Intubation Versus Tracheotomy Outcomes in Critically Ill COVID-19 Patients in Low-Resource Settings: What Do We Know?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, Population
2.2. Percutaneous Tracheotomy Procedure
2.3. Primary Outcome
2.4. Secondary Outcome
2.5. Statistical Analysis
3. Results
3.1. Study Population
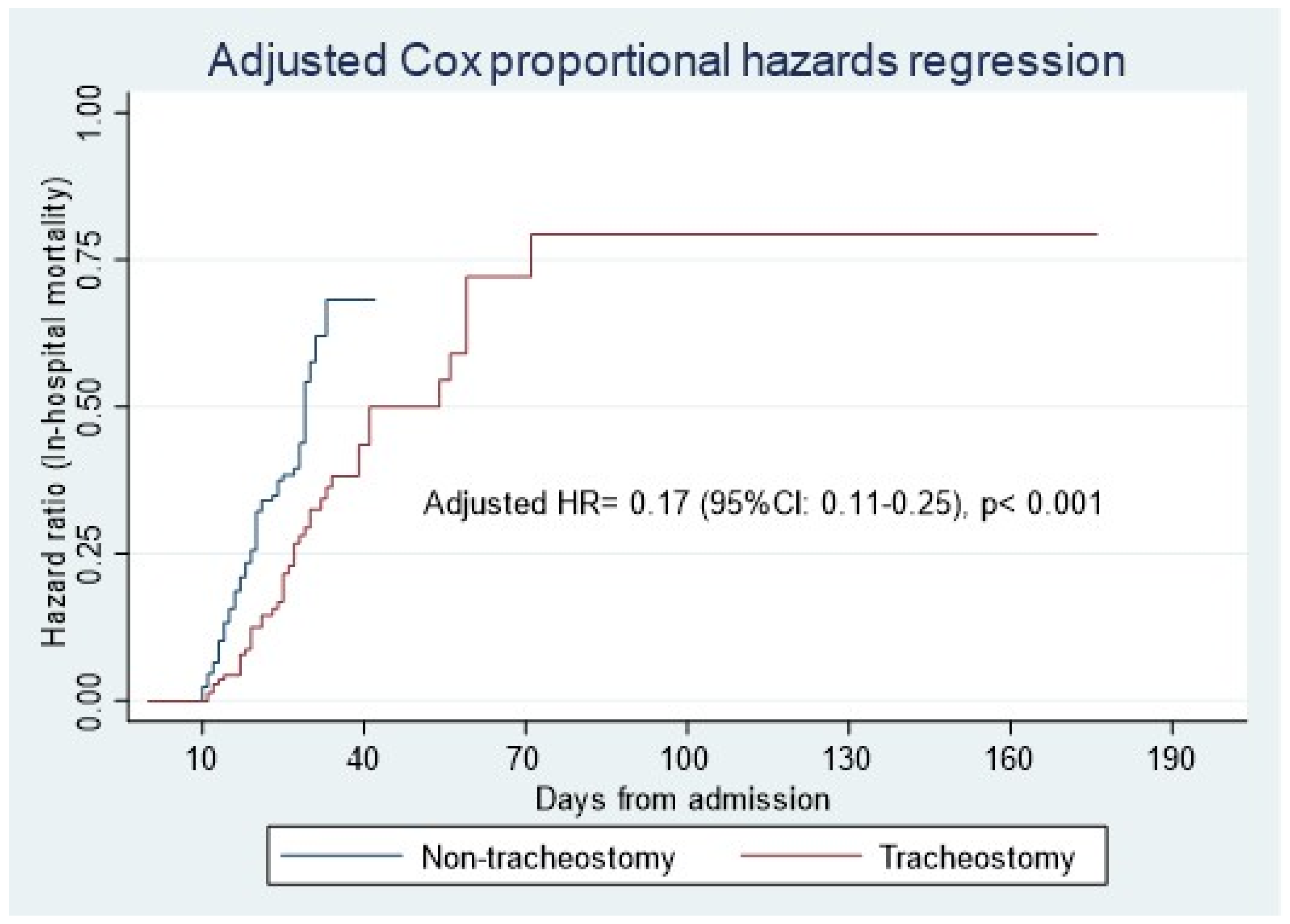
3.2. Primary Outcome
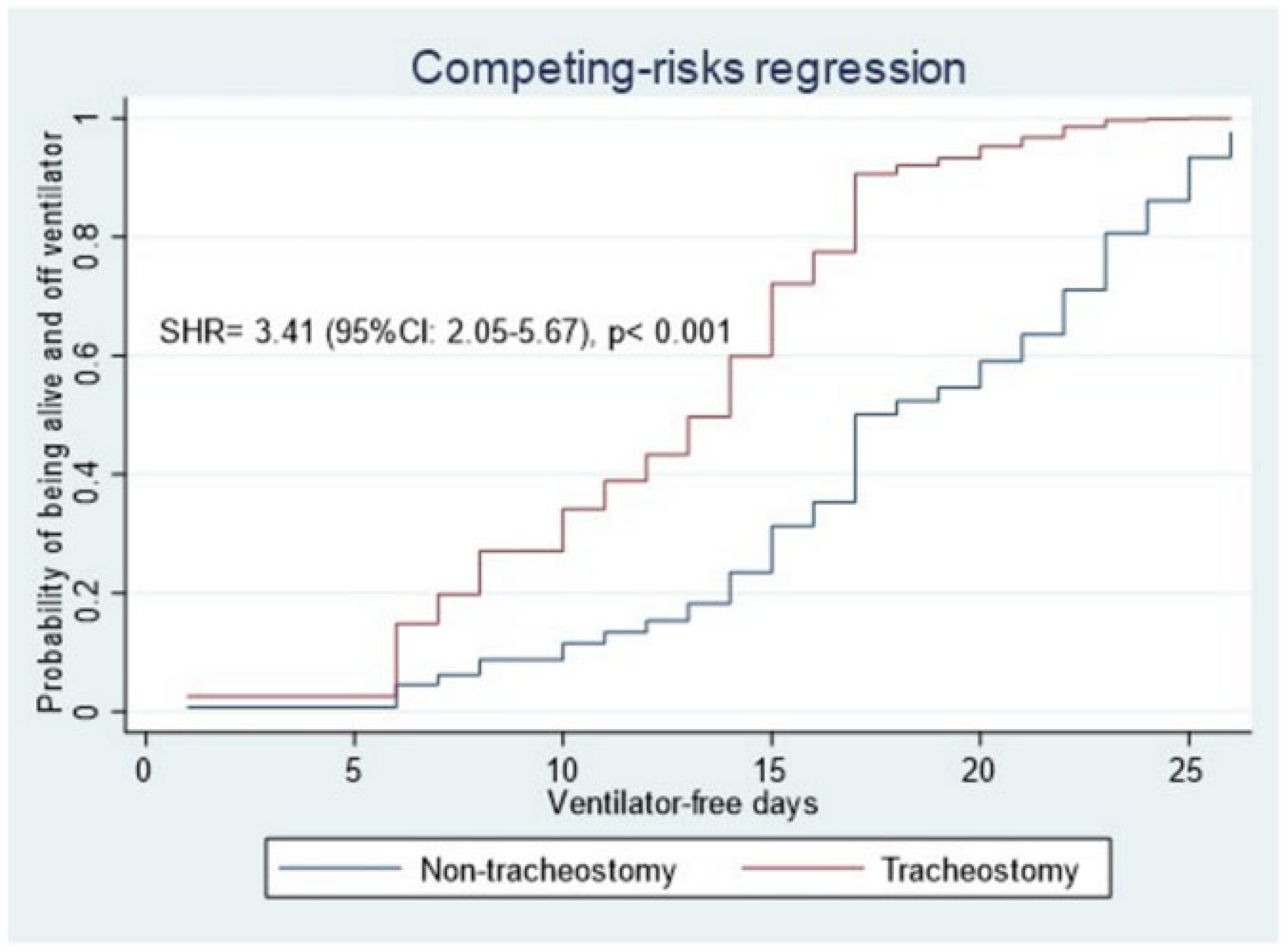
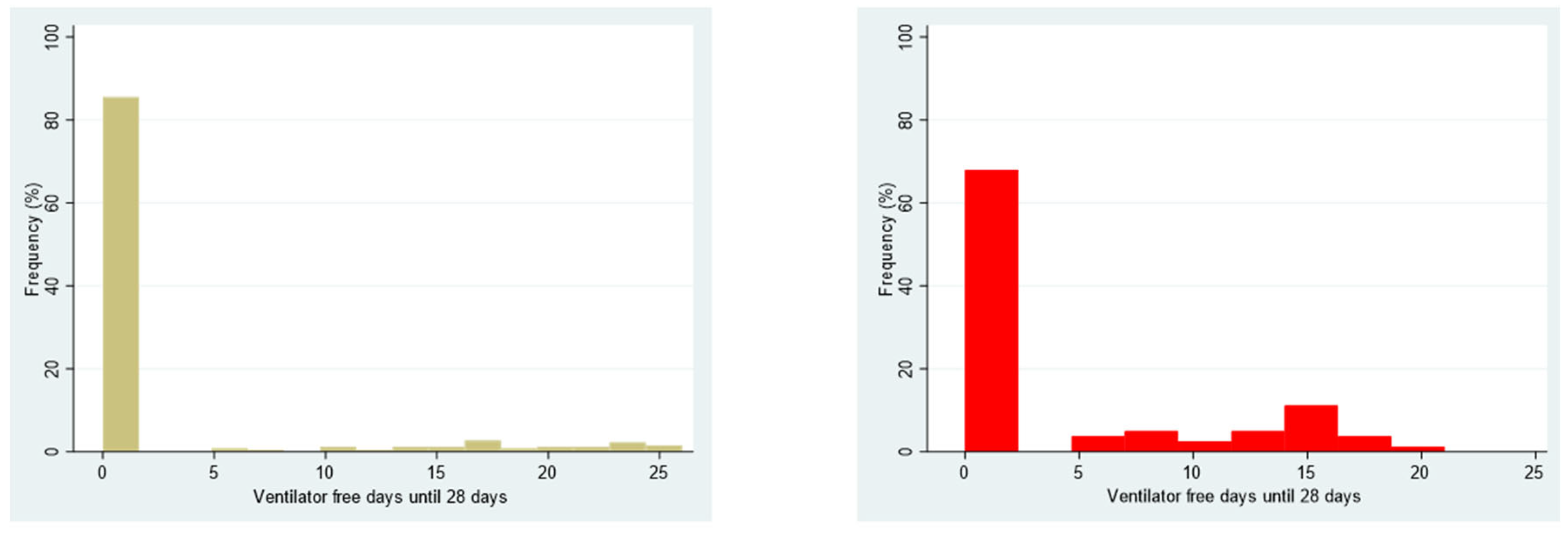
3.3. Secondary Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ranieri, V.M.; Rubenfeld, G.D.; Taylor Thompson, B.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Čučković, M.; Drmić, Ž.; Pražetina, M.; Tipura, D.; Ćurčić, M.; Miko, I.; Mihelčić, A.; Romić, A.; Kukoč, A.; Blagaj, V.; et al. Epidemiological characteristics, baseline clinical features, and outcomes of critically ill patients treated in a coronavirus disease 2019 tertiary center in continental Croatia. Croat. Med J. 2022, 63, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kovacevic, P. Can the terms “low resource setting” and “low-income country” be used interchangeably in the context of intensive care medicine? Intensive Care Med. 2023, 49, 1274–1275. [Google Scholar] [CrossRef]
- Cobb, N.L.; Sathe, N.A.; Duan, K.I.; Seitz, K.P.; Thau, M.R.; Sung, C.C.; Morrell, E.D.; Mikacenic, C.; Kim, H.N.; Liles, W.C.; et al. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Ann. Am. Thorac. Soc. 2021, 18, 632–640. [Google Scholar] [CrossRef]
- Durbin, C.G., Jr. Tracheostomy: Why, when, and how? Respir. Care 2010, 55, 1056–1068. [Google Scholar]
- Cheung, N.H.; Napolitano, L.M. Tracheostomy: Epidemiology, indications, timing, technique, and outcomes. Respir. Care 2014, 59, 895–915, discussion 916–919. [Google Scholar] [CrossRef]
- Brass, P.; Hellmich, M.; Ladra, A.; Ladra, J.; Wrzosek, A. Percutaneous techniques versus surgical techniques for tracheostomy. Cochrane Database Syst. Rev. 2016, 7, CD008045. [Google Scholar] [CrossRef]
