Role of C-Reactive Protein as a Predictor of Early Revascularization and Mortality in Advanced Peripheral Arterial Disease
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Overall Population
3.2. Baseline Characteristics of Group 1 and Group 2
3.3. Baseline Comorbidities and Prior Treatments of the Overall Population
3.4. Baseline Comorbidities and Prior Treatments in Group 1 and Group 2
3.5. Outcomes for the Overall Population
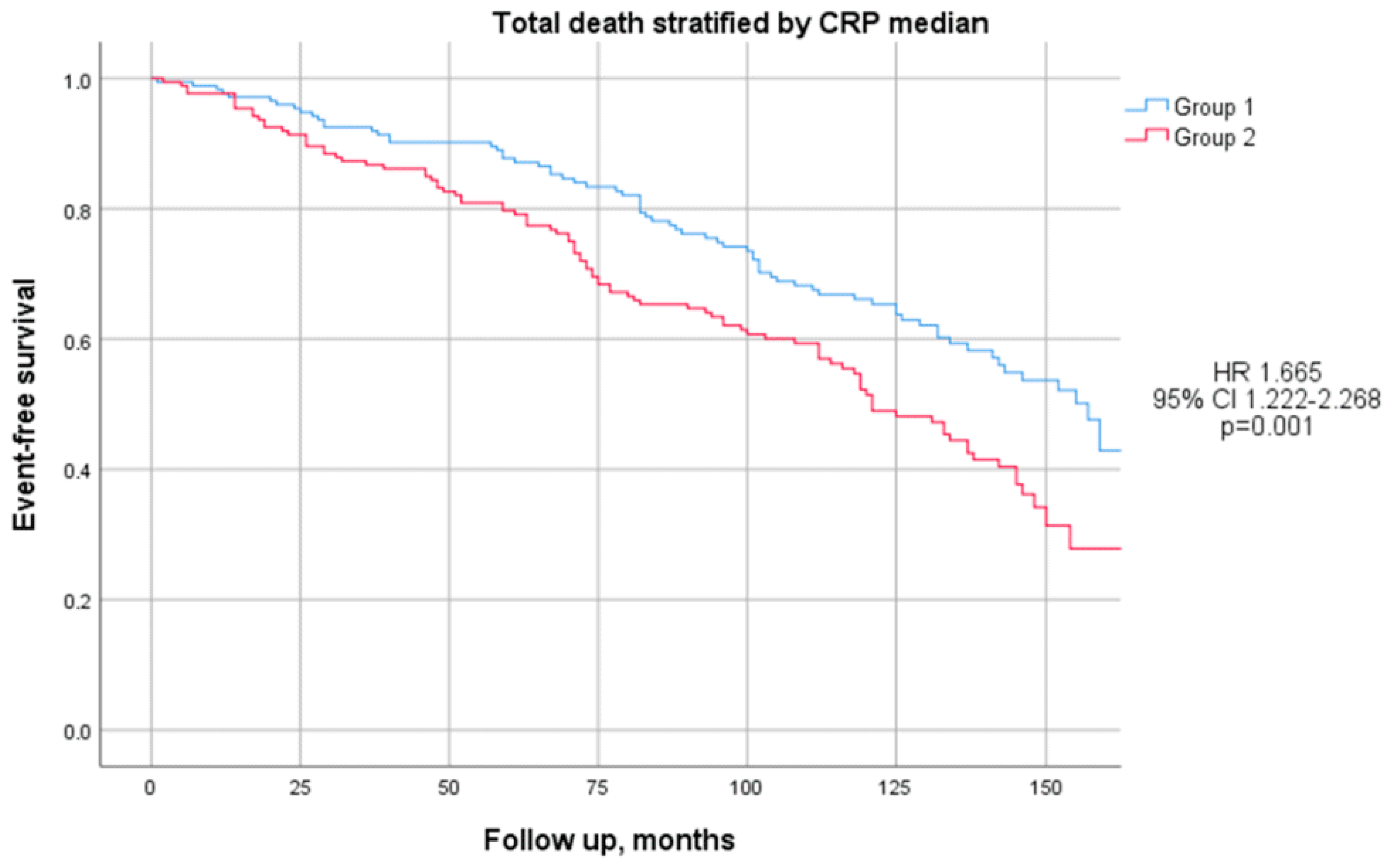
3.6. Outcomes for Group 1 and Group 2
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ridker, P.M.; Lüscher, T.F. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014, 35, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and Atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Khuseyinova, N.; Koenig, W. Biomarkers of outcome from cardiovascular disease. Curr. Opin. Crit. Care 2006, 12, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Inflammation, C-Reactive Protein, and Cardiovascular Disease. Circ. Res. 2014, 114, 594–595. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Marino, E.; Scuto, S.; Raimondo, D.D. Pathophysiology of Peripheral Arterial Disease (PAD): A Review on Oxidative Disorders. Int. J. Mol. Sci. 2020, 21, 4393. [Google Scholar] [CrossRef]
- Golledge, J. Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef] [PubMed]
- Sigvant, B.; Lundin, F.; Wahlberg, E. The Risk of Disease Progression in Peripheral Arterial Disease is Higher than Expected: A Meta-Analysis of Mortality and Disease Progression in Peripheral Arterial Disease. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Ankle Brachial Index Collaboration. Ankle Brachial Index Combined with Framingham Risk Score to Predict Cardiovascular Events and Mortality: A Meta-analysis. JAMA J. Am. Med. Assoc. 2008, 300, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.M.; Pradhan, A.D.; Dorresteijn, J.A.N.; Koudstaal, S.; Teraa, M.; de Borst, G.J.; van der Meer, M.G.; Mosterd, A.; Ridker, P.M.; Visseren, F.L.J. C-Reactive Protein and Risk of Cardiovascular Events and Mortality in Patients with Various Cardiovascular Disease Locations. Am. J. Cardiol. 2023, 197, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.P.; Solomon, D.H. Traditional cardiovascular risk factors, inflammation and cardiovascular risk in rheumatoid arthritis. Rheumatology 2013, 52, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.; Williams, K.; Hunt, K.J.; Haffner, S.M. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 2007, 30, 8–13. