supplementary Materials
Funding
Conflicts of Interest
References
- Baloglu, O.; Latifi, S.Q.; Nazha, A. What is machine learning? Arch. Dis. Child. Educ. Pract. Ed. 2022, 107, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Okwor, I.A.; Hitch, G.; Hakkim, S.; Akbar, S.; Sookhoo, D.; Kainesie, J. Digital Technologies Impact on Healthcare Delivery: A Systematic Review of Artificial Intelligence (AI) and Machine-Learning (ML) Adoption, Challenges, and Opportunities. AI 2024, 5, 1918–1941. [Google Scholar] [CrossRef]
- Bekbolatova, M.; Mayer, J.; Ong, C.W.; Toma, M. Transformative Potential of AI in Healthcare: Definitions, Applications, and Navigating the Ethical Landscape and Public Perspectives. Healthcare 2024, 12, 125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klassen, T.P.; Hartling, L.; Craig, J.C.; Offringa, M. Children are not just small adults: The urgent need for high-quality trial evidence in children. PLoS Med. 2008, 5, e172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narita, R.E. Consumption of Healthcare Services in the United States: The Impact of Health Insurance. J. Risk Financ. Manag. 2023, 16, 277. [Google Scholar] [CrossRef]
- Martin, B.; Kaminski-Ozturk, N.; O’Hara, C.; Smiley, R. Examining the Impact of the COVID-19 Pandemic on Burnout and Stress Among U.S. Nurses. J. Nurs. Regul. 2023, 14, 4–12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, F.; Ahimaz, P.; Nguyen, Q.M.; Lewis, R.; Chung, W.K.; Ta, C.N.; Szigety, K.M.; Sheppard, S.E.; Campbell, I.M.; Wang, K.; et al. Phenotype driven molecular genetic test recommendation for diagnosing pediatric rare disorders. NPJ Digit. Med. 2024, 7, 333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarinabad, N.; Abernethy, L.J.; Avula, S.; Davies, N.P.; Gutierrez, D.R.; Jaspan, T.; MacPherson, L.; Mitra, D.; Rose, H.E.; Wilson, M.; et al. Application of pattern recognition techniques for classification of pediatric brain tumors by in vivo, 3.T. Magn. Reson. Med. 2018, 79, 2359–2366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jang, S.; Yu, J.; Park, S.; Lim, H.; Koh, H.; Park, Y.R. Development of Time-Aggregated Machine Learning Model for Relapse Prediction in Pediatric Crohn’s Disease. Clin. Transl. Gastroenterol. 2024, 16, e00794. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Strobl, R.; Berner, R.; Armann, J.; Scheithauer, S.; Grill, E. Six clinical phenotypes with prognostic implications were identified by unsupervised machine learning in children and adolescents with SARS-CoV-2 infection: Results from a German nationwide registry. Respir. Res. 2024, 25, 392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, G.; Stone, B.L.; Fassl, B.; Maloney, C.G.; Gesteland, P.H.; Yerram, S.R.; Nkoy, F.L. Predicting asthma control deterioration in children. BMC Med. Inform. Decis. Mak. 2015, 15, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chadaga, K.; Khanna, V.; Prabhu, S.; Sampathila, N.; Chadaga, R.; Umakanth, S.; Bhat, D.; Swathi, K.S.; Kamath, R. An interpretable and transparent machine learning framework for appendicitis detection in pediatric patients. Sci. Rep. 2024, 14, 24454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marassi, C.; Socia, D.; Larie, D.; An, G.; Cockrell, R.C. Children are small adults (when properly normalized): Transferrable/generalizable sepsis prediction. Surg. Open Sci. 2023, 16, 77–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, R.; Greenstein, J.L.; Fackler, J.C.; Bergmann, J.; Bembea, M.M.; Winslow, R.L. Prediction of Impending Septic Shock in Children with Sepsis. Crit. Care Explor. 2021, 3, e0442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Y.; Jin, L.; Li, X. Differential diagnosis of pediatric cervical lymph node lesions based on simple clinical features. Eur. J. Pediatr. 2024, 183, 4929–4938. [Google Scholar] [CrossRef] [PubMed]