- Fernandez-Bussy, S.; Mahajan, B.; Folch, E.; Caviedes, I.; Guerrero, J.; Majid, A. Tracheostomy tube placement: Early and late complications. J. Bronchol. Interv. Pulmonol. 2015, 22, 357–364. [Google Scholar] [CrossRef]
- Delaney, A.; Bagshaw, S.M.; Nalos, M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: A systematic review and meta-analysis. Crit. Care 2006, 10, R55. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.J.; Wolff, C.J.; Bechtold, H.M.; Free, D.; Lorenzo, J.; Minot, P.R.; Maggio, P.G.; Spain, D.A.; Weiser, T.G.; Forrester, J.D. Modified percutaneous tracheostomy in patients with COVID-19. Trauma Surg. Acute Care Open 2020, 5, e000625. [Google Scholar] [CrossRef] [PubMed]
- Staibano, P.; Levin, M.; McHugh, T.; Gupta, M.; Sommer, D.D. Association of tracheostomy with outcomes in patients with COVID-19 and SARS-CoV-2 transmission among health care professionals: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021, 147, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Livneh, N.; Mansour, J.; Kassif Lerner, R.; Feinmesser, G.; Alon, E. Early vs. late tracheostomy in ventilated COVID-19 patients—A retrospective study. Am. J. Otolaryngol. 2021, 42, 103102. [Google Scholar] [CrossRef]
- Rozenblat, T.; Reifen, E.; Benov, A.; Shaul, C.; Neuman, U.; Karol, D.; Schvartz, R.; Bachar, G. The value of tracheostomy of critically ill COVID-19 patients—A multicentral study. Am. J. Otolaryngol. 2022, 43, 103230. [Google Scholar] [CrossRef]
- Flinspach, A.N.; Booke, H.; Zacharowski, K.; Balaban, Ü.; Herrmann, E.; Adam, E.H. Association of mortality and early tracheostomy in patients with COVID-19: A retrospective analysis. Sci. Rep. 2022, 12, 15406. [Google Scholar] [CrossRef]
- Ji, Y.; Fang, Y.; Cheng, B.; Li, L.; Fang, X. Tracheostomy timing and clinical outcomes in ventilated COVID-19 patients: A systematic review and meta-analysis. Crit Care 2022, 26, 40. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, Y.; Zhu, F.; Yang, X.; Huang, C.; Hou, G.; Xu, W.; Hu, M.; Zhang, L.; Cheng, A.; et al. Tracheostomy in 80 COVID-19 patients: A multicenter, retrospective, observational study. Front. Med. 2020, 7, 615845. [Google Scholar] [CrossRef]