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Thomas, J.; Ravichandran, R.; Singh, M.; Nag, A.; Panjiyar, B.K. Inflammation in Cardiovascular Disease: A Comprehensive Review of Biomarkers and Therapeutic Targets. Cureus 2023, 15, e45483. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 522637. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, F.J.; Kullo, I.J. Novel markers of peripheral arterial disease. Vasc. Med. 2009, 14, 381. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012, 367, 1310–1320. [Google Scholar] [CrossRef]
- Maga, P.; Wachsmann-Maga, A.; Włodarczyk, A.; Maga, M.; Batko, K.; Bogucka, K.; Kapusta, M.; Terlecki, P. Leukotrienes E4 and B4 and vascular endothelium—New insight into the link between vascular inflammation and peripheral arterial. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 23, 200343. [Google Scholar] [CrossRef]
- Pino, A.D.; DeFronzo, R.A. Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents. Endocr. Rev. 2019, 40, 1447. [Google Scholar] [CrossRef] [PubMed]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef] [PubMed]
- Bienstock, S.; Lee, S.E.; Lin, F.; Blankstein, R.; Leipsic, J.; Patel, K.; Narula, J.; Chandrashekhar, Y.S.; Fuster, V.; Shaw, L.J. Systemic Inflammation with High-Sensitivity C-Reactive Protein and Atherosclerotic Plaque Progression. JACC Cardiovasc. Imaging 2024, 17, 212–213. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Nahrendorf, M.; Swirski, F.K. Leukocytes Link Local and Systemic Inflammation in Ischemic Cardiovascular Disease: An Expanded “Cardiovascular Continuum”. J. Am. Coll. Cardiol. 2016, 67, 1091–1103. [Google Scholar] [CrossRef]
- Bernabe-Ortiz, A.; Carrillo-Larco, R.M.; Gilman, R.H.; Smeeth, L.; Checkley, W.; Miranda, J.J. High-sensitivity C-reactive protein and all-cause mortality in four diverse populations: The CRONICAS Cohort Study. Ann. Epidemiol. 2021, 67, 13. [Google Scholar] [CrossRef]
- Chen, Y.; Ju, H.; Xie, K.; Zhao, X. Association of inflammatory score with all-cause and cardiovascular mortality in patients with metabolic syndrome: NHANES longitudinal cohort study. Front. Immunol. 2024, 15, 1410871. [Google Scholar]
- Sharif, S.; Van der Graaf, Y.; Cramer, M.J.; Kapelle, L.J.; Borst GJ de Visseren, F.L.J.; Wasternik, J.; SMART Study Group. Low-grade inflammation as a risk factor for cardiovascular events and all-cause mortality in patients with type 2 diabetes. Cardiovasc. Diabetology. 2021, 20, 220. [Google Scholar] [CrossRef]
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522. [Google Scholar] [CrossRef]
- Zhang, F.S.; He, Q.Z.; Qin, C.H.; Little, P.J.; Weng, J.P.; Xu, S.W. Therapeutic potential of colchicine in cardiovascular medicine: A pharmacological review. Acta Pharmacol. Sin. 2022, 43, 2173. [Google Scholar] [CrossRef] [PubMed]
