Unveiling the Resistome Landscape in Peri-Implant Health and Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Information
2.2. Data Analysis and Statistics
3. Results
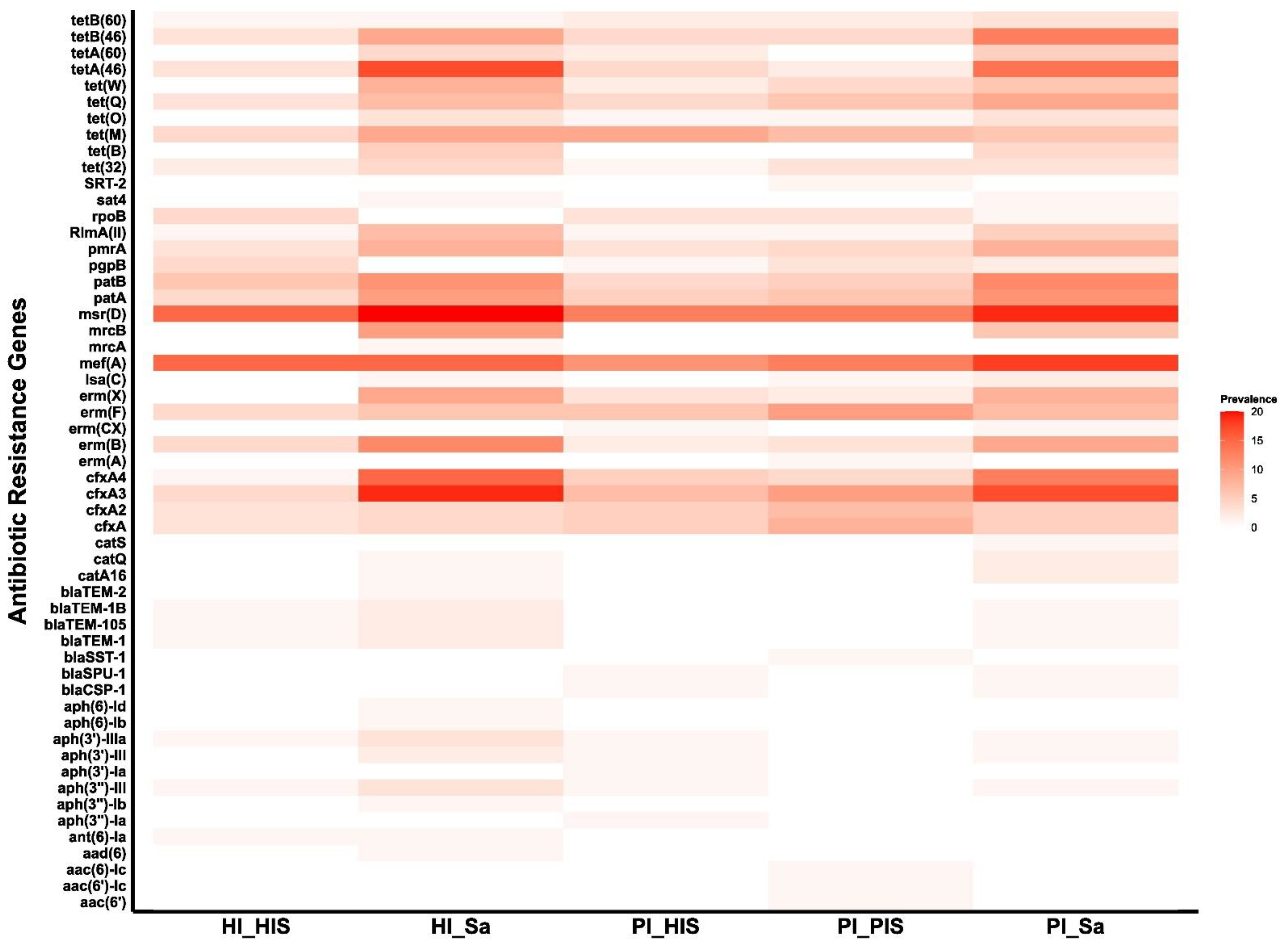
3.1. Detection and Prevalence of ARGs and Plasmids Across Study Groups
3.2. Taxonomic Assignment of Identified ARGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, A.P.; Mullany, P. Oral Biofilms: A Reservoir of Transferable, Bacterial, Antimicrobial Resistance. Expert Rev. Anti Infect. Ther. 2010, 8, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral Microbiome and Health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I. Biofilm-Specific Antibiotic Tolerance and Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Truong, D.T.; Franzosa, E.A.; Tickle, T.L.; Scholz, M.; Weingart, G.; Pasolli, E.; Tett, A.; Huttenhower, C.; Segata, N. MetaPhlAn2 for Enhanced Metagenomic Taxonomic Profiling. Nat. Methods 2015, 12, 902–903. [Google Scholar] [CrossRef]
- Bessa, L.J.; Botelho, J.; Machado, V.; Alves, R.; Mendes, J.J. Managing Oral Health in the Context of Antimicrobial Resistance. Int. J. Environ. Res. Public Health 2022, 19, 16448. [Google Scholar] [CrossRef]
- Kang, Y.; Sun, B.; Chen, Y.; Lou, Y.; Zheng, M.; Li, Z. Dental Plaque Microbial Resistomes of Periodontal Health and Disease and Their Changes after Scaling and Root Planing Therapy. mSphere 2021, 6, e0016221. [Google Scholar] [CrossRef]
- Sukumar, S.; Roberts, A.P.; Martin, F.E.; Adler, C.J. Metagenomic Insights into Transferable Antibiotic Resistance in Oral Bacteria. J. Dent. Res. 2016, 95, 969–976. [Google Scholar] [CrossRef]
- Anderson, A.C.; von Ohle, C.; Frese, C.; Boutin, S.; Bridson, C.; Schoilew, K.; Peikert, S.A.; Hellwig, E.; Pelz, K.; Wittmer, A.; et al. The Oral Microbiota Is a Reservoir for Antimicrobial Resistance: Resistome and Phenotypic Resistance Characteristics of Oral Biofilm in Health, Caries, and Periodontitis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 37. [Google Scholar] [CrossRef]
- Sukumar, S.; Wang, F.; Simpson, C.A.; Willet, C.E.; Chew, T.; Hughes, T.E.; Bockmann, M.R.; Sadsad, R.; Martin, F.E.; Lydecker, H.W.; et al. Development of the Oral Resistome during the First Decade of Life. Nat. Commun. 2023, 14, 1291. [Google Scholar] [CrossRef]
