Pre-Clinical and Clinical Advances in Gene Therapy of X-Linked Retinitis Pigmentosa: Hope on the Horizon
Abstract
1. Introduction
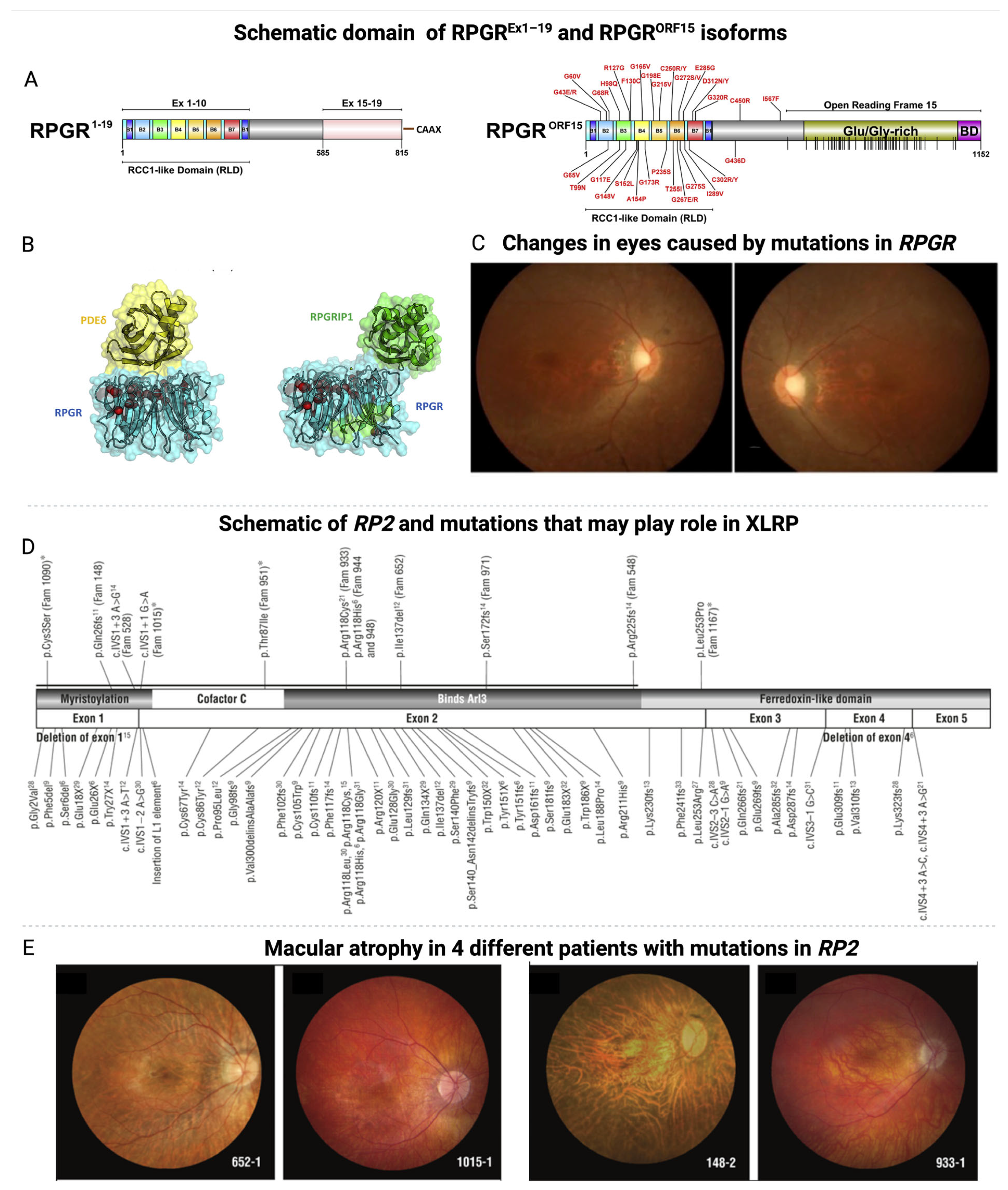
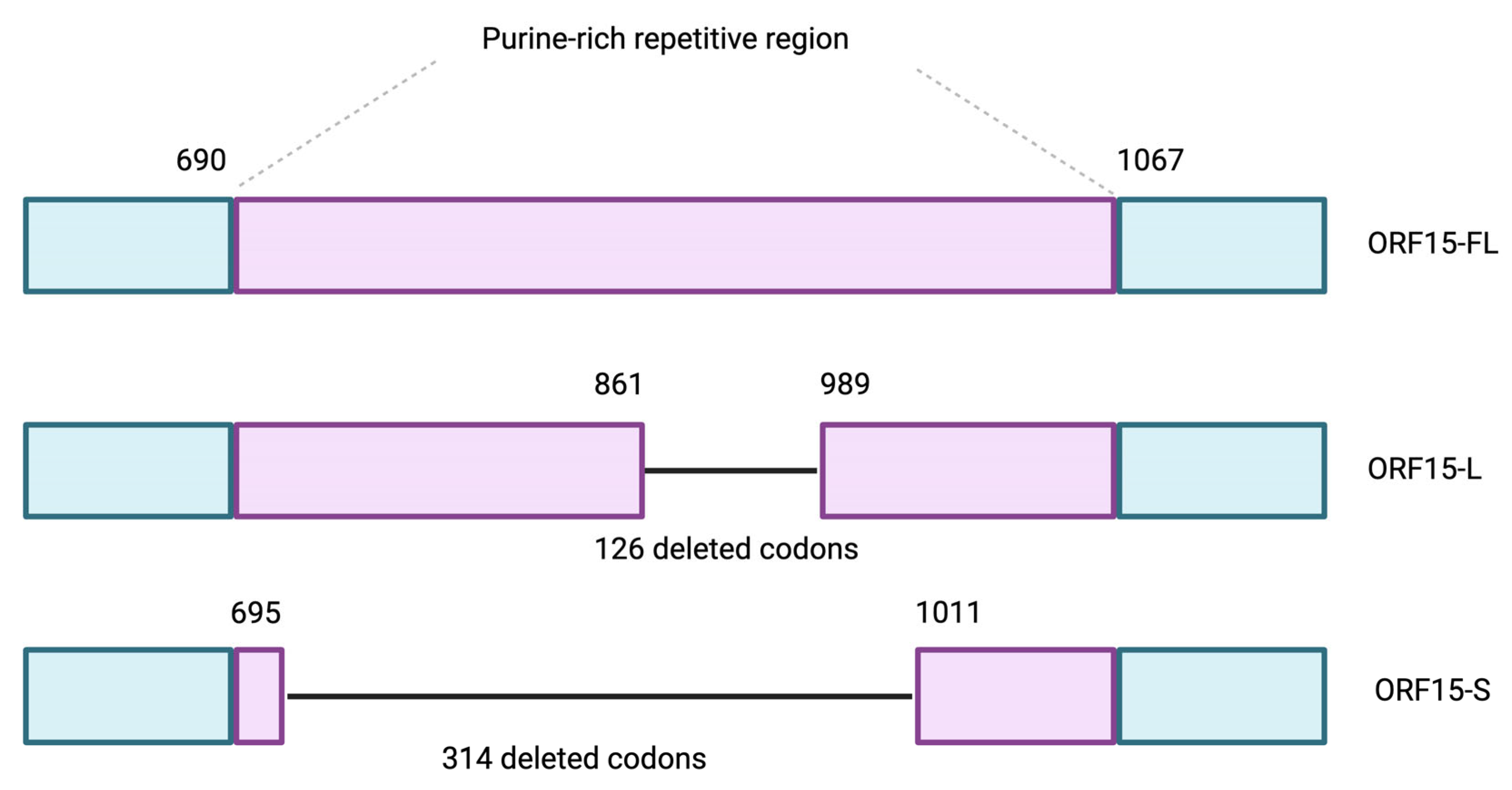
2. Etiology and Pathophysiology of XLRP
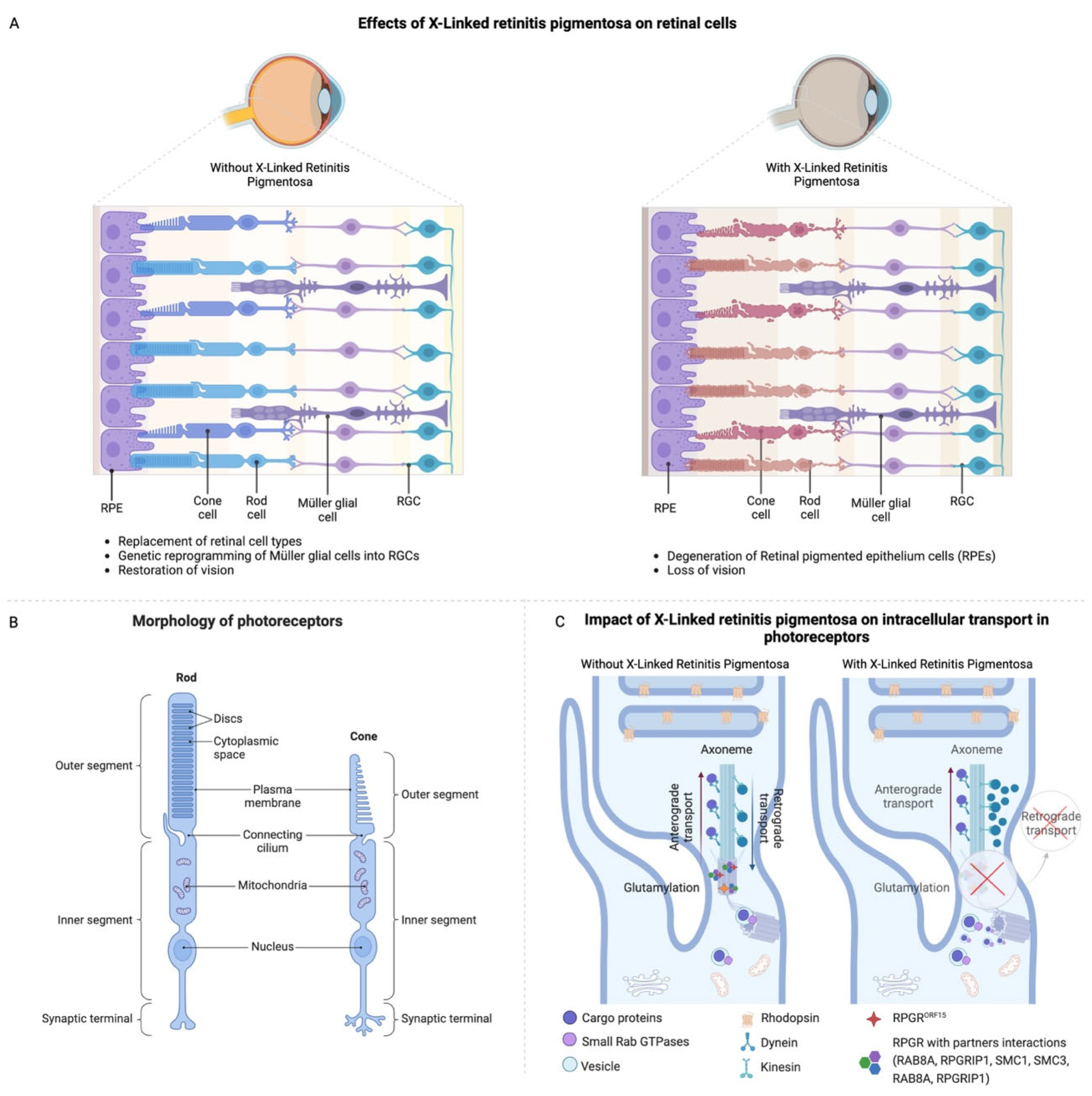
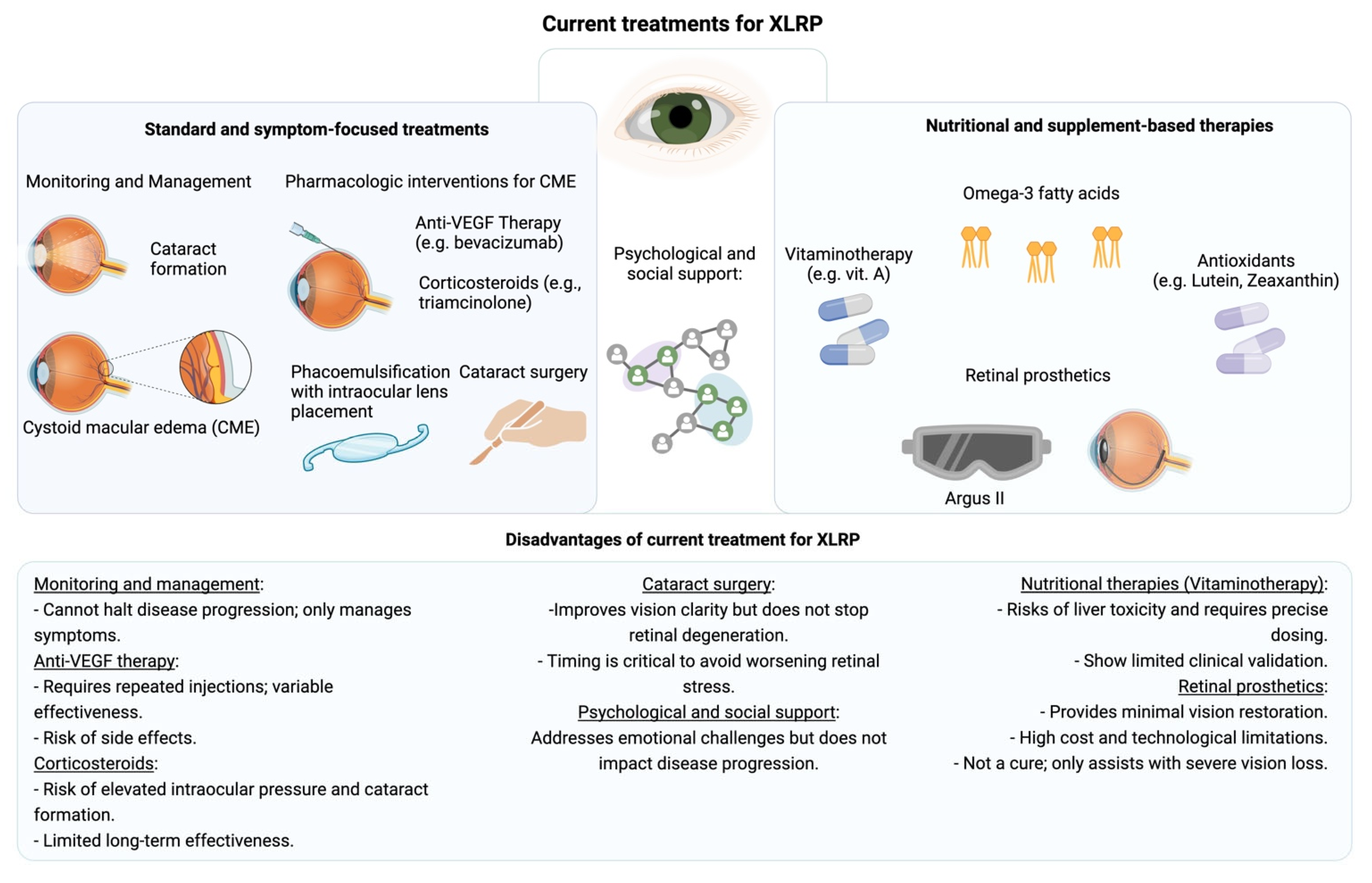
3. The Current Treatments of XLRP and Their Challenges
4. Gene Treatment of XLRP
4.1. Vectors Utilized in Ocular Gene Therapy
4.2. Immune Challenges and Side Effects of AAV-Based Gene Therapies
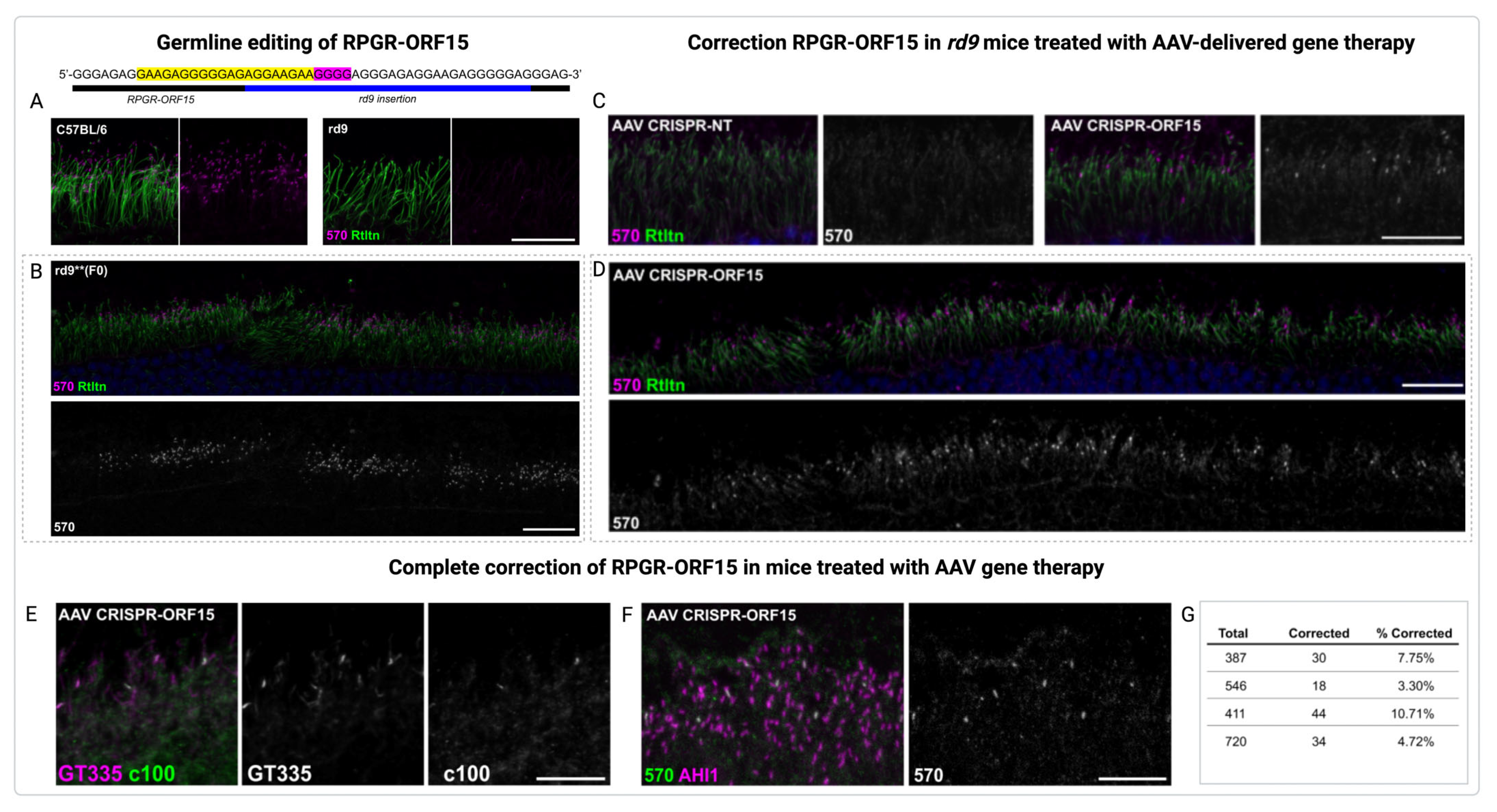
4.3. Gene Therapy of XLRP in Pre-Clinical Trials
5. Recent Clinical Advances in Gene Therapy for XLRP Care
6. Conclusions and Future Perspectives
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| AEs | Adverse effects |
| ARL3 | ADP-ribosylation factor-like GTPase 3 |
| CME | Cystoid macular edema |
| dB | Decibels |
| GAP | GTPase-activating protein |
| GEF | Guanine nucleotide exchange factor |
| GRK1 | G protein-coupled receptor kinase 1 |
| IRBP | Interphotoreceptor retinoid-binding protein |
| iPSCs | Induced pluripotent stem cells |
| KO | Knockout |
| LCA | Leber congenital amaurosis |
| MAIA | Macular integrity assessment |
| nAbs | Neutralizing antibodies |
| ONL | Outer nuclear layer |
| PAM | Protospacer adjacent motif |
| PDE δ | Phosphodiesterase δ |
| RGCs | Retinal ganglion cells |
| rAAV | Recombinant AAV |
| ROS | Retinal organoids |
| RPE | Retinal pigment epithelium |
| RP | Retinitis pigmentosa |
| RPGR | Retinitis pigmentosa GTPase regulator |
| ROS | Reactive oxygen species |
| sgRNA | Single guide RNA |
| TEAEs | Treatment-emergent adverse events |
| TLRs | Toll-like receptors |
| UNC119 | Uncoordinated 119 protein |
| VF | Visual field |
| VEGF | Vascular endothelial growth factor |
| XLRP | X-linked retinitis pigmentosa |
References
- Chivers, M.; Li, N.; Pan, F.; Wieffer, H.; Slowik, R.; Leartsakulpanitch, J. The Burden of X-Linked Retinitis Pigmentosa on Patients and Society: A Narrative Literature Review. Clin. Outcomes Res. 2021, 13, 565–572. [Google Scholar] [CrossRef] [PubMed]
- de La Camara, C.M.-F.; Nanda, A.; Salvetti, A.P.; Fischer, M.D.; MacLaren, R.E. Gene therapy for the treatment of X-linked retinitis pigmentosa. Expert Opin. Orphan Drugs 2018, 6, 167–177. [Google Scholar] [CrossRef]
- Pungor, K.; Lee, J.; Denee, T.; Kambarov, Y.; Nissinen, R.; Ampeh, K.; Pellegrini, M.; Parmeggiani, F. Impacts of X-linked Retinitis Pigmentosa and Patient Pathways in European Countries: Results from the Cross-sectional EXPLORE XLRP-1 Physician Survey. Adv. Ther. 2024, 41, 3378–3395. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, H.; Wang, L.; Tao, T.; Xu, J.; Sun, X.; Yang, L.; Li, G. Novel mutations of RPGR in Chinese families with X-linked retinitis pigmentosa. BMC Ophthalmol. 2019, 19, 240. [Google Scholar] [CrossRef]
- Zahid, S.; Khan, N.; Branham, K.; Othman, M.; Karoukis, A.J.; Sharma, N.; Moncrief, A.; Mahmood, M.N.; Sieving, P.A.; Swaroop, A.; et al. Phenotypic Conservation in Patients With X-Linked Retinitis Pigmentosa Caused by RPGR Mutations. JAMA Ophthalmol. 2013, 131, 1016. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, L.; Zhang, D.; Tan, Y.; Li, D.; He, F.; Jiao, X.; Yang, M.; Hejtmancik, J.F.; Liu, X. A novel mutation of the RPGR gene in a Chinese X-linked retinitis pigmentosa family and possible involvement of X-chromosome inactivation. Eye 2021, 35, 1688–1696. [Google Scholar] [CrossRef]