- Razavi, E.; Ramezani, A.; Kazemi, A.; Attar, A. Effect of Treatment with Colchicine after Acute Coronary Syndrome on Major Cardiovascular Events: A Systematic Review and Meta-Analysis of Clinical Trials. Cardiovasc. Ther. 2022, 2022, 8317011. [Google Scholar] [CrossRef]
- Mewton, N.; Roubille, F.; Bresson, D.; Prieur, C.; Bouleti, C.; Bochaton, T.; Ivanes, F.; Dubreuil, O.; Biere, L.; Hayek, A.; et al. Effect of Colchicine on Myocardial Injury in Acute Myocardial Infarction. Circulation 2021, 144, 859. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.E. Colchicine Reduces Cardiovascular Events in Patients with Diabetes and Recent MI. NEJM J. Watch. 2024, 47, 467. Available online: https://www.jwatch.org/NA57207/2024/03/06 (accessed on 6 November 2024).
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; Salem, H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. New Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef]
- Bian, F.; Yang, X.Y.; Xu, G.; Zheng, T.; Jin, S. CRP-Induced NLRP3 Inflammasome Activation Increases LDL Transcytosis Across Endothelial Cells. Front. Pharmacol. 2019, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Athavale, A.; Fukaya, E.; Leeper, N.J. Peripheral Artery Disease: Molecular Mechanisms and Novel Therapies. ATVB 2024, 44, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Chan, N. Low Dose ColchicinE in pAtients with Peripheral Artery DiseasE to Address Residual Vascular Risk: A Randomized Trial; Report NCT04774159; National Institutes of Health: Bethesda, MD, USA, 2024. Available online: https://clinicaltrials.gov/study/NCT04774159 (accessed on 6 November 2024).
- Lin, D.S.H.; Huang, K.C.; Lin, T.T.; Lee, J.K.; Lin, L.Y. Effects of Colchicine on Major Adverse Limb and Cardiovascular Events in Patients with Peripheral Artery Disease. Mayo Clin. Proc. 2024, 99, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Tramujas, L.; Nogueira, A.; Felix, N.; Abizaid, A.; Cavalcanti, A.B. Association of colchicine use with cardiovascular and limb events in peripheral artery disease: Insights from a retrospective cohort study. Atherosclerosis 2024, 398, 118563. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Téllez, S.A.; Sekheri, M.; Filep, J.G. C-reactive protein: A target for therapy to reduce inflammation. Front. Immunol. 2023, 14, 1237729. [Google Scholar] [CrossRef]