- Zamzmi, G.; Venkatesh, K.; Nelson, B.; Prathapan, S.; Yi, P.; Sahiner, B.; Delfino, J.G. Out-of-Distribution Detection and Radiological Data Monitoring Using Statistical Process Control. J. Imaging Inform. Med. 2024; Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Mumenin, K.M.; Biswas, P.; Khan, M.A.; Alammary, A.S.; Nahid, A.A. A Modified Aquila-Based Optimized XGBoost Framework for Detecting Probable Seizure Status in Neonates. Sensors 2023, 23, 7037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hunfeld, M.; Verboom, M.; Josemans, S.; van Ravensberg, A.; Straver, D.; Lückerath, F.; Jongbloed, G.; Buysse, C.; Berg, R.v.D. Prediction of Survival After Pediatric Cardiac Arrest Using Quantitative EEG and Machine Learning Techniques. Neurology 2024, 103, e210043. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y. Identification of Pediatric Bacterial Gastroenteritis From Blood Counts and Interviews Based on Machine Learning. Cureus 2023, 15, e43644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, L.; Kauer, J.; Chen, X.; Chu, K.; Dou, H.; Smith, Z.J. Screening of nutritional and genetic anemias using elastic light scattering. Lab Chip 2018, 18, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Hegde, N.; Zhang, T.; Uswatte, G.; Taub, E.; Barman, J.; McKay, S.; Taylor, A.; Morris, D.M.; Griffin, A.; Sazonov, E.S. The Pediatric SmartShoe: Wearable Sensor System for Ambulatory Monitoring of Physical Activity and Gait. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.D.; Ferro, D.; Mullaney, C.; Skrabonja, L.; Barnes, M.S.; Patton, S.R.; Lockee, B.; Tallon, E.M.; A Vandervelden, C.; Schweisberger, C.; et al. An “All-Data-on-Hand” Deep Learning Model to Predict Hospitalization for Diabetic Ketoacidosis in Youth with Type 1 Diabetes: Development and Validation Study. JMIR Diabetes 2023, 8, e47592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Meslamani, A.Z. How AI is advancing asthma management? Insights into economic and clinical aspects. J. Med. Econ. 2023, 26, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Kifle, N.; Teti, S.; Ning, B.; Donoho, D.A.; Katz, I.; Keating, R.; Cha, R.J. Pediatric Brain Tissue Segmentation Using a Snapshot Hyperspectral Imaging (sHSI) Camera and Machine Learning Classifier. Bioengineering 2023, 10, 1190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adamson, P.M.; Bhattbhatt, V.; Principi, S.; Beriwal, S.; Strain, L.S.; Offe, M.; Wang, A.S.; Vo, N.; Schmidt, T.G.; Jordan, P. Technical note: Evaluation of a V-Net autosegmentation algorithm for pediatric CT scans: Performance, generalizability, and application to patient-specific CT dosimetry. Med. Phys. 2022, 49, 2342–2354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deoni, S.C.L.; Bruchhage, M.M.K.; Beauchemin, J.; Volpe, A.; D’Sa, V.; Huentelman, M.; Williams, S.C. Accessible pediatric neuroimaging using a low field strength MRI scanner. Neuroimage 2021, 238, 118273. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, H.A.; Latifi, S.Q.; Baloglu, O. Pediatric Intensive Care Unit Length of Stay Prediction by Machine Learning. Bioengineering 2024, 11, 962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mocrii, A.A.; Chirila, O.S. AI-Assisted Application for Pediatric Drug Dosing. Stud. Health Technol. Inform. 2024, 321, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Pembegul Yildiz, E.; Coskun, O.; Kurekci, F.; Maras Genc, H.; Ozaltin, O. Machine learning models for predicting treatment response in infantile epilepsies. Epilepsy Behav. 2024, 160, 110075. [Google Scholar] [CrossRef] [PubMed]