- Kovacevic, P.; Meyer, F.J.; Gajic, O. Challenges, obstacles, and unknowns in implementing principles of modern intensive care medicine in low-resource settings: An insider’s perspective. Intensive Care Med. 2024, 50, 141–143. [Google Scholar] [CrossRef]
- Kovacevic, P.; Meyer, F.J.; Gajic, O. Successful implementation of modern critical care in the low-resources country Bosnia and Herzegovina: Single-center experience. Med. Klin. Intensivmed. Notfmed. 2021, 117, 269–275. [Google Scholar] [CrossRef]
- Kovacevic, P.; Djajic, V.; Momcicevic, D.; Zlojutro, B.; Jandric, M.; Kovacevic, T.; Latinovic, M.; Seranic, A.; Bokonjic, D.; Skrbic, R.; et al. Boosting ICU capacity during the COVID-19 pandemic in the western Balkan region, The Republic of Srpska experience. J. Public Health Res. 2023, 12, 22799036231151762. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.; Mehta, Y. Percutaneous tracheostomy. Ann. Card. Anaesth. 2017, 20, S19–S25. [Google Scholar] [CrossRef] [PubMed]
- Ghattas, C.; Alsunaid, S.; Pickering, E.M.; Holden, V.K. State of the art: Percutaneous tracheostomy in the intensive care unit. J. Thorac. Dis. 2021, 13, 5261–5276. [Google Scholar] [CrossRef]
- Yehya, N.; Harhay, M.O.; Curley, M.A.Q.; Schoenfeld, D.A.; Reeder, R.W. Reappraisal of Ventilator-Free Days in Critical Care Research. Am. J. Respir. Crit. Care Med. 2019, 200, 828–836. [Google Scholar] [CrossRef]
- Molin, N.; Myers, K.; Soliman, A.M.S.; Schmalbach, C.E. COVID-19 tracheostomy outcomes. Otolaryngol. Head. Neck Surg. 2022, 167, 923–928. [Google Scholar] [CrossRef]
- Alnemri, A.; Ricciardelli, K.; Wang, S.; Baumgartner, M.; Chao, T.N. Tracheostomy is associated with decreased in-hospital mortality during severe COVID-19 infection. World J. Otorhinolaryngol. Head. Neck Surg. 2023, 10, 253–260. [Google Scholar] [CrossRef]