- Blaum, C.; Brunner, F.J.; Kröger, F.; Braetz, J.; Lorenz, T.; Goßling, A.; Ojeda, F.; Koester, L.; Karakas, M.; Zeller, T.; et al. Modifiable lifestyle risk factors and C-reactive protein in patients with coronary artery disease: Implications for an anti-inflammatory treatment target population. Eur. J. Prev. Cardiol. 2021, 28, 152–158. [Google Scholar] [CrossRef]

| All Patients 1 | Group 1 1 | Group 2 1 | p-Value 2 | |
|---|---|---|---|---|
| Clinical Parameters | ||||
| Men (M) | 272 (79%) | 135 (78%) | 137 (79%) | 0.79 |
| Age, yrs | 72.11 ± 7.90 | 71.32 ± 7.65 | 72.89 ± 8.08 | 0.07 |
| BMI, Kg/m2 | 28.43 ± 4.06 | 27.87 ± 3.74 | 28.99 ± 4.30 | 0.01 |
| Waist circumference, cm | 101.22 ± 10.19 | 99.19 ± 9.91 | 103.32 ± 10.08 | <0.01 |
| Waist–hip ratio, cm | 0.97 ± 0.07 | 0.96 ± 0.07 | 0.97 ± 0.06 | 0.03 |
| Blood Pressure | ||||
| Systolic, mmHg | 132.08 ± 17.39 | 132.93 ± 17.58 | 131.16 ± 17.20 | 0.37 |
| Diastolic, mmHg | 79.61 ± 6.25 | 80.03 ± 6.63 | 79.15 ± 5.80 | 0.21 |
| Mean, mmHg | 97.06 ± 8.67 | 97.61 ± 9.04 | 96.45 ± 8.22 | 0.23 |
| PWV, m/s | 15.20 ± 6.97 | 15.22 ± 7.68 | 15.17 ± 6.17 | 0.96 |
| Augmentation index, % | 23.17 ± 7.06 | 23.01 ± 6.98 | 23.34 ± 7.17 | 0.75 |
| Pulse pressure, mmHg | 52.65 ± 15.45 | 53.24 ± 15.39 | 52.01 ± 15.54 | 0.48 |
| Echocardiographic Parameters | ||||
| Interventricular septum, mm | 11.83 ± 1.33 | 11.82 ± 1.34 | 11.85 ± 1.33 | 0.83 |
| Posterior wall, mm | 11.14 ± 1.14 | 11.14 ± 1.17 | 11.14 ± 1.12 | 0.99 |
| Ejection fraction, % | 0.58 ± 0.06 | 0.59 ± 0.05 | 0.58 ± 0.06 | 0.35 |
| Ventricular mass index, g/m2 | 77.83 ± 20.29 | 76.48 ± 18.60 | 79.20 ± 21.86 | 0.23 |
| Electrocardiographic Parameters | ||||
| Heart rate, bpm | 69.82 ± 10.42 | 70.26 ± 10.50 | 69.37 ± 10.35 | 0.43 |
| Sinus rhythm, n (%) | 323 (93%) | 164 (95%) | 159 (92%) | 0.28 |
| Right bundle branch block, n (%) | 20 (5.8%) | 12 (6.9%) | 8 (4.6%) | 0.36 |
| Left bundle branch block, n (%) | 16 (4.6%) | 8 (4.6%) | 8 (4.6%) | 1.00 |
| PR interval, ms | 167.99 ± 34.09 | 166.34 ± 27.89 | 169.66 ± 39.38 | 0.39 |
| QRS duration, ms | 101.90 ± 21.54 | 101.26 ± 19.63 | 102.54 ± 23.32 | 0.59 |
| QTc, ms | 414.00 ± 39.69 | 411.59 ± 44.01 | 416.39 ± 34.85 | 0.28 |
| Blood Chemistry Parameters | ||||
| Hemoglobin, g/dL | 13.78 ± 1.61 | 13.86 ± 1.53 | 13.70 ± 1.70 | 0.34 |
| Triglycerides, mg/dL | 120.60 ± 57.83 | 120.68 ± 59.43 | 120.52 ± 56.35 | 0.95 3 |
| Cholesterol, mg/dL | 164.53 ± 39.46 | 164.42 ± 39.95 | 164.64 ± 39.09 | 0.96 |
| HDL cholesterol, mg/dL | 48.83 ± 12.50 | 49.19 ± 12.46 | 48.47 ± 12.58 | 0.59 |
| LDL cholesterol, mg/dL | 92.75 ± 33.69 | 92.72 ± 34.01 | 92.78 ± 33.46 | 0.99 |
| Fasting glucose, mmol/L | 118.87 ± 39.38 | 119.41 ± 36.49 | 118.34 ± 42.15 | 0.80 |
| HbA1c, % | 6.95 ± 1.22 | 6.78 ± 1.12 | 7.12 ± 1.29 | 0.05 |