- Sharon, D.; Sandberg, M.A.; Rabe, V.W.; Stillberger, M.; Dryja, T.P.; Berson, E.L. RP2 and RPGR mutations and clinical correlations in patients with X-linked retinitis pigmentosa. Am. J. Hum. Genet. 2003, 73, 1131–1146. [Google Scholar] [CrossRef]
- Birch, D.G.; Cheetham, J.K.; Daiger, S.P.; Hoyng, C.; Kay, C.; MacDonald, I.M.; Pennesi, M.E.; Sullivan, L.S. Overcoming the Challenges to Clinical Development of X-Linked Retinitis Pigmentosa Therapies: Proceedings of an Expert Panel. Transl. Vis. Sci. Technol. 2023, 12, 5. [Google Scholar] [CrossRef]
- Nguyen, X.-T.-A.; Moekotte, L.; Plomp, A.S.; Bergen, A.A.; van Genderen, M.M.; Boon, C.J.F. Retinitis Pigmentosa: Current Clinical Management and Emerging Therapies. Int. J. Mol. Sci. 2023, 24, 7481. [Google Scholar] [CrossRef]
- Mansouri, V. X-Linked Retinitis Pigmentosa Gene Therapy: Preclinical Aspects. Ophthalmol. Ther. 2022, 12, 7–34. [Google Scholar] [CrossRef]
- Liu, Y.; Zong, X.; Cao, W.; Zhang, W.; Zhang, N.; Yang, N. Gene Therapy for Retinitis Pigmentosa: Current Challenges and New Progress. Biomolecules 2024, 14, 903. [Google Scholar] [CrossRef] [PubMed]
- Padhy, S.K.; Takkar, B.; Narayanan, R.; Venkatesh, P.; Jalali, S. Voretigene Neparvovec and Gene Therapy for Leber’s Congenital Amaurosis: Review of Evidence to Date. Appl. Clin. Genet. 2020, 13, 179–208. [Google Scholar] [CrossRef]
- Wang, J.-H.; Gessler, D.J.; Zhan, W.; Gallagher, T.L.; Gao, G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target. Ther. 2024, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- de la Camara, C.M.-F.; Cehajic-Kapetanovic, J.; MacLaren, R.E. Emerging gene therapy products for RPGR-associated X-linked retinitis pigmentosa. Expert Opin. Emerg. Drugs 2022, 27, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Du, J.; Chen, N.; Jia, R.; Zhang, J.; Liu, X.; Yang, L. In Vivo CRISPR/Cas9-Mediated Genome Editing Mitigates Photoreceptor Degeneration in a Mouse Model of X-Linked Retinitis Pigmentosa. Investig. Opthalmology Vis. Sci. 2020, 61, 31. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet. J. Rare Dis. 2006, 1, 1795–1809. [Google Scholar] [CrossRef]
- Vinikoor-Imler, L.C.; Simpson, C.; Narayanan, D.; Abbasi, S.; Lally, C. Prevalence of RPGR-mutated X-linked retinitis pigmentosa among males. Ophthalmic Genet. 2022, 43, 581–588. [Google Scholar] [CrossRef]
- Lam, B.L.; Scholl, H.P.N.; Doub, D.; Sperling, M.; Hashim, M.; Li, N. A systematic literature review of disease progression reported in RPGR-associated X-linked retinitis pigmentosa. Retina 2023, 44, 1097. [Google Scholar] [CrossRef]
- de Silva, S.R.; Arno, G.; Robson, A.G.; Fakin, A.; Pontikos, N.; Mohamed, M.D.; Bird, A.C.; Moore, A.T.; Michaelides, M.; Webster, A.R.; et al. The X-linked retinopathies: Physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Prog. Retin. Eye Res. 2020, 82, 100898. [Google Scholar] [CrossRef]
- Georgiou, M.; Robson, A.G.; Uwaydat, S.H.; Ji, M.H.; Shakarchi, A.F.; Pontikos, N.; Mahroo, O.A.; Cheetham, M.E.; Webster, A.R.; Hardcastle, A.J.; et al. RP2-Associated X-linked Retinopathy: Clinical Findings, Molecular Genetics, and Natural History in a Large Cohort of Female Carriers. Am. J. Ophthalmol. 2024, 261, 112–120. [Google Scholar] [CrossRef]
- Gocuk, S.A.; Edwards, T.L.; Jolly, J.K.; Ayton, L.N. A global survey of visual symptoms in female carriers of choroideremia and X-linked retinitis pigmentosa. AJO Int. 2024, 1, 100002. [Google Scholar] [CrossRef]
- Gocuk, S.A.; Jolly, J.K.; Edwards, T.L.; Ayton, L.N. Female carriers of X-linked inherited retinal diseases—Genetics, diagnosis, and potential therapies. Prog. Retin. Eye Res. 2023, 96, 101190. [Google Scholar] [CrossRef] [PubMed]