- Bokov, P.; Mahut, B.; Flaud, P.; Delclaux, C. Wheezing recognition algorithm using recordings of respiratory sounds at the mouth in a pediatric population. Comput. Biol. Med. 2016, 70, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; DeSalvo, D.J.; Haridas, B.; McKay, S.; Shenoy, A.; Koh, C.J.; Lawley, M.; Erraguntla, M. Feature-Based Machine Learning Model for Real-Time Hypoglycemia Prediction. J. Diabetes Sci. Technol. 2021, 15, 842–855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hattab, Z.; Moler-Zapata, S.; Doherty, E.; Sadique, Z.; Ramnarayan, P.; O’Neill, S. Exploring Heterogeneity in the Cost-Effectiveness of High-Flow Nasal Cannula Therapy in Acutely Ill Children-Insights From the Step-Up First-line Support for Assistance in Breathing in Children Trial Using a Machine Learning Method. Value Health 2024, 28, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Wang, R.Q.; Wu, L.Q.; Chen, D.M. Transfer learning-enabled outcome prediction for guiding CRRT treatment of the pediatric patients with sepsis. BMC Med. Inform. Decis. Mak. 2024, 24, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chollet, F. Deep Learning with Python, 2nd ed.; Manning Publications Co.: Shelter Island, NY, USA, 2021. [Google Scholar]
- Liu, G.; Poon, M.; Zapala, M.A.; Temple, W.C.; Vo, K.T.; Matthay, K.K.; Mitra, D.; Seo, Y. Incorporating Radiomics into Machine Learning Models to Predict Outcomes of Neuroblastoma. J. Digit. Imaging 2022, 35, 605–612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Namdar, K.; Wagner, M.W.; Kudus, K.; Hawkins, C.; Tabori, U.; Ertl-Wagner, B.B.; Khalvati, F. Improving Deep Learning Models for Pediatric Low-Grade Glioma Tumours Molecular Subtype Identification Using MRI-based 3D Probability Distributions of Tumour Location. Can. Assoc. Radiol. J. 2024; Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Xu, P.; Cai, Y.; Wang, F.; Li, M.; Zeng, X.; Liu, C. A Deep Learning Model for the Diagnosis and Discrimination of Gram-Positive and Gram-Negative Bacterial Pneumonia for Children Using Chest Radiography Images and Clinical Information. Infect. Drug Resist. 2023, 16, 4083–4092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xin, K.Z.; Li, D.; Yi, P.H. Limited generalizability of deep learning algorithm for pediatric pneumonia classification on external data. Emerg. Radiol. 2022, 29, 107–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habib, N.; Hasan, M.M.; Reza, M.M.; Rahman, M.M. Ensemble of CheXNet and VGG-19 Feature Extractor with Random Forest Classifier for Pediatric Pneumonia Detection. SN Comput. Sci. 2020, 1, 359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghomrawi, H.M.K.; O’Brien, M.K.; Carter, M.; Macaluso, R.; Khazanchi, R.; Fanton, M.; DeBoer, C.; Linton, S.C.; Zeineddin, S.; Pitt, J.B.; et al. Applying machine learning to consumer wearable data for the early detection of complications after pediatric appendectomy. NPJ Digit. Med. 2023, 6, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onorati, F.; Regalia, G.; Caborni, C.; LaFrance, W.C.; Blum, A.S.; Bidwell, J.; De Liso, P.; El Atrache, R.; Loddenkemper, T.; Mohammadpour-Touserkani, F.; et al. Prospective Study of a Multimodal Convulsive Seizure Detection Wearable System on Pediatric and Adult Patients in the Epilepsy Monitoring Unit. Front. Neurol. 2021, 12, 724904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garbern, S.C.; Mamun, G.M.S.; Shaima, S.N.; Hakim, N.; Wegerich, S.; Alla, S.; Sarmin, M.; Afroze, F.; Sekaric, J.; Genisca, A.; et al. A novel digital health approach to improving global pediatric sepsis care in Bangladesh using wearable technology and machine learning. PLoS Digit. Health 2024, 3, e0000634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lemmon, J.; Guo, L.L.; Steinberg, E.; Morse, K.E.; Fleming, S.L.; Aftandilian, C.; Pfohl, S.R.; Posada, J.D.; Shah, N.; Fries, J.; et al. Self-supervised machine learning using adult inpatient data produces effective models for pediatric clinical prediction tasks. J. Am. Med. Inform. Assoc. 