| Serum insulin, mmol/L | 19.78 ± 72.29 | 20.35 ± 98.69 | 19.19 ± 26.22 | 0.88 |
| HOMA-IR | 5.08 ± 8.45 | 4.05 ± 5.40 | 6.12 ± 10.60 | 0.06 3 |
| HOMA-β | 115.49 ± 103.72 | 99.13 ± 83.61 | 132.25 ± 118.85 | 0.01 3 |
| Serum creatinine, mg/dL | 1.07 ± 0.55 | 1.02 ± 0.41 | 1.13 ± 0.66 | 0.06 |
| Potassium, mmol/L | 4.45 ± 0.44 | 4.45 ± 0.42 | 4.46 ± 0.47 | 0.71 |
| Serum albumin, g/dL | 4.54 ± 0.36 | 4.56 ± 0.35 | 4.51 ± 0.36 | 0.22 |
| Fibrinogen, mg/dL | 345.07 ± 76.74 | 328.18 ± 69.73 | 361.77 ± 79.84 | <0.01 |
| ESR, mm | 25.84 ± 17.15 | 21.71 ± 13.38 | 29.92 ± 19.39 | <0.01 3 |
| Vitamin D (25OH), ng/mL | 16.32 ± 12.22 | 16.34 ± 11.87 | 16.30 ± 12.61 | 0.98 |
| Urinary Parameters | ||||
| Urinary creatinine | 97.65 ± 56.16 | 98.49 ± 49.33 | 96.82 ± 62.38 | 0.78 |
| eGFR, mL/min per 1.73 m2 | 79.72 ± 27.43 | 82.28 ± 26.06 | 77.07 ± 28.61 | 0.08 |
| Microalbuminuria, µg/min | 118.54 ± 457.89 | 139.07 ± 619.39 | 98.38 ± 197.79 | 0.10 3 |
| ACR | 17.87 ± 54.90 | 16.31 ± 61.21 | 19.59 ± 47.14 | 0.10 3 |
| All Patients 1 | Group 1 1 | Group 2 1 | p-Value 2 | |
|---|---|---|---|---|
| Comorbidity | ||||
| Hypertension, n (%) | 332 (96%) | 166 (96%) | 166 (96%) | 1.00 |
| Years of hypertension, yrs | 11.10 ± 7.70 | 10.15 ± 7.73 | 11.99 ± 7.59 | 0.07 |
| Type 2 diabetes, n (%) | 161 (47%) | 81 (47%) | 80 (46%) | 0.91 |
| Years of diabetes, yrs | 13.09 ± 10.09 | 11.77 ± 9.05 | 14.31 ± 10.90 | 0.15 |
| Dyslipidemia, n (%) | 327 (95%) | 165 (95%) | 162 (94%) | 0.48 |
| Smoking, n (%) | 75 (22%) | 42 (24%) | 33 (19%) | 0.24 |
| Cancer, n (%) | 66 (19%) | 33 (19%) | 33 (19%) | 1.00 |
| Cardiovascular disease | ||||
| Ischemic heart disease, n (%) | 113 (33%) | 52 (30%) | 61 (35%) | 0.30 |
| Onset of ischemic heart disease, yrs | 62.54 ± 9.66 | 61.21 ± 8.82 | 63.84 ± 10.34 | 0.19 |
| Cerebral ischemia, n (%) | 68 (20%) | 38 (22%) | 30 (17%) | 0.28 |
| Onset of cerebral ischemia, yrs | 67.02 ± 10.44 | 64.74 ± 10.86 | 69.85 ± 9.32 | 0.05 |
| Type of cerebral ischemia, n (%) | 0.49 | |||
| Ictus | 35 (51%) | 18 (47%) | 17 (57%) | |
| Leukoencephalopathy | 17 (25%) | 9 (24%) | 8 (27%) | |
| TIA | 16 (24%) | 11 (29%) | 5 (17%) | |
| Myocardial revascularization | ||||
| Myocardial revascularization, n (%) | 98 (28%) | 45 (26%) | 53 (31%) | 0.34 |
| Age at myocardial revascularization, yrs | 64.61 ± 8.66 | 63.79 ± 8.40 | 65.32 ± 8.90 | 0.39 |
| Type of revascularization, n (%) | 0.36 | |||
| PCI | 48 (50%) | 25 (57%) | 23 (44%) | |
| CABG | 38 (40%) | 14 (32%) | 24 (46%) | |
| CABG + PCI | 10 (10%) | 5 (11%) | 5 (9.6%) | |
| Limb revascularization | ||||