- Megaw, R.D.; Soares, D.C.; Wright, A.F. RPGR: Its role in photoreceptor physiology, human disease, and future therapies. Exp. Eye Res. 2015, 138, 32–41. [Google Scholar] [CrossRef]
- Jayasundera, T.; Branham, K.E.H.; Othman, M.; Rhoades, W.R.; Karoukis, A.J.; Khanna, H.; Swaroop, A.; Heckenlively, J.R. RP2 Phenotype and Pathogenetic Correlations in X-Linked Retinitis Pigmentosa. Arch. Ophthalmol. 2010, 128, 915–923. [Google Scholar] [CrossRef]
- Murga-Zamalloa, C.; Swaroop, A.; Khanna, H. Multiprotein Complexes of Retinitis Pigmentosa GTPase Regulator (RPGR), a Ciliary Protein Mutated in X-Linked Retinitis Pigmentosa (XLRP). Adv. Exp. Med. Biol. 2010, 664, 105. [Google Scholar]
- Melamud, A.; Shen, G.Q.; Chung, D.; Xi, Q.; Simpson, E.; Li, L.; Peachey, N.S.; Zegarra, H.; Hagstrom, S.A.; Wang, Q.K.; et al. Mapping a new genetic locus for X linked retinitis pigmentosa to Xq28. J. Med. Genet. 2006, 43, e27. [Google Scholar] [CrossRef]
- Georgiou, M.; Robson, A.G.; Uwaydat, S.H.; Ji, M.H.; Shakarchi, A.F.; Pontikos, N.; Mahroo, O.A.; Cheetham, M.E.; Webster, A.R.; Hardcastle, A.J.; et al. RP2-Associated X-linked Retinopathy: Clinical Findings, Molecular Genetics, and Natural History. Ophthalmology 2023, 130, 413. [Google Scholar] [CrossRef]
- Hadalin, V.; Buscarino, M.; Sajovic, J.; Meglič, A.; Jarc-Vidmar, M.; Hawlina, M.; Volk, M.; Fakin, A. Genetic Characteristics and Long-Term Follow-Up of Slovenian Patients with RPGR Retinal Dystrophy. Int. J. Mol. Sci. 2023, 24, 3840. [Google Scholar] [CrossRef]
- Meindl, A.; Dry, K.; Herrmann, K.; Manson, E.; Ciccodicola, A.; Edgar, A.; Carvalho, M.; Achatz, H.; Hellebrand, H.; Lennon, A.; et al. A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X–linked retinitis pigmentosa (RP3). Nat. Genet. 1996, 13, 35–42. [Google Scholar] [CrossRef]
- Schmid, F.; Glaus, E.; Cremers, F.P.; Kloeckener-Gruissem, B.; Berger, W.; Neidhardt, J. Mutation- and Tissue-Specific Alterations of RPGR Transcripts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1628–1635. [Google Scholar] [CrossRef][Green Version]
- Sladen, P.E.; Naeem, A.; Adefila-Ideozu, T.; Vermeule, T.; Busson, S.L.; Michaelides, M.; Naylor, S.; Forbes, A.; Lane, A.; Georgiadis, A. AAV-RPGR Gene Therapy Rescues Opsin Mislocalisation in a Human Retinal Organoid Model of RPGR-Associated X-Linked Retinitis Pigmentosa. Int. J. Mol. Sci. 2024, 25, 1839. [Google Scholar] [CrossRef] [PubMed]
- Gallenga, C.E.; Lonardi, M.; Pacetti, S.; Violanti, S.S.; Tassinari, P.; Di Virgilio, F.; Tognon, M.; Perri, P. Molecular Mechanisms Related to Oxidative Stress in Retinitis Pigmentosa. Antioxidants 2021, 10, 848. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, C.; Yan, N. Neuroinflammation in retinitis pigmentosa: Therapies targeting the innate immune system. Front. Immunol. 2022, 13, 1059947. [Google Scholar] [CrossRef]
- Raparia, E.; Ballios, B.G.; Place, E.M.; Husain, D.; Huckfeldt, R.M. RP2 X-linked retinitis pigmentosa carrier state presenting with vascular leakage and unilateral macular atrophy. Retin. Cases Brief. Rep. 2022, 17, 533–537. [Google Scholar] [CrossRef]
- Lane, A.; Jovanovic, K.; Shortall, C.; Ottaviani, D.; Panes, A.B.; Schwarz, N.; Guarascio, R.; Hayes, M.J.; Palfi, A.; Chadderton, N.; et al. Modeling and Rescue of RP2 Retinitis Pigmentosa Using iPSC-Derived Retinal Organoids. Stem. Cell Rep. 2020, 15, 67–79. [Google Scholar] [CrossRef]
- Beltran, W.A.; Cideciyan, A.V.; Iwabe, S.; Swider, M.; Kosyk, M.S.; McDaid, K.; Martynyuk, I.; Ying, G.-S.; Shaffer, J.; Deng, W.-T.; et al. Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease. Proc. Natl. Acad. Sci. USA 2015, 112, E5844–E5853. [Google Scholar] [CrossRef]