2023, 30, 2004–2011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khondker, A.; Kwong, J.C.C.; Rickard, M.; Erdman, L.; Gabrielson, A.T.; Nguyen, D.D.; Kim, J.K.; Abbas, T.; Fernandez, N.; Fischer, K.; et al. AI-PEDURO-Artificial intelligence in pediatric urology: Protocol for a living scoping review and online repository. J. Pediatr. Urol. 2024; Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Wang, H.; Wan, J.; Zhang, L.; Huang, J.; Shen, Y. Efficient federated learning for pediatric pneumonia on chest X-ray classification. Sci. Rep. 2024, 14, 23272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rb-Silva, R.; Ribeiro, X.; Almeida, F.; Ameijeiras-Rodriguez, C.; Souza, J.; Conceição, L.; Taveira-Gomes, T.; Marreiros, G.; Freitas, A. Secur-e-Health Project: Towards Federated Learning for Smart Pediatric Care. In Caring is Sharing–Exploiting the Value in Data for Health and Innovation; Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherland, 2023; Volume 302, pp. 516–520. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Macias, C.; Barda, A.; Fung, C.M. Federated data health networks hold potential for accelerating emergency research. J. Am. Coll. Emerg. Physicians Open 2023, 4, e12968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Girard, C.I.; Romanchuk, N.J.; Del Bel, M.J.; Carsen, S.; Chan, A.D.C.; Benoit, D.L. Classifiers of anterior cruciate ligament status in female and male adolescents using return-to-activity criteria. Knee Surg. Sports Traumatol. Arthrosc. 2024; Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, T.; Zhou, Y.; Liu, T.; Feng, S.; Xiong, X.; Fan, J.; Liang, Q.; Cui, Y.; Zhang, Y. A host immune-related LncRNA and mRNA signature as a discriminant classifier for bacterial from non-bacterial sepsis in children. Heliyon 2024, 10, e38728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simonini, A.; Murugan, J.; Vittori, A.; Pallotto, R.; Bignami, E.G.; Calevo, M.G.; Piazza, O.; Cascella, M. Data-driven Machine Learning Models for Risk Stratification and Prediction of Emergence Delirium in Pediatric Patients Underwent Tonsillectomy/Adenotonsillectomy. Ann. Ital. Chir. 2024, 95, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Kashani, B.; Cordova-Delgado, M.; Scott, E.N.; Alemi, K.; Trueman, J.; Groeneweg, G.; Chang, W.-C.; Loucks, C.M.; Ross, C.J.; et al. Machine learning model identifies genetic predictors of cisplatin-induced ototoxicity in CERS6 and TLR4. Comput. Biol. Med. 2024, 183, 109324. [Google Scholar] [CrossRef] [PubMed]
- Elnoor, Z.I.A.; Abdelmajeed, O.; Mustafa, A.; Gasim, T.; Musa, S.A.M.; Abdelmoneim, A.H.; Omer, I.I.A.; Fadl, H.A.O. Hematological picture of pediatric Sudanese patients with visceral leishmaniasis and prediction of leishmania donovani parasite load. World J. Clin. Cases 2024, 12, 6374–6382. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gray, G.M.; Ahumada, L.M.; Rehman, M.A.; Varughese, A.; Fernandez, A.M.; Fackler, J.; Yates, H.M.; Habre, W.; Disma, N.; Lonsdale, H. A machine-learning approach for decision support and risk stratification of pediatric perioperative patients based on the APRICOT dataset. Paediatr. Anaesth. 2023, 33, 710–719. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reddy, S.; Fox, J.; Purohit, M.P. Artificial intelligence-enabled healthcare delivery. J. R. Soc. Med. 2019, 112, 22–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beam, A.L.; Kohane, I.S. Big Data and Machine Learning in Health Care. JAMA 2018, 319, 1317–1318. [Google Scholar] [CrossRef] [PubMed]