| Lower limb revascularization, n (%) | 57 (16%) | 32 (18%) | 25 (14%) | 0.31 |
| Age at lower limb revascularization, yrs | 66.67 ± 9.84 | 65.52 ± 9.01 | 68.14 ± 10.81 | 0.32 |
| Type of revascularization, n (%) | 0.21 | |||
| Bypass | 7 (12%) | 2 (6.3%) | 5 (20%) | |
| PTA | 49 (86%) | 29 (91%) | 20 (80%) | |
| TEA | 1 (1.8%) | 1 (3.1%) | 0 (0%) | |
| Site of lower limb revascularization, n(%) | 0.33 | |||
| Common femoral | 7 (11%) | 2 (6.3%) | 5 (17%) | |
| Iliac | 25 (41%) | 16 (50%) | 9 (31%) | |
| Popliteal | 3 (4.9%) | 1 (3.1%) | 2 (6.9%) | |
| Superficial femoral | 26 (43%) | 13 (41%) | 13 (45%) | |
| Carotid revascularization | ||||
| Carotid revascularization, n (%) | 85 (25%) | 39 (23%) | 46 (27%) | 0.38 |
| Age at carotid revascularization, yrs | 67.55 ± 7.75 | 68.40 ± 6.48 | 66.82 ± 8.69 | 0.35 |
| Type of revascularization, n (%) | 0.14 | |||
| Bypass | 2 (2.4%) | 2 (5.1%) | 0 (0%) | |
| PTA | 40 (47%) | 21 (54%) | 19 (41%) | |
| TEA | 40 (47%) | 14 (36%) | 26 (57%) | |
| TEA + PTA | 3 (3.5%) | 2 (5.1%) | 1 (2.2%) | |
| Total peripheral revascularizations | ||||
| Total peripheral revascularizations, n (%) | 128 (37%) | 62 (36%) | 66 (38%) | 0.66 |
| Age at total peripheral revascularizations, yrs | 66.98 ± 8.71 | 66.65 ± 7.96 | 67.28 ± 9.43 | 0.68 |
| All Patients 1 | Before Enrollment | After Enrollment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Medical Treatments | Before Enrollment | After Enrollment | p-Value 2 | Group 1 1 | Group 2 1 | p-Value 2 | Group 1 1 | Group 2 1 | p-Value 2 |
| Antihypertensive treatment, n (%) | 290 (84%) | 322 (93%) | <0.01 | 146 (84%) | 144 (83%) | 0.77 | 162 (94%) | 160 (92%) | 0.67 |
| β-blockers, n (%) | 93 (27%) | 102 (29%) | 0.02 | 45 (26%) | 48 (28%) | 0.72 | 51 (29%) | 51 (29%) | 1.00 |
| ACE inhibitors, n (%) | 132 (38%) | 157 (45%) | <0.01 | 64 (37%) | 68 (39%) | 0.66 | 77 (45%) | 80 (46%) | 0.75 |
| Diuretics, n (%) | 149 (43%) | 163 (47%) | 0.03 | 71 (41%) | 78 (45%) | 0.45 | 72 (42%) | 91 (53%) | 0.04 |
| ARBs, n (%) | 134 (39%) | 158 (46%) | <0.01 | 70 (40%) | 64 (37%) | 0.51 | 80 (46%) | 78 (45%) | 0.83 |
| Calcium channel blockers, n (%) | 110 (32%) | 120 (35%) | 0.07 | 47 (27%) | 63 (36%) | 0.06 | 55 (32%) | 65 (38%) | 0.26 |
| Nitrates, n (%) | 55 (16%) | 54 (16%) | 0.56 | 28 (16%) | 27 (16%) | 0.88 | 27 (16%) | 27 (16%) | 1.00 |
| Antiplatelet, n (%) | 284 (82%) | 317 (92%) | <0.01 | 142 (82%) | 142 (82%) | 1.00 | 160 (92%) | 157 (91%) | 0.56 |
| Dual antiplatelet therapy, n (%) | 255 (74%) | 289 (84%) | <0.01 | 130 (75%) | 125 (72%) | 0.72 | 145 (84%) | 144 (83%) | 0.63 |