- Wu, Z.; Hiriyanna, S.; Qian, H.; Mookherjee, S.; Campos, M.M.; Gao, C.; Fariss, R.; Sieving, P.A.; Li, T.; Colosi, P.; et al. A long-term efficacy study of gene replacement therapy for RPGR-associated retinal degeneration. Hum. Mol. Genet. 2015, 24, 3956–3970. [Google Scholar] [CrossRef]
- Bartolini, F.; Bhamidipati, A.; Thomas, S.; Schwahn, U.; Lewis, S.A.; Cowan, N.J. Functional Overlap between Retinitis Pigmentosa 2 Protein and the Tubulin-specific Chaperone Cofactor C. J. Biol. Chem. 2002, 277, 14629–14634. [Google Scholar] [CrossRef]
- Sharon, D.; Bruns, G.A.; McGee, T.L.; Sandberg, M.A.; Berson, E.L.; Dryja, T.P. X-Linked Retinitis Pigmentosa: Mutation Spectrum of the RPGR and RP2 Genes and Correlation with Visual Function. Investig. Ophthalmol. Vis. Sci. 2020, 41, 2712–2721. [Google Scholar]
- Shu, X.; Zeng, Z.; Gautier, P.; Lennon, A.; Gakovic, M.; Patton, E.E.; Wright, A.F. Zebrafish Rpgr is required for normal retinal development and plays a role in dynein-based retrograde transport processes. Hum. Mol. Genet. 2010, 19, 657–670. [Google Scholar] [CrossRef]
- Vingolo, E.M.; Mascolo, S.; Miccichè, F.; Manco, G. Retinitis Pigmentosa: From Pathomolecular Mechanisms to Therapeutic Strategies. Medicina 2024, 60, 189. [Google Scholar] [CrossRef] [PubMed]
- Bakthavatchalam, M.; Lai, F.H.P.; Rong, S.S.; Ng, D.S.; Brelen, M.E. Treatment of cystoid macular edema secondary to retinitis pigmentosa: A systematic review. Surv. Ophthalmol. 2018, 63, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Beltran, W.A.; Cideciyan, A.V.; Boye, S.E.; Ye, G.-J.; Iwabe, S.; Dufour, V.L.; Marinho, L.F.; Swider, M.; Kosyk, M.S.; Sha, J.; et al. Optimization of Retinal Gene Therapy for X-Linked Retinitis Pigmentosa Due to RPGR Mutations. Mol. Ther. 2017, 25, 1866–1880. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Uauy, R.; Birch, D.G. Metabolism of omega-3 fatty acids in patients with autosomal dominant retinitis pigmentosa. Exp. Eye Res. 1995, 60, 279–289. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Hughbanks-Wheaton, D.K.; Spencer, R.; Fish, G.E.; Pearson, N.S.; Wang, Y.-Z.; Klein, M.; Takacs, A.; Locke, K.G.; Birch, D.G. Docosahexaenoic Acid Slows Visual Field Progression in X-Linked Retinitis Pigmentosa: Ancillary Outcomes of the DHAX Trial. Investig. Opthalmol. Vis. Sci. 2015, 56, 6646–6653. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Hughbanks-Wheaton, D.K.; Pearson, N.S.; Fish, G.E.; Spencer, R.; Takacs, A.; Klein, M.; Locke, K.G.; Birch, D.G. Four-Year Placebo-Controlled Trial of Docosahexaenoic Acid in X-Linked Retinitis Pigmentosa (DHAX Trial): A Randomized Clinical Trial. JAMA Ophthalmol. 2014, 132, 866–873. [Google Scholar] [CrossRef]
- Luo, Y.H.L.; Zhong, J.J.; da Cruz, L. The use of Argus® II retinal prosthesis by blind subjects to achieve localisation and prehension of objects in 3-dimensional space. Graefe Arch. Clin. Exp. Ophthalmol. 2015, 253, 1907–1914. [Google Scholar] [CrossRef]
- Issa, S.S.; Shaimardanova, A.A.; Solovyeva, V.V.; Rizvanov, A.A. Various AAV Serotypes and Their Applications in Gene Therapy: An Overview. Cells 2023, 12, 785. [Google Scholar] [CrossRef]
- Fuller-Carter, P.I.; Basiri, H.; Harvey, A.R.; Carvalho, L.S. Focused Update on AAV-Based Gene Therapy Clinical Trials for Inherited Retinal Degeneration. BioDrugs 2020, 34, 763–781. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, Z.; Zheng, M.; Shi, L.; Gu, M.; Liu, G.; Miao, F.; Chang, Y.; Huang, F.; Tang, N. Recombinant adeno-associated virus 8 vector in gene therapy: Opportunities and challenges. Genes Dis. 2024, 11, 283–293. [Google Scholar] [CrossRef]