- Shortliffe, E.H.; Sepúlveda, M.J. Clinical Decision Support in the Era of Artificial Intelligence. JAMA 2018, 320, 2199–2200. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Huang, W.; Luo, Y.; Xiong, S.; Tang, Y.; Yang, G.; Luo, D.; Zhou, X.; Zhang, Z.; Liu, H. Utility of Loneliness Status to Risk Stratification and Prediction of Recurrent Atrial Fibrillation After Catheter Ablation. Adv. Ther. 2024; Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sim, J.A.; Wang, J.X.; Forrest, C.B.; Krull, K.R.; Srivastava, D.; Hudson, M.M.; Robison, L.L.; Baker, J.N.; Huang, I.-C. Natural Language Processing and Machine Learning Methods to Characterize Unstructured Patient-Reported Outcomes: Validation Study. J. Med. Internet Res. 2021, 23, e26777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ying, J.; Wang, Q.; Xu, T.; Lu, Z. Diagnostic potential of a gradient boosting-based model for detecting pediatric sepsis. Genomics 2021, 113 Pt 2, 874–883. [Google Scholar] [CrossRef] [PubMed]
| Count (Percentage) | |
|---|---|
| Pediatric Subspecialty | |
| Radiology | 225 (19.1%) |
| Genetics | 139 (11.8%) |
| Infectious diseases | 105 (8.9%) |
| Hematology/oncology | 104 (8.8%) |
| Cardiology | 99 (8.4%) |
| Surgery | 90 (7.6%) |
| Neurology | 85 (7.2%) |
| Gastroenterology/nutrition | 84 (7.1%) |
| Respiratory/pulmonology | 67 (5.7%) |
| Critical care | 34 (2.9%) |
| Nephrology | 23 (2.0%) |
| Administrative | 22 (1.9%) |
| Endocrinology | 19 (1.6%) |
| Psychiatry/mental health | 16 (1.4%) |
| Neonatology | 16 (1.4%) |
| Ophthalmology | 14 (1.2%) |
| Orthopedic/musculoskeletal | 12 (1.0%) |
| Multiple specialties | 10 (0.8%) |
| Pharmacology | 4 (0.3%) |
| Dentistry | 4 (0.3%) |
| Obstetric/gynecology | 3 (0.3%) |
| Emergency medicine | 2 (0.2%) |
| Urology | 2 (0.2%) |
| Theme | |
| Prognostics | 833 (70.7%) |
| Diagnostics | 767 (65.1%) |
| Screening | 511 (43.3%) |
| Treatment | 443 (37.6%) |
| ML methods | 46 (3.9%) |
| Algorithm | Primary Applications | Advantages | Challenges |
|---|---|---|---|
| Random forest | Predictive modeling, risk stratification | Robust, handles missing data well, interpretable. | May struggle with very high-dimensional data. |
| Neural networks | Imaging (e.g., X-rays, MRIs), diagnostics | Excels in complex data analysis, capable of identifying intricate patterns in imaging data. | Requires large datasets, computationally intense. |
| Support vector machines | Classification tasks | Effective with smaller datasets, performs well on high-dimensional data. | Limited scalability to large datasets. |
| Gradient boosting | Risk prediction, regression analysis | High accuracy, can handle mixed data types. | Prone to overfitting if not properly tuned. |
| Decision trees | Simple decision-making models | Easy to interpret, trains quickly on small datasets. | Prone to overfitting, low predictive power. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganatra, H.A. Machine Learning in Pediatric Healthcare: Current Trends, Challenges, and Future Directions. J. Clin. Med. 2025, 14, 807. https://doi.org/10.3390/jcm14030807
Ganatra HA. Machine Learning in Pediatric Healthcare: Current Trends, Challenges, and Future Directions. Journal of Clinical Medicine. 2025; 14(3):807. https://doi.org/10.3390/jcm14030807
Chicago/Turabian StyleGanatra, Hammad A. 2025. "Machine Learning in Pediatric Healthcare: Current Trends, Challenges, and Future Directions" Journal of Clinical Medicine 14, no. 3: 807. https://doi.org/10.3390/jcm14030807
APA StyleGanatra, H. A. (2025). Machine Learning in Pediatric Healthcare: Current Trends, Challenges, and Future Directions. Journal of Clinical Medicine, 14(3), 807. https://doi.org/10.3390/jcm14030807