| Anticoagulant, n (%) | 15 (4.3%) | 19 (5.5%) | 0.05 | 7 (4.0%) | 8 (4.6%) | 0.79 | 7 (4.0%) | 12 (6.9%) | 0.24 |
| Lipid-lowering drug, n (%) | 280 (81%) | 330 (95%) | <0.01 | 146 (84%) | 134 (77%) | 0.10 | 168 (97%) | 162 (94%) | 0.12 |
| SGLT2i/iDDP4/other, n (%) | 0 (0%) | 17 (4.9%) | <0.01 | 0 (0%) | 0 (0%) | - | 10 (5.8%) | 7 (4.0%) | 0.46 |
| Subcutaneous insulin, n (%) | 13 (3.8%) | 58 (17%) | <0.01 | 5 (2.9%) | 8 (4.6%) | 0.40 | 26 (15%) | 32 (18%) | 0.39 |
| Oral antidiabetic therapy, n (%) | 85 (25%) | 100 (29%) | 0.05 | 43 (25%) | 42 (24%) | 0.90 | 53 (31%) | 47 (27%) | 0.48 |
| Metformin, n (%) | 81 (23%) | 90 (26%) | 0.04 | 45 (26%) | 36 (21%) | 0.25 | 50 (29%) | 40 (23%) | 0.22 |
| All Patients 1 | Group 1 1 | Group 2 1 | p-Value 2 | |
|---|---|---|---|---|
| Follow-up, months | 102.70 ± 44.13 | 108.82 ± 43.56 | 96.59 ± 43.98 | 0.01 |
| Death, n (%) | 169 (49%) | 72 (42%) | 97 (56%) | <0.01 |
| Time of death, months | 80.11 ± 43.78 | 84.86 ± 5.18 | 76.58 ± 43.53 | 0.22 |
| Cause of death, n (%) | 0.63 | |||
| Cardiovascular events | 59 (35%) | 28 (39%) | 31 (32%) | |
| Cancer | 39 (23%) | 15 (21%) | 24 (25%) | |
| Other causes | 71 (42%) | 29 (40%) | 42 (43%) | |
| Clinical events during follow-up | ||||
| Ischemic heart disease, n (%) | 41 (12%) | 20 (12%) | 21 (12%) | 0.87 |
| Cerebral ischemia, n (%) | 26 (7.5%) | 10 (5.8%) | 16 (9.2%) | 0.22 |
| New cancer diagnosis, n (%) | 46 (13%) | 20 (12%) | 26 (15%) | 0.34 |
| Myocardial revascularization during follow-up | ||||
| Myocardial revascularization, n (%) | 44 (13%) | 24 (14%) | 20 (12%) | 0.52 |
| Time of myocardialrevascularization, months | 33.64 ± 34.07 | 35.50 ± 36.37 | 31.40 ± 31.88 | 0.70 |
| Type of revascularization, n (%) | 0.28 | |||
| PCI | 34 (76%) | 19 (79%) | 15 (71%) | |
| CABG | 7 (16%) | 2 (8.3%) | 5 (24%) | |
| CABG + PCI | 4 (8.9%) | 3 (13%) | 1 (4.8%) | |
| Second myocardial revascularization, N (%) | 9 (2.6%) | 7 (4.0%) | 2 (1.2%) | 0.09 |
| Time of second myocardial revascularization, months | 79.78 ± 41.31 | 87.29 ± 40.85 | 53.50 ± 43.13 | 0.34 |
| Limb revascularization during follow-up | ||||
| Lower limb revascularization, n (%) | 29 (8.4%) | 16 (9.2%) | 13 (7.5%) | 0.56 |
| Time of lower limb revascularization, months | 61.59 ± 45.09 | 73.94 ± 48.96 | 46.39 ± 35.96 | 0.10 |
| Type of revascularization, n (%) | 0.18 | |||
| Bypass | 3 (10%) | 0 (0%) | 3 (23%) | |
| PTA | 23 (79%) | 14 (88%) | 9 (69%) | |
| TEA | 1 (3.4%) | 1 (6.3%) | 0 (0%) | |
| TEA + amputation | 2 (6.9%) | 1 (6.3%) | 1 (7.7%) | |
| Carotid revascularization during follow-up | ||||
| Carotid revascularization, n (%) | 28 (8.1%) | 13 (7.5%) | 15 (8.7%) | 0.69 |