- Ohlhausen, M.; Conrady, C.D. Clinical and Ocular Inflammatory Inhibitors of Viral-Based Gene Therapy of the Retina. Acta Microbiol. Hell. 2024, 69, 187–203. [Google Scholar] [CrossRef]
- Bucher, K.; Rodríguez-Bocanegra, E.; Dauletbekov, D.; Fischer, M.D. Immune responses to retinal gene therapy using adeno-associated viral vectors—Implications for treatment success and safety. Prog. Retin. Eye Res. 2021, 83, 100915. [Google Scholar] [CrossRef] [PubMed]
- Ail, D.; Ren, D.; Brazhnikova, E.; Nouvel-Jaillard, C.; Bertin, S.; Mirashrafi, S.B.; Fisson, S.; Dalkara, D. Systemic and local immune responses to intraocular AAV vector administration in non-human primates. Mol. Ther. Methods Clin. Dev. 2022, 24, 306–316. [Google Scholar] [CrossRef]
- Ren, D.; Fisson, S.; Dalkara, D.; Ail, D. Immune Responses to Gene Editing by Viral and Non-Viral Delivery Vectors Used in Retinal Gene Therapy. Pharmaceutics 2022, 14, 1973. [Google Scholar] [CrossRef]
- Fischer, M.D.; Ochakovski, G.A.; Beier, B.; Seitz, I.P.; Vaheb, Y.; Kortuem, C.; Reichel, F.F.L.; Kuehlewein, L.; Kahle, N.A.; Peters, T.; et al. Efficacy and Safety of Retinal Gene Therapy Using Adeno-Associated Virus Vector for Patients with Choroideremia: A Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 1247–1254. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhan, W.; Gallagher, T.L.; Gao, G. Recombinant adeno-associated virus as a delivery platform for ocular gene therapy: A comprehensive review. Mol. Ther. 2024, 32, 4185–4207. [Google Scholar] [CrossRef]
- Sobh, M.; Lagali, P.S.; Ghiasi, M.; Montroy, J.; Dollin, M.; Hurley, B.; Leonard, B.C.; Dimopoulos, I.; Lafreniere, M.; Fergusson, D.A.; et al. Safety and Efficacy of Adeno-Associated Viral Gene Therapy in Patients with Retinal Degeneration: A Systematic Review and Meta-Analysis. Transl. Vis. Sci. Technol. 2023, 12, 24. [Google Scholar] [CrossRef]
- Ramachandran, P.S.; Lee, V.; Wei, Z.; Song, J.Y.; Casal, G.; Cronin, T.; Willett, K.; Huckfeldt, R.; Morgan, J.I.W.; Aleman, T.S.; et al. Evaluation of Dose and Safety of AAV7m8 and AAV8BP2 in the Non-Human Primate Retina. Hum. Gene Ther. 2017, 28, 154–167. [Google Scholar] [CrossRef]
- Xia, X.; Guo, X. Adeno-associated virus vectors for retinal gene therapy in basic research and clinical studies. Front. Med. 2023, 10, 1310050. [Google Scholar] [CrossRef]
- Maurya, S.; Sarangi, P.; Jayandharan, G.R. Safety of Adeno-associated virus-based vector-mediated gene therapy—Impact of vector dose. Cancer Gene Ther. 2022, 29, 1305–1306. [Google Scholar] [CrossRef]
- Beltran, W.A.; Cideciyan, A.V.; Lewin, A.S.; Iwabe, S.; Khanna, H.; Sumaroka, A.; Chiodo, V.A.; Fajardo, D.S.; Román, A.J.; Deng, W.T.; et al. Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2012, 109, 2132–2137. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, T.; Madigan, M.C.; Fernando, N.; Aggio-Bruce, R.; Zhou, F.; Pierce, M.; Chen, Y.; Huang, L.; Natoli, R.; et al. Interphotoreceptor Retinoid-Binding Protein (IRBP) in Retinal Health and Disease. Front. Cell Neurosci. 2020, 14, 577935. [Google Scholar] [CrossRef]
- Young, J.E.; Vogt, T.; Gross, K.W.; Khani, S.C. A Short, Highly Active Photoreceptor-Specific Enhancer/Promoter Region Upstream of the Human Rhodopsin Kinase Gene. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4076–4085. [Google Scholar] [CrossRef]