- Sukumar, S.; Rahmanyar, Z.; El Jurf, H.Q.; Akil, W.S.; Hussain, J.; Elizabeth Martin, F.; Ekanayake, K.; Martinez, E. Mapping the Oral Resistome: A Systematic Review. J. Med. Microbiol. 2024, 73, 001866. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Bostanci, N.; Marsh, P.D.; Zaura, E. Applications of the Oral Microbiome in Personalized Dentistry. Arch. Oral Biol. 2019, 104, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Ma, J.; Kim, W.; Kim, J.; Belenky, P.; Lee, I. Genome-Resolved Metagenomics: A Game Changer for Microbiome Medicine. Exp. Mol. Med. 2024, 56, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.; Gonzalo, E.; Villagra, L.J.G.; Miegimolle, B.; Suarez, M.J. What Is the Prevalence of Peri-Implantitis? A Systematic Review and Meta-Analysis. BMC Oral Health 2022, 22, 449. [Google Scholar] [CrossRef]
- Dieckow, S.; Szafrański, S.P.; Grischke, J.; Qu, T.; Doll-Nikutta, K.; Steglich, M.; Yang, I.; Häussler, S.; Stiesch, M. Structure and Composition of Early Biofilms Formed on Dental Implants Are Complex, Diverse, Subject-Specific, and Dynamic. NPJ Biofilms Microbiomes 2024, 10, 155. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Charalampakis, G.; Bostanci, N.; Stadlinger, B. Peri-Implant Infections of Oral Biofilm Etiology. Adv. Exp. Med. Biol. 2015, 830, 69–84. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pisano, M.; Di Palo, M.P.; Franci, G.; Rupe, A.; Fiorino, A.; Rengo, C. Peri-Implantitis-Associated Microbiota Before and After Peri-Implantitis Treatment, the Biofilm “Competitive Balancing” Effect: A Systematic Review of Randomized Controlled Trials. Microorganisms 2024, 12, 1965. [Google Scholar] [CrossRef]
- Thompson, W.; Teoh, L.; Pulcini, C.; Williams, D.; Pitkeathley, C.; Carter, V.; Sanderson, S.; Torres, G.; Walsh, T. Dental Antibiotic Stewardship: Study Protocol for Developing International Consensus on a Core Outcome Set. Trials 2022, 23, 116. [Google Scholar] [CrossRef]
- Teoh, L.; Thompson, W.; Suda, K. Antimicrobial Stewardship in Dental Practice. J. Am. Dent. Assoc. 2020, 151, 589–595. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and Memory-Efficient Alignment of Short DNA Sequences to the Human Genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P.A. metaSPAdes: A New Versatile Metagenomic Assembler. Genome Res. 2017, 27, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. ABRicate: Mass Screening of Contigs for Antibiotic Resistance Genes. 2016. Available online: https://github.com/tseemann/abricate.