- Corona, A.; De Santis, V.; Vitale, D.; Nencini, C.; Potalivo, A.; Prete, A.; Barzaghi, N.; Tardivo, S.; Terzitta, M.; Malfatto, A.; et al. Tracheostomy in critically ill patients with SARS 2 COVID-19 infection: A prospective observational multi-center study of short- and long-term outcomes. Can. J. Respir. Ther. 2022, 58, 155–161. [Google Scholar] [CrossRef]
- Romero, C.M.; Gajardo, A.I.; Cruz, A.; Tobar, E.; Godoy, J.; Medel, N.; Zamorano, R.; Rappoport, D.; Rojas, V.; Herrera, M.C.; et al. Mortality in patients with severe COVID-19 who underwent tracheostomy due to prolonged mechanical ventilation. Rev. Medica Chil. 2023, 151, 151–159. [Google Scholar] [CrossRef]
- Battaglini, D.; Premraj, L.; White, N.; Sutt, A.L.; Robba, C.; Cho, S.M.; Di Giacinto, I.; Bressan, F.; Sorbello, M.; Cuthbertson, B.H.; et al. Tracheostomy outcomes in critically ill patients with COVID-19: A systematic review, meta-analysis, and meta-regression. Br. J. Anaesth. 2022, 129, 679–692. [Google Scholar] [CrossRef]
- Hernandez, G.; Ramos, F.J.; Añon, J.M.; Ortiz, R.; Colinas, L.; Masclans, J.R.; De Haro, C.; Ortega, A.; Peñuelas, O.; Cruz-Delgado, M.D.M.; et al. Early Tracheostomy for Managing ICU Capacity During the COVID-19 Outbreak: A Propensity-Matched Cohort Study. Chest 2022, 161, 121–129. [Google Scholar] [CrossRef]
- Taha, M.; Elahi, M.; Wahby, K.; Samavati, L. Incidence and risk factors of COVID-19 associated pneumothorax. PLoS ONE 2022, 17, e0271964. [Google Scholar] [CrossRef] [PubMed]
- Jirak, P.; Mirna, M.; Van Almsick, V.; Shomanova, Z.; Mahringer, M.; Lichtenauer, M.; Kopp, K.; Topf, A.; Sieg, F.; Kraus, J.; et al. Gender-Specific Differences in the Intensive Care Treatment of COVID-19 Patients. J. Pers. Med. 2022, 12, 849. [Google Scholar] [CrossRef] [PubMed]
- Fabião, J.; Sassi, B.; Pedrollo, E.F.; Gerchman, F.; Kramer, C.K.; Leitão, C.B.; Pinto, L.C. Why do men have worse COVID-19-related outcomes? A systematic review and meta-analysis with sex adjusted for age. Braz. J. Med Biol. Res. 2022, 55, e11711. [Google Scholar] [CrossRef]