- Pawlyk, B.S.; Bulgakov, O.V.; Sun, X.; Adamian, M.; Shu, X.; Smith, A.J.; Berson, E.L.; Ali, R.R.; Khani, S.; Wright, A.F.; et al. Photoreceptor rescue by an abbreviated human RPGR gene in a murine model of X-linked retinitis pigmentosa. Gene Ther. 2015, 23, 196–204. [Google Scholar] [CrossRef]
- Fischer, M.D.; McClements, M.E.; Martinez-Fernandez de la Camara, C.; Bellingrath, J.S.; Dauletbekov, D.; Ramsden, S.C.; Hickey, D.G.; Barnard, A.R.; MacLaren, R.E. Codon-Optimized RPGR Improves Stability and Efficacy of AAV8 Gene Therapy in Two Mouse Models of X-Linked Retinitis Pigmentosa. Mol. Ther. 2017, 25, 1854–1865. [Google Scholar] [CrossRef]
- Gumerson, J.D.; Alsufyani, A.; Yu, W.; Lei, J.; Sun, X.; Dong, L.; Wu, Z.; Li, T. Restoration of RPGR expression in vivo using CRISPR/Cas9 gene editing. Gene Ther. 2021, 29, 81–93. [Google Scholar] [CrossRef]
- van Wyk, M.; Hulliger, E.C.; Girod, L.; Ebneter, A.; Kleinlogel, S. Present molecular limitations of ON-bipolar cell targeted gene therapy. Front. Neurosci. 2017, 11, 257919. [Google Scholar] [CrossRef]
- Mookherjee, S.; Hiriyanna, S.; Kaneshiro, K.; Li, Y.; Li, W.; Qian, H.; Li, T.; Colosi, P.; Swaroop, A.; Wu, Z.; et al. Long-term rescue of cone photoreceptor degeneration in retinitis pigmentosa 2 (RP2)-knockout mice by gene replacement therapy. Hum. Mol. Genet. 2015, 24, 6446. [Google Scholar] [CrossRef]
- Kang, C.; Scott, L.J. Voretigene Neparvovec: A Review in RPE65 Mutation-Associated Inherited Retinal Dystrophy. Mol. Diagn. Ther. 2020, 24, 487–495. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Lauer, A.K.; Yang, P.; Sisk, R.; Anand, R.; Curtiss, D.; Christenson, A.; Waheed, N.K. Subretinal gene therapy AGTC-501 for X-linked retinitis pigmentosa in the Phase 1/2 Horizon study: Post-hoc analysis of microperimetry results in the high dose groups. Investig. Ophthalmol. Vis. Sci. 2024, 65, 2133. [Google Scholar]
- Michaelides, M.; Besirli, C.G.; Yang, Y.E.S.A.; de Guimaraes, T.A.C.; Wong, S.C.; Huckfeldt, R.M.; Comander, J.I.; Shah, S.J.; Shah, S.M.; Tee, J.J.L.; et al. Phase 1/2 AAV5-hRKp.RPGR (Botaretigene Sparoparvovec) Gene Therapy: Safety and Efficacy in RPGR-Associated X-Linked Retinitis Pigmentosa. Am. J. Ophthalmol. 2024, 267, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Xu, J.; Wang, D.; Wong, P.; Fung, A.; Forbes, A.; Naylor, S.; Zeldin, R.; Parker, M.A.; Weleber, R.; et al. AAV5-RPGR (botaretigene sparoparvovec) gene therapy for X-linked retinitis pigmentosa (XLRP) demonstrates localized improvements in static perimetry. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3846. [Google Scholar]
- Von Krusenstiern, L.; Liu, J.; Liao, E.; Gow, J.A.; Chen, G.; Ong, T.; Lotery, A.J.; Jalil, A.; Lam, B.L.; Maclaren, R.E. Changes in retinal sensitivity associated with cotoretigene toliparvovec in X-linked retinitis pigmentosa with RPGR gene variations. JAMA Ophthalmol. 2023, 141, 275. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef]
- Dufour, V.L.; Artur, V.C.; Ye, G.J.; Song, C.; Timmers, A.; Habecker, P.L.; Pan, W.; Weinstein, N.M.; Swider, M.; Durham, A.C.; et al. Toxicity and Efficacy Evaluation of an Adeno-Associated Virus Vector Expressing Codon-Optimized RPGR Delivered by Subretinal Injection in a Canine Model of X-linked Retinitis Pigmentosa. Hum. Gene Ther. 2020, 31, 253–267. [Google Scholar] [CrossRef]
- Weber, T. Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions. Front. Immunol. 2021, 12, 658399. [Google Scholar] [CrossRef]
- Hampson, G.; Towse, A.; Pearson, S.D.; Dreitlein, W.B.; Henshall, C. Gene therapy: Evidence, value and affordability in the US health care system. J. Comp. Eff. Res. 2018, 7, 15–28. [Google Scholar] [CrossRef]