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and Model-Centric Curation of the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef]
- Feldgarden, M.; Brover, V.; Haft, D.H.; Prasad, A.B.; Slotta, D.J.; Tolstoy, I.; Tyson, G.H.; Zhao, S.; Hsu, C.H.; McDermott, P.F.; et al. Validating the AMRFinder Tool and Resistance Gene Database by Using Antimicrobial Resistance Genotype-Phenotype Correlations in a Collection of Isolates. Antimicrob. Agents Chemother. 2019, 63, e00483-19, Erratum in Antimicrob. Agents Chemother. 2020, 64, e00361-20. [Google Scholar] [CrossRef]
- Gupta, S.K.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, J.M. ARG-ANNOT: A New Bioinformatic Tool to Discover Antibiotic Resistance Genes in Bacterial Genomes. Antimicrob. Agents Chemother. 2014, 58, 212–220. [Google Scholar] [CrossRef]
- Bharat, A.; Petkau, A.; Avery, B.P.; Chen, J.C.; Folster, J.P.; Carson, C.A.; Kearney, A.; Nadon, C.; Mabon, P.; Thiessen, J.; et al. Correlation between Phenotypic and In Silico Detection of Antimicrobial Resistance in Salmonella enterica in Canada Using Staramr. Microorganisms 2022, 10, 292. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In Silico Detection and Typing of Plasmids Using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R Package Version 2.6-4. 2020. Available online: https://CRAN.R-project.org/package=vegan.
- Heinze, G.; Ploner, M. logistf: Firth’s Bias-Reduced Logistic Regression. R Package Version 1.23.1. 2020. Available online: https://CRAN.R-project.org/package=logistf.
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/.
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2023; Available online: https://posit.co/.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Rofael, S.; Leboreiro Babe, C.; Davrandi, M.; Kondratiuk, A.L.; Cleaver, L.; Ahmed, N.; Atkinson, C.; McHugh, T.; Lowe, D.M. Antibiotic Resistance, Bacterial Transmission and Improved Prediction of Bacterial Infection in Patients with Antibody Deficiency. JAC Antimicrob. Resist. 2023, 5, dlad135. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, P.A.; Zhang, T.; Järhult, J.D.; Joffré, E.; Wang, H. Heterogeneity and Metabolic Diversity Among Enterococcus Species During Long-Term Colonization. bioRxiv 2024. [Google Scholar] [CrossRef]
- WHO. Critically Important Antimicrobials for Human Medicine: 6th Revision; World Health Organisation: Geneva, Switzerland, 2019. [Google Scholar]
- Fox, V.; Santoro, F.; Pozzi, G.; Iannelli, F. Predicted Transmembrane Proteins with Homology to Mef(A) Are Not Responsible for Complementing mef(A) Deletion in the mef(A)–msr(D) Macrolide Efflux System in Streptococcus pneumoniae. BMC Res. Notes 2021, 14, 432. [Google Scholar] [CrossRef] [PubMed]
- Vitali, L.A.; Di Luca, M.C.; Prenna, M.; Petrelli, D. Correlation Between Genetic Features of the mef(A)–msr(D) Locus and Erythromycin Resistance in Streptococcus pyogenes. Diagn. Microbiol. Infect. Dis. 2016, 84, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, F.; Santoro, F.; Santagati, M.; Docquier, J.-D.; Lazzeri, E.; Pastore, G.; Cassone, M.; Oggioni, M.R.; Rossolini, G.M.; Stefani, S.; et al. Type M Resistance to Macrolides Is Due to a Two-Gene Efflux Transport System of the ATP-Binding Cassette (ABC) Superfamily. Front. Microbiol. 2018, 9, 1670. [Google Scholar] [CrossRef]
- Correia, F.; Ribeiro-Vidal, H.; Gouveia, S.; Faria Almeida, R. Prescription of Antibiotic Prophylaxis in Implant Placement Among Portuguese Dentists: A Web Survey. Clin. Oral Implants Res. 2024, 35, 242–250. [Google Scholar] [CrossRef]
- Becker, K.; Gurzawska-Comis, K.; Klinge, B.; Lund, B.; Brunello, G. Patterns of Antibiotic Prescription in Implant Dentistry and Antibiotic Resistance Awareness Among European Dentists: A Questionnaire-Based Study. Clin. Oral Implants Res. 2024, 35, 771–780. [Google Scholar] [CrossRef]