- Taniguchi, L.U.; Aliberti, M.J.R.; Dias, M.B.; Jacob-Filho, W.; Avelino-Silva, T.J. Twelve Months and Counting: Following Clinical Outcomes in Critical COVID-19 Survivors. Ann. Am. Thorac. Soc. 2023, 20, 289–295. [Google Scholar] [CrossRef]
- Gmanyami, J.M.; Quentin, W.; Lambert, O.; Jarynowski, A.; Belik, V.; Amuasi, J.H. Excess mortality during the COVID-19 pandemic in low-and lower-middle-income countries: A systematic review and meta-analysis. BMC Public Health. 2024, 24, 1643. [Google Scholar] [CrossRef]
- Kovacevic, P.; Vidovic, J.; Tomic, B.; Mallat, J.; Hssain, A.A.; Rotimi, M.; Akindele, O.T.; Doi, K.; Mishra, R.; Meyer, F.J.; et al. Consensus statements for the establishment of medical intensive care in low-resource settings: International study using modified Delphi methodology. Crit. Care 2024, 28, 323. [Google Scholar] [CrossRef]
- Cagino, L.M.; Kercheval, J.B.; Kenes, M.T.; McSparron, J.I.; Blank, R.; Chinn, S.B.; Claar, D.D.; Iwashyna, T.J.; De Cardenas, J. Association of Tracheostomy with Changes in Sedation during COVID-19: A Quality Improvement Evaluation at the University of Michigan. Ann. Am. Thorac. Soc. 2021, 18, 907–909. [Google Scholar] [CrossRef]
- Sutt, A.L.; Tronstad, O.; Barnett, A.G.; Kitchenman, S.; Fraser, J.F. Earlier tracheostomy is associated with an earlier return to walking, talking, and eating. Aust. Crit. Care 2020, 33, 213–218. [Google Scholar] [CrossRef]

| All Patients (n = 343) | Non-Tracheotomy (n = 262) | Tracheotomy (n = 81) | p-Value | |
|---|---|---|---|---|
| Age, y | 64 [56–71] | 64 [57–72] | 64 [53–69] | 0.160 |
| Male, n (%) | 230 (67.1) | 183 (69.5) | 47 (58.0) | 0.048 |
| Diabetes, n (%) | 103 (30.0) | 81 (30.9) | 22 (27.2) | 0.52 |
| Chronic heart failure, n (%) | 154 (44.9) | 122 (46.6) | 32 (39.5) | 0.26 |
| Chronic lung disease, n (%) | 18 (5.2) | 9 (3.4) | 9 (11.1) | 0.02 |
| Chronic liver disease, n (%) | 8 (2.3) | 7 (2.7) | 1 (1.2) | 0.69 |
| Chronic kidney disease, n (%) | 22 (6.4) | 13 (5.0) | 9 (11.1) | 0.07 |
| Obesity, n (%) | 42 (12.2) | 33 (12.6) | 9 (11.1) | 0.85 |