- Gopinath, C.; Rompicherla, R.; Mathias, G.P.; Patil, R.; Poornachandra, B.; Vinekar, A.; Mochi, T.B.; Braganza, S.; Shetty, K.B.; Kumaramanickavel, G.; et al. Inherited retinal disorders: A genotype–phenotype correlation in an Indian cohort and the importance of genetic testing and genetic counselling. Graefe’S Arch. Clin. Exp. Ophthalmol. 2023, 261, 2003–2017. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, E.K. Asymmetric presentation with a novel RP2 gene mutation in X-Linked retinitis pigmentosa: A case report. BMC Ophthalmol. 2023, 23, 221. [Google Scholar] [CrossRef]
- Liu, X.; Jia, R.; Meng, X.; Wang, L.; Yang, L. Analysis of RPGR gene mutations in 41 Chinese families affected by X-linked inherited retinal dystrophy. Front. Genet. 2022, 13, 999695. [Google Scholar] [CrossRef] [PubMed]

| Therapeutics | Company/ University | Clinical Identifier (Phase) | Date and Status | Purpose |
|---|---|---|---|---|
| AAV8-RPGR | Biogen | NCT03116113 (I/II) | 2017–2020, completed | posted |
| AAV2/5-RPGR | MeiraGTx UK II Ltd. | NCT03252847 (I/II) | 2017–2021, completed | submitted |
| BIIB112 (AAV8-RPGR) | NightstaRx Ltd., a Biogen Company | NCT04926129 (Observational) | 2017–2022, completed | no posted |
| AAV5 hRKp.RPGR | Janssen Research & Development, LLC | NCT06646289 (II) | 2024–2030, Not yet recruiting | Safety and tolerability |
| FT-002 | Frontera Therapeutics | NCT06492850 (I/II) | 2024–2026 Recruiting | Safety, tolerability, and efficacy |
| rAAV2tYF-GRK1-RPGR | Beacon Therapeutics | NCT06333249 (II) | 2021–2027 Active, not recruiting | Safety and efficacy |
| rAAV2tYF-GRK1-RPGR | Beacon Therapeutics | NCT06275620 (II) | 2023–2029 Enrolling by invitation | Safety |
| AAV5-hRKp.RPGR | Janssen Pharmaceutical K.K. | NCT05926583 (III) | 2023–2030 Recruiting | Safety, tolerability |
| FT-002 | Frontera Therapeutics | NCT05874310 (I) | 2023–2027 Recruiting | Safety, tolerability, and preliminary efficacy |
| rAAV2tYF-GRK1-RPGR | Beacon Therapeutics | NCT03316560 (I/II) | 2018–2025 Active, not recruiting | Safety and efficacy |
| rAAV2tYF-GRK1-hRPGRco (AGTC-501) | Beacon Therapeutics | NCT04850118 (II/III) | 2024–2029 Recruiting | Safety, efficacy, and tolerability |
| AAV5-hRKp.RPGR | Janssen Research & Development, LLC | NCT04794101 (III) | 2020–2029 Active, not recruiting | Safety |
| AAV5-hRKp.RPGR | Janssen Research & Development, LLC | NCT04671433 (III) | 2020–2024 Active, not recruiting | Safety |
| 4D-125 | 4D Molecular Therapeutics | NCT04517149 (I/II) | 2020–2029, Active, not recruiting | Evaluate natural disease progression, safety and tolerability |
| AAV5-hRKp.RPGR | Janssen Research & Development, LLC | NCT04312672 (Observational) | 2017–2026 Active, not recruiting | Safety up to 60 months |
| AAV2-REP1, previously treated AAV8-RPGR | NightstaRx Ltd., a Biogen Company | NCT03584165 (III) | 2018–2026 Active, not recruiting | Long-term safety and efficacy |
| Drug | Comments | Side Effects | Results | Ref. |
|---|---|---|---|---|
| LUXTURNA (voretigene neparvovecrzyl, AAV2-hRPE65v2) | AAV2 vector containing human RPE65 cDNA with a modified Kozak sequence engineered at the translational start site, under control of a hybrid chicken β-actin promoter with a cytomegalovirus enhancer. | Common: conjunctival hyperemia, cataract, increased intraocular pressure, retinal tear, dellen (thinning of the corneal stroma), macular hole, subretinal deposits, eye inflammation, eye irritation, eye pain, and maculopathy (wrinkling on the surface of the macula). | 65% of patients were responded on therapy. Full-field stimulus testing (FST) and baseline at years 1 and 3: −18.41 dB at year 1 and −14.73 dB at year 3. Visual acuity remained stable without decrease, and visual function showed improvement in patients with chorioretinal atrophy over the 3-year period. MAIA at 12 months: 4.1 dB of gene therapy and 0 without therapy. | [69,70] |
