Exploring Resting Sinus Tachycardia in Cancer Care: A Comprehensive Review
Abstract
1. Introduction
2. Methodology
3. Hemodynamic Variability in Cancer
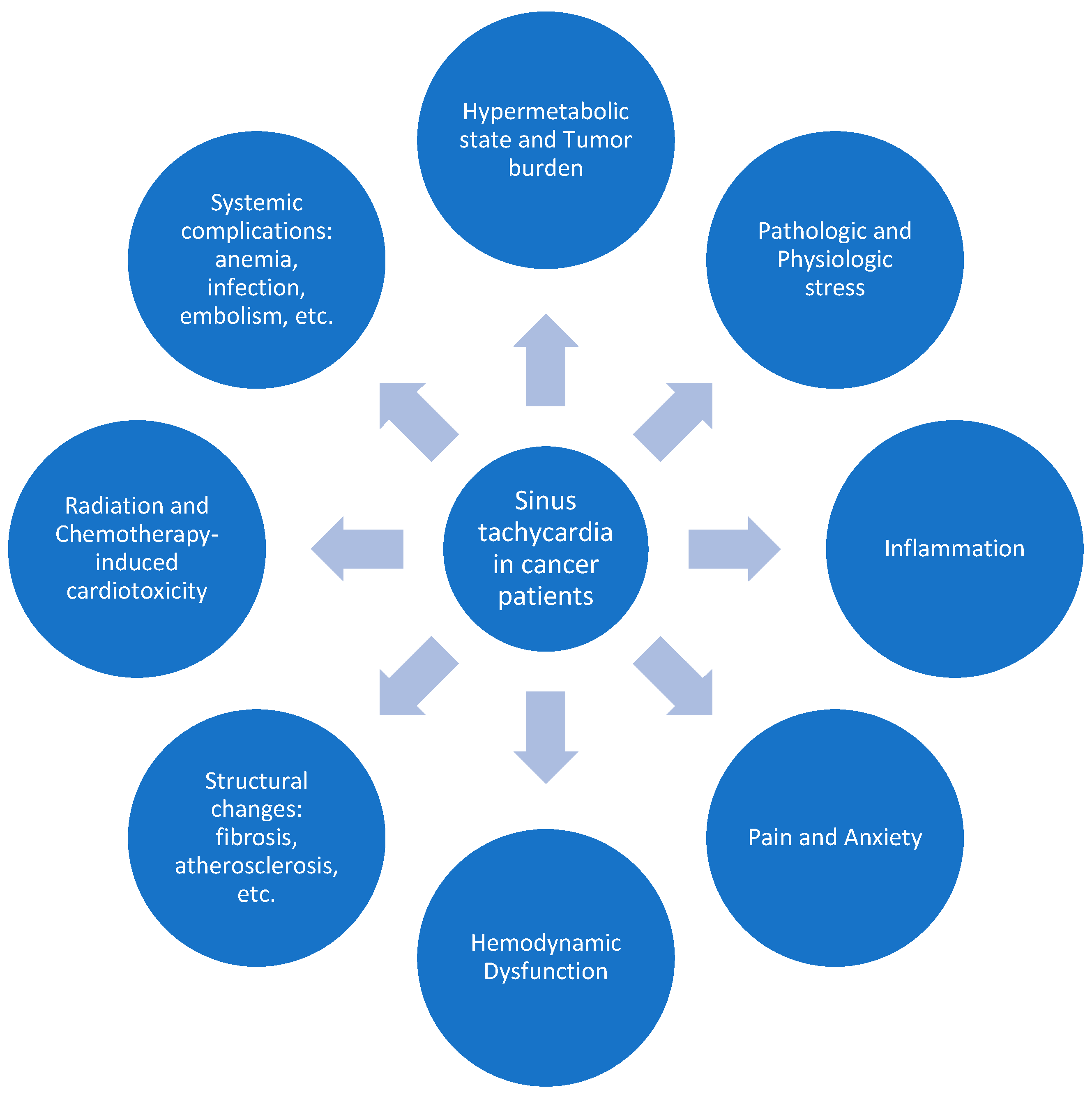
3.1. Sinus Tachycardia in Cancer
3.2. Association Between Resting Sinus Tachycardia and Cardiovascular Outcomes in Cancer Patients
3.3. Association Between Elevated Resting Heart Rate/Sinus Tachycardia and Overall Survival in Cancer Patients
4. Therapeutic Opportunities
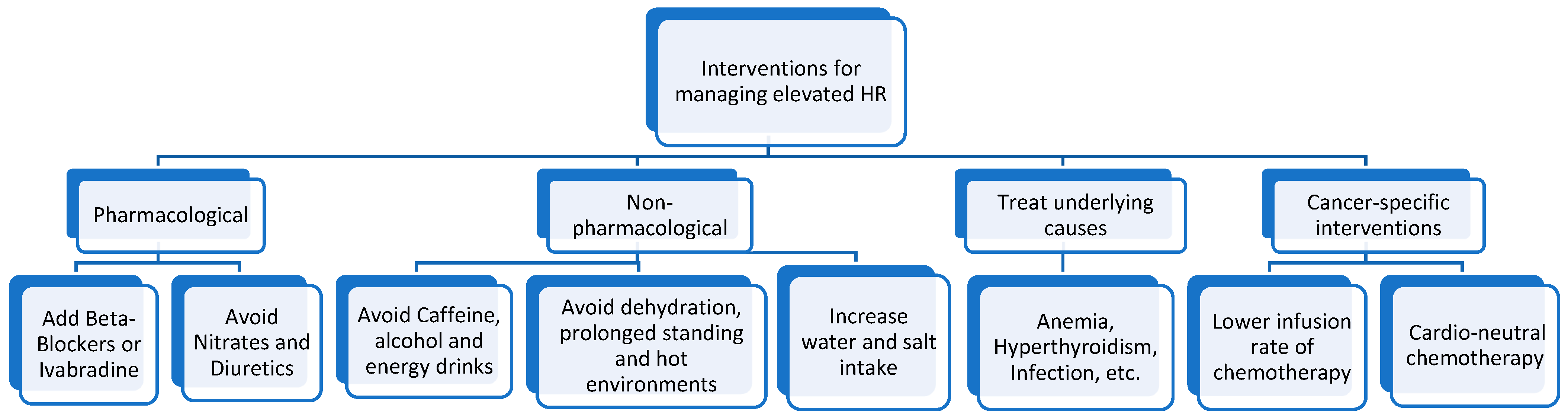
4.1. General Therapeutic Approaches for Managing Sinus Tachycardia
4.2. Cancer-Specific Therapeutic Approaches for Managing Resting Sinus Tachycardia
5. Knowledge Gaps and Further Research
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Davis, M.K.; Law, A.; Sulpher, J. Shared risk factors for cardiovascular disease and cancer: Implications for preventive health and clinical care in oncology patients. Can. J. Cardiol. 2016, 32, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Kobold, S. Inflammation: A common contributor to cancer, aging, and cardiovascular diseases—Expanding the concept of cardio-oncology. Cardiovasc. Res. 2019, 115, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Sueta, D.; Tabata, N.; Akasaka, T.; Yamashita, T.; Ikemoto, T.; Hokimoto, S. The dawn of a new era in onco-cardiology: The Kumamoto Classification. Int. J. Cardiol. 2016, 220, 837–841. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Hoffmeister, H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur. Heart J. 1997, 18, 1404–1410. [Google Scholar] [CrossRef]
- Kannel, W.B.; Kannel, C.; Paffenbarger, R.S., Jr.; Cupples, L. Heart rate and cardiovascular mortality: The Framingham study. Am. Heart J. 1987, 113, 1489–1494. [Google Scholar] [CrossRef]
- Lonn, E.M.; Rambihar, S.; Gao, P.; Custodis, F.F.; Sliwa, K.; Teo, K.K.; Yusuf, S.; Böhm, M. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: An analysis of ONTARGET/TRANSCEND. Clin. Res. Cardiol. 2014, 103, 149–159. [Google Scholar] [CrossRef]
- Opdahl, A.; Ambale Venkatesh, B.; Fernandes, V.R.; Wu, C.O.; Nasir, K.; Choi, E.Y.; Almeida, A.L.C.; Rosen, B.; Carvalho, B.; Lima, J.A.; et al. Resting heart rate as predictor for left ventricular dysfunction and heart failure: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2014, 63, 1182–1189. [Google Scholar] [CrossRef]