| Rheumatologic disease, n (%) | 16 (4.7) | 13 (5.0) | 3 (3.7) | 0.77 |
| Hypothyroid, n (%) | 19 (5.5) | 16 (6.1) | 3 (3.7) | 0.58 |
| Malignancy, n (%) | 15 (4.6) | 12 (4.8) | 3 (3.7) | 1.00 |
| Time from symptoms to hospital admission, days | 6 [4–8] | 6 [4–8] | 6 [3–7] | 0.21 |
| Time from hospital to ICU admission, days | 4 [1–7] | 4 [1–7] | 4 [1–6.5] | 0.57 |
| SOFA score | 4 [2–7] | 4 [2–7] | 4 [2–7] | 0.84 |
| SAPS II score | 27 [18–39] | 28 [19–39] | 24 [18–37] | 0.11 |
| Tocilizumab, n (%) | 14 (4.1) | 11 (4.2) | 3 (3.7) | 1.00 |
| Corticosteroids, n (%) | 0.39 | |||
| Methylprednisolone | 283 (82.5) | 220 (84.0) | 63 (77.8) | |
| Dexamethasone | 44 (12.8) | 31 (11.8) | 13 (16.0) | |
| None | 16 (4.7) | 11 (4.2) | 5 (6.2) | |
| C-reactive protein, mg/L | 96.1 [43.8–169.0] | 100.0 [43.8–171.0] | 87.0 [45.0–147.0] | 0.44 |
| Procalcitonin, ng/mL | 0.2 [0.1–0.6] | 0.2 [0.1–0.6] | 0.2 [0.1–0.7] | 0.83 |
| Leucocyte count, ×109/L | 11.6 [8.4–16.3] | 11.7 [8.4–16.7] | 11.5 [8.4–15.1] | 0.54 |
| Lymphocyte count, ×109/L | 0.65 [0.42–0.98] | 0.63 [0.41–0.98] | 0.71 [0.50–0.90] | 0.26 |
| Neutrophil count, ×109/L | 9.8 [6.6–13.7] | 9.8 [6.6–13.7] | 9.7 [6.5–13.9] | 0.91 |
| D-dimer, µg/mL | 2.5 [1.2–10.6] | 2.5 [1.1–10.0] | 2.0 [1.2–11.7] | 0.92 |
| Lactate dehydrogenase, U/L | 540 [413–695] | 552 [441–701] | 487 [324–674] | 0.04 |
| Fibrinogen, g/L | 5.2 [3.9–6.1] | 5.3 [3.8–6.2] | 4.8 [3.9–5.5] | 0.04 |
| Lactate, mmol/L | 2.0 [1.5–2.6] | 2.0 [1.5–2.6] | 1.8 [1.3–2.5] | 0.03 |
| Acute kidney injury, n (%) | 110 (32.1) | 86 (32.8) | 24 (29.6) | 0.59 |
| Continuous renal replacement therapy, n (%) | 62 (18.1) | 44 (16.8) | 18 (22.2) | 0.27 |
| Cytosorb filter, n (%) | 15 (4.4) | 5 (1.9) | 10 (12.3) | <0.001 |
| Deep venous thromboembolism, n (%) | 7 (2.0) | 7 (2.7) | 0 (0.0) | 0.20 |
| Pulmonary embolism, n (%) | 15 (4.4) | 11 (4.2) | 4 (4.9) | 0.76 |
| Pneumothorax, n (%) | 22 (6.4) | 11 (4.2) | 11 (13.6) | 0.007 |
| Non-Survival (n = 268) | Survival (n = 75) | p-Value | |
|---|---|---|---|