| Time of carotid revascularization, months | 37.33 ± 52.81 | 63.00 ± 59.08 | 16.80 ± 37.69 | 0.03 |
| Type of revascularization, n (%) | 0.47 | |||
| Bypass | 1 (3.6%) | 0 (0%) | 1 (6.7%) | |
| PTA | 10 (36%) | 4 (31%) | 6 (40%) | |
| TEA | 16 (57%) | 9 (69%) | 7 (47%) | |
| TEA + PTA | 1 (3.6%) | 0 (0%) | 1 (6.7%) | |
| Second carotid revascularization, n (%) | 2 (0.6%) | 0 (0%) | 2 (1.2%) | 0.16 |
| Time of second carotid revascularization, months | 83.00 ± 87.68 | - | 83.00 ± 87.68 | - |
| Peripheral revascularization during follow-up | ||||
| Peripheral revascularization, n (%) | 52 (15%) | 27 (52%) | 25 (48%) | 0.76 |
| Time of peripheral revascularization, months | 48.39 ± 49.97 | 67.04 ± 52.45 | 28.24 ± 38.87 | <0.01 |
| Major adverse cardiovascular events during follow-up | ||||
| MACEs, n (%) | 129 (37%) | 64 (37%) | 65 (38%) | 0.91 |
| MACEs, months | 48.04 ± 43.31 | 55.89 ± 46.33 | 40.31 ± 38.95 | 0.04 |
| Major adverse peripheral events during follow-up | ||||
| MAPEs, n (%) | 78 (23%) | 38 (22%) | 40 (23%) | 0.80 |
| MAPEs, months | 51.02 ± 45.21 | 66.19 ± 48.18 | 36.60 ± 37.35 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stolfo, G.; Mastroianno, M.; Pacilli, M.A.; De Luca, G.; Coli, C.R.; Bevere, E.M.L.; Pacilli, G.; Potenza, D.R.; Mastroianno, S. Role of C-Reactive Protein as a Predictor of Early Revascularization and Mortality in Advanced Peripheral Arterial Disease. J. Clin. Med. 2025, 14, 815. https://doi.org/10.3390/jcm14030815
Di Stolfo G, Mastroianno M, Pacilli MA, De Luca G, Coli CR, Bevere EML, Pacilli G, Potenza DR, Mastroianno S. Role of C-Reactive Protein as a Predictor of Early Revascularization and Mortality in Advanced Peripheral Arterial Disease. Journal of Clinical Medicine. 2025; 14(3):815. https://doi.org/10.3390/jcm14030815
Chicago/Turabian StyleDi Stolfo, Giuseppe, Mario Mastroianno, Michele Antonio Pacilli, Giovanni De Luca, Carlo Rosario Coli, Ester Maria Lucia Bevere, Gabriella Pacilli, Domenico Rosario Potenza, and Sandra Mastroianno. 2025. "Role of C-Reactive Protein as a Predictor of Early Revascularization and Mortality in Advanced Peripheral Arterial Disease" Journal of Clinical Medicine 14, no. 3: 815. https://doi.org/10.3390/jcm14030815
APA StyleDi Stolfo, G., Mastroianno, M., Pacilli, M. A., De Luca, G., Coli, C. R., Bevere, E. M. L., Pacilli, G., Potenza, D. R., & Mastroianno, S. (2025). Role of C-Reactive Protein as a Predictor of Early Revascularization and Mortality in Advanced Peripheral Arterial Disease. Journal of Clinical Medicine, 14(3), 815. https://doi.org/10.3390/jcm14030815