| AGTC-501 (rAAV2tYF-GRK1-RPGR) | rAAV2tYF-GRK1-RPGR expresses the human RPGRORF15 driven by the GRK1 promoter and packaged in AAV2 capsids with single tyrosine to phenylalanine (YF) mutations. | Not registered in human | 63% of eyes treated with high dose were responded on therapy. Retinal Sensitivity at 12 months: >7 dB in at least five loci. MAIA 12 months: 1.96 dB of gene therapy and −0.39 dB of without therapy. | [71] |
| AAV5- RPGR (Botaretigene Sparoparvovec, AAV2/5.hRKp.RPGR) | AAV2/5.hRKp.RPGR is based on the AAV2/5 serotype but the RPGR sequence is mutated in the ORF15 region and carries a random deletion of 126 codons. | 96.9% participants in the treatment groups reported an AE considered related to surgery. These AEs were transient and resolved without intervention: conjunctival hemorrhage, reduced VA, and the presence of anterior chamber cells | ~50% of patients treated with AAV5-RPGR demonstrated significant improvements in visual function. Retinal sensitivity at 12 months: 0.95 dB of gene therapy and –0.59 dB of without therapy. MAIA: 12 months: 5.6 dB of gene therapy and–1.2 dB of without therapy. Functional vision assessment at 9 months: 16.4” in gene therapy with no errors and 61.7” with 2 errors of baseline. (”—seconds) | [72] |
| BIIB112 (Cotoretigene toliparvovec, AAV8-RPGR) | AAV8-RPGR vector containing the codon-optimized human RPGRORF15 under the control of the GRK1 promoter | 94% experienced 35 TEAEs associated with study procedure: 67% inflammation, Serious TEAEs 28%: visual acuity reduced, noninfective retinitis, retinal detachment, visual impairment. | ~33% of patients treated with BIIB112demonstrated significant improvements in visual function. Retinal Sensitivity at 12 months: 2.8 dB of gene therapy and 0.1 dB of without therapy. MAIA 12 months: +5.1 dB of gene therapy and –0.9 dB of without therapy. | [74,75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pechnikova, N.A.; Poimenidou, M.; Iliadis, I.; Zafeiriou-Chatziefraimidou, M.; Iaremenko, A.V.; Yaremenko, T.V.; Domvri, K.; Yaremenko, A.V. Pre-Clinical and Clinical Advances in Gene Therapy of X-Linked Retinitis Pigmentosa: Hope on the Horizon. J. Clin. Med. 2025, 14, 898. https://doi.org/10.3390/jcm14030898
Pechnikova NA, Poimenidou M, Iliadis I, Zafeiriou-Chatziefraimidou M, Iaremenko AV, Yaremenko TV, Domvri K, Yaremenko AV. Pre-Clinical and Clinical Advances in Gene Therapy of X-Linked Retinitis Pigmentosa: Hope on the Horizon. Journal of Clinical Medicine. 2025; 14(3):898. https://doi.org/10.3390/jcm14030898
Chicago/Turabian StylePechnikova, Nadezhda A., Malamati Poimenidou, Ioannis Iliadis, Maria Zafeiriou-Chatziefraimidou, Aleksandra V. Iaremenko, Tamara V. Yaremenko, Kalliopi Domvri, and Alexey V. Yaremenko. 2025. "Pre-Clinical and Clinical Advances in Gene Therapy of X-Linked Retinitis Pigmentosa: Hope on the Horizon" Journal of Clinical Medicine 14, no. 3: 898. https://doi.org/10.3390/jcm14030898
APA StylePechnikova, N. A., Poimenidou, M., Iliadis, I., Zafeiriou-Chatziefraimidou, M., Iaremenko, A. V., Yaremenko, T. V., Domvri, K., & Yaremenko, A. V. (2025). Pre-Clinical and Clinical Advances in Gene Therapy of X-Linked Retinitis Pigmentosa: Hope on the Horizon. Journal of Clinical Medicine, 14(3), 898. https://doi.org/10.3390/jcm14030898