| Age, y | 64 [57–72] | 64 [54–70] | 0.37 |
| Male, n (%) | 178 (66.4) | 52 (69.3) | 0.63 |
| Diabetes, n (%) | 75 (28.0) | 28 (37.3) | 0.12 |
| Chronic heart failure, n (%) | 119 (44.4) | 35 (46.7) | 0.73 |
| Chronic lung disease, n (%) | 12 (4.5) | 6 (8.0) | 0.24 |
| Chronic liver disease, n (%) | 6 (2.2) | 2 (2.7) | 0.69 |
| Chronic kidney disease, n (%) | 11 (4.1) | 11 (14.7) | 0.002 |
| Obesity, n (%) | 35 (13.1) | 7 (9.3) | 0.43 |
| Rheumatologic disease, n (%) | 13 (4.8) | 3 (4.0) | 1.00 |
| Hypothyroid, n (%) | 14 (5.2) | 5 (6.7) | 0.58 |
| Malignancy, n (%) | 13 (5.1) | 2 (2.7) | 0.53 |
| Time from symptoms to hospital admission, days | 6 [4–8] | 6 [4–10] | 0.55 |
| Time from hospital to ICU admission, days | 4 [1–7] | 3 [1–7] | 0.58 |
| SOFA score | 4 [2–7] | 4 [3–8] | 0.18 |
| SAPS II score | 27 [18–38] | 28 [18–39] | 0.97 |
| Tocilizumab, n (%) | 11 (4.1) | 3 (4.0) | 1.00 |
| Corticosteroids, n (%) | 0.02 | ||
| Methylprednisolone | 227 (84.7) | 56 (74.7) | |
| Dexamethasone | 33 (12.3) | 11 (14.7) | |
| None | 8 (3.0) | 8 (10.7) | |
| C-reactive protein, mg/L | 97 [43–169] | 85 [45–173] | 0.71 |
| Procalcitonin, ng/mL | 0.2 [0.1–0.6] | 0.2 [0.1–0.7] | 0.34 |
| Leucocyte count, ×109/L | 11.5 [8.3–16.2] | 11.9 [8.5–16.7] | 0.32 |
| Lymphocyte count, ×109/L | 0.64 [0.42–0.94] | 0.67 [0.41–1.08] | 0.58 |
| Neutrophile count, ×109/L | 9.7 [6.5–13.7] | 10.2 [7.4–13.6] | 0.32 |
| D-dimer, µg/mL | 2.5 [1.1–10.6] | 2.5 [1.2–10.6] | 0.90 |
| Lactate dehydrogenase, U/L | 554 [441–701] | 479 [309–680] | 0.006 |
| Fibrinogen, g/L | 5.3 [3.9–6.2] | 5.0 [3.9–5.7] | 0.25 |
| Lactate, mmol/L | 2.0 [1.5–2.7] | 1.8 [1.3–2.2] | 0.003 |
| Acute kidney injury, n (%) | 101 (37.7) | 9 (12.0) | <0.001 |
| Continuous renal replacement therapy, n (%) | 49 (18.3) | 13 (17.3) | 0.85 |
| Cytosorb filter, n (%) | 14 (5.3) | 1 (1.3) | 0.21 |
| Deep venous thromboembolism, n (%) | 5 (1.9) | 2 (2.7) | 0.65 |
| Pulmonary embolism, n (%) | 13 (4.8) | 2 (2.7) | 0.54 |
| Pneumothorax, n (%) | 19 (7.1) | 3 (4.0) | 0.43 |