- Gager, Y.; Koppe, J.; Vogl, I.; Gabert, J.; Jentsch, H. Antibiotic Resistance Genes in the Subgingival Microbiome and Implications for Periodontitis Therapy. J. Periodontol. 2023, 94, 1295–1301. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; Accolti, M.D.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the Oral Microbiome by Whole-Genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Abramova, A.; Karkman, A.; Bengtsson-Palme, J. Metagenomic Assemblies Tend to Break around Antibiotic Resistance Genes. BMC Genom. 2024, 25, 959. [Google Scholar] [CrossRef]
- De Abreu, V.A.C.; Perdigão, J.; Almeida, S. Metagenomic Approaches to Analyze Antimicrobial Resistance: An Overview. Front. Genet. 2021, 11, 575592. [Google Scholar] [CrossRef] [PubMed]
- Iwahara, K.; Kuriyama, T.; Shimura, S.; Williams, D.W.; Yanagisawa, M.; Nakagawa, K.; Karasawa, T. Detection of cfxA and cfxA2, the β-Lactamase Genes of Prevotella spp., in Clinical Samples from Dentoalveolar Infection by Real-Time PCR. J. Clin. Microbiol. 2006, 44, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Hayashi, M.; Goto, T.; Muto, Y.; Tanaka, K. Identification of cfxA Gene Variants and Susceptibility Patterns in β-Lactamase-Producing Prevotella Strains. Anaerobe 2023, 79, 102688. [Google Scholar] [CrossRef] [PubMed]
- Serwold-Davis, T.M.; Groman, N.B. Identification of a Methylase Gene for Erythromycin Resistance Within the Sequence of a Spontaneously Deleting Fragment of Corynebacterium diphtheriae Plasmid pNG2. FEMS Microbiol. Lett. 1988, 56, 7–13. [Google Scholar] [CrossRef]
- Trieu-Cuot, P.; Gerbaud, G.; Lambert, T.; Courvalin, P. In Vivo Transfer of Genetic Information Between Gram-Positive and Gram-Negative Bacteria. EMBO J. 1985, 4, 3583–3587. [Google Scholar] [CrossRef]
- Fu, C.X.; Chen, C.; Xiang, Q.; Wang, Y.F.; Wang, L.; Qi, F.Y.; Zhu, D.; Li, H.Z.; Cui, L.; Hong, W.L.; et al. Antibiotic Resistance at Environmental Multi-Media Interfaces Through Integrated Genotype and Phenotype Analysis. J. Hazard. Mater. 2024, 480, 136160. [Google Scholar] [CrossRef]
- Scannapieco, F.A. Role of Oral Bacteria in Respiratory Infection. J. Periodontol. 1999, 70, 793–802. [Google Scholar] [CrossRef]
- Han, Y.W.; Wang, X. Mobile Microbiome: Oral Bacteria in Extra-Oral Infections and Inflammation. J. Dent. Res. 2013, 92, 485–491. [Google Scholar] [CrossRef]
- Molina, A.; Huck, O.; Herrera, D.; Montero, E. The Association Between Respiratory Diseases and Periodontitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2023, 50, 842–887. [Google Scholar] [CrossRef]
- Sánchez-Peña, M.K.; Orozco-Restrepo, L.A.; Suárez-Brochero, Ó.F.; Barrios-Arroyave, F.A. Association Between Oral Health, Pneumonia and Mortality in Patients of Intensive Care. Rev. Med. Inst. Mex. Seguro Soc. 2020, 58, 468–476. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessa, L.J.; Egas, C.; Botelho, J.; Machado, V.; Alcoforado, G.; Mendes, J.J.; Alves, R. Unveiling the Resistome Landscape in Peri-Implant Health and Disease. J. Clin. Med. 2025, 14, 931. https://doi.org/10.3390/jcm14030931
Bessa LJ, Egas C, Botelho J, Machado V, Alcoforado G, Mendes JJ, Alves R. Unveiling the Resistome Landscape in Peri-Implant Health and Disease. Journal of Clinical Medicine. 2025; 14(3):931. https://doi.org/10.3390/jcm14030931
Chicago/Turabian StyleBessa, Lucinda J., Conceição Egas, João Botelho, Vanessa Machado, Gil Alcoforado, José João Mendes, and Ricardo Alves. 2025. "Unveiling the Resistome Landscape in Peri-Implant Health and Disease" Journal of Clinical Medicine 14, no. 3: 931. https://doi.org/10.3390/jcm14030931
APA StyleBessa, L. J., Egas, C., Botelho, J., Machado, V., Alcoforado, G., Mendes, J. J., & Alves, R. (2025). Unveiling the Resistome Landscape in Peri-Implant Health and Disease. Journal of Clinical Medicine, 14(3), 931. https://doi.org/10.3390/jcm14030931