- Palatini, P. Heart Rate and the Cardiometabolic Risk. Curr. Hypertens. Rep. 2013, 15, 253–259. [Google Scholar] [CrossRef]
- Hori, M.; Okamoto, H. Heart rate as a target of treatment of chronic heart failure. J. Cardiol. 2012, 60, 86–90. [Google Scholar] [CrossRef]
- Julius, S.; Randall, O.S.; Esler, M.D.; Kashima, T.; Ellis, C.; Bennett, J. Altered cardiac responsiveness and regulation in the normal cardiac output type of borderlind hlpertension. Circ. Res. 1975, 36 (Suppl. S1), 199–207. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Levine, T.B.; Olivari, M.T.; Garberg, V.; Lura, D.; Francis, G.S.; Simon, A.B.; Rector, T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. New Engl. J. Med. 1984, 311, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Hemu, M.; Chiang, C.J.; Bhatt, P.K.; Ahmed, A.; Hein, K.Z.; Mourad, T.; Randall, M.E.; Palomo, A.P.; Kramer, J.B.; Fughhi, I.; et al. Associations between sinus tachycardia and adverse cardiovascular outcomes and mortality in cancer patients. J. Thorac. Dis. 2021, 13, 4845–4852. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Lainscak, M.; Kung, T.; Cramer, L.; Fülster, S.; Pelzer, U.; Hildebrandt, B.; Sandek, A.; Schefold, J.C.; Rauchhaus, M.; et al. Non-invasive assessment of cardiac hemodynamics in patients with advanced cancer and with chronic heart failure: A pilot feasibility study. Arch. Med. Sci. 2013, 2, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Geiger, S.; Stemmler, H.J.; Suhl, P.; Stieber, P.; Lange, V.; Baur, D.; Hausmann, A.; Tischer, J.; Horster, S. Anthracycline-induced cardiotoxicity: Cardiac monitoring by continuous wave-doppler ultrasound cardiac output monitoring and correlation to echocardiography. Onkologie 2012, 35, 241–246. [Google Scholar] [CrossRef]
- Trimarchi, G.; Carerj, S.; Di Bella, G.; Manganaro, R.; Pizzino, F.; Restelli, D.; Pelaggi, G.; Lofrumento, F.; Licordari, R.; Taverna, G.; et al. Clinical Applications of Myocardial Work in Echocardiography: A Comprehensive Review. J. Cardiovasc. Echogr. 2024, 34, 99–113. [Google Scholar] [CrossRef]
- Bhagat, A.; Kleinerman, E.S. Anthracycline-Induced Cardiotoxicity: Causes, Mechanisms, and Prevention. Adv. Exp. Med. Biol. 2020, 1257, 181–192. [Google Scholar] [CrossRef]