| Tracheotomy, n (%) | 44 (16.4) | 37 (49.3) | <0.001 |
| Variable | Hazards Ratio (95% Confidence Interval) | p-Value |
|---|---|---|
| Tracheostomy (reference: non-tracheostomy) | 0.17 (0.11–0.25) | <0.001 |
| Age, years | 1.02 (1.00–1.03) | 0.006 |
| SAPS II score | 0.99 (0.98–1.00) | 0.19 |
| SOFA score | 1.02 (0.98–1.07) | 0.25 |
| Lactate | 1.01 (1.00–1.02) | 0.03 |
| Acute kidney injury | 0.91 (0.78–1.14) | 0.23 |
| Lactate dehydrogenase | 1.00 (0.99–1.00) | 0.06 |
| Corticosteroids | 1.01 (0.77–1.34) | 0.92 |
| Tocilizumab | 1.00 (0.53–1.90) | 0.99 |
| Gender | 1.11 (0.85–1.46) | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacevic, P.; Baric, G.; Dragic, S.; Momcicevic, D.; Zlojutro, B.; Jandric, M.; Kovacevic, T.; Lovric, D.; Palibrk, I.; Mallat, J. Intubation Versus Tracheotomy Outcomes in Critically Ill COVID-19 Patients in Low-Resource Settings: What Do We Know? J. Clin. Med. 2025, 14, 978. https://doi.org/10.3390/jcm14030978
Kovacevic P, Baric G, Dragic S, Momcicevic D, Zlojutro B, Jandric M, Kovacevic T, Lovric D, Palibrk I, Mallat J. Intubation Versus Tracheotomy Outcomes in Critically Ill COVID-19 Patients in Low-Resource Settings: What Do We Know? Journal of Clinical Medicine. 2025; 14(3):978. https://doi.org/10.3390/jcm14030978
Chicago/Turabian StyleKovacevic, Pedja, Goran Baric, Sasa Dragic, Danica Momcicevic, Biljana Zlojutro, Milka Jandric, Tijana Kovacevic, Daniel Lovric, Ivan Palibrk, and Jihad Mallat. 2025. "Intubation Versus Tracheotomy Outcomes in Critically Ill COVID-19 Patients in Low-Resource Settings: What Do We Know?" Journal of Clinical Medicine 14, no. 3: 978. https://doi.org/10.3390/jcm14030978
APA StyleKovacevic, P., Baric, G., Dragic, S., Momcicevic, D., Zlojutro, B., Jandric, M., Kovacevic, T., Lovric, D., Palibrk, I., & Mallat, J. (2025). Intubation Versus Tracheotomy Outcomes in Critically Ill COVID-19 Patients in Low-Resource Settings: What Do We Know? Journal of Clinical Medicine, 14(3), 978. https://doi.org/10.3390/jcm14030978