- Coumbe, B.G.T.; Groarke, J.D. Cardiovascular Autonomic Dysfunction in Patients with Cancer. Curr. Cardiol. Rep. 2018, 20, 69. [Google Scholar] [CrossRef]
- Navarro, X. Fisiologia del sistema nervioso autónomo [Physiology of the autonomic nervous system]. Rev. De Neurol. 2002, 35, 553–562. [Google Scholar] [CrossRef]
- Randall, D.C.; Brown, D.R.; McGuirt, A.S.; Thompson, G.W.; Armour, J.A.; Ardell, J.L. Interactions within the intrinsic cardiac nervous system contribute to chronotropic regulation. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R1066–R1075. [Google Scholar] [CrossRef]
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015, 7, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Stauss, H.M. Heart rate variability. Am. J. Physiol. Integr. Comp. Physiol. 2003, 285, R927–R931. [Google Scholar] [CrossRef] [PubMed]
- Savić, M.; Milivojević, M.; D’Onofrio, R. Changes in heart rate during activity and Recovery. Ital. J. Sports Rehabil. Posturology 2022, 9, 1–9. [Google Scholar]
- Sakellakis, M.; Reet, J.; Kladas, M.; Hoge, G.; Chalkias, A.; Radulovic, M. Cancer-Induced Resting Sinus Tachycardia: An Overlooked Clinical Diagnosis. Oncol. Rev. 2024, 18, 1439415. [Google Scholar] [CrossRef]
- Anker, M.S.; Frey, M.K.; Goliasch, G.; Bartko, P.E.; Prausmüller, S.; Gisslinger, H.; Kornek, G.; Strunk, G.; Raderer, M.; Zielinski, C.; et al. Increased resting heart rate and prognosis in treatment-naïve unselected cancer patients: Results from a prospective observational study. Eur. J. Heart Fail. 2020, 22, 1230–1238. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Tian, F.; Fang, F.; Shen, Q.; Ye, W.; Valdimarsdóttir, U.A.; Song, H. Stress-related disorders and subsequent cancer risk and mortality: A population-based and sibling-controlled cohort study in Sweden. Eur. J. Epidemiol. 2022, 37, 947–958. [Google Scholar] [CrossRef]
- Tian, F.; Shen, Q.; Hu, Y.; Ye, W.; Valdimarsdóttir, U.A.; Song, H.; Fang, F. Association of stress-related disorders with subsequent risk of all-cause and cause-specific mortality: A population-based and sibling-controlled cohort study. Lancet Reg. Health Eur. 2022, 18, 100402. [Google Scholar] [CrossRef]
- Jain, D.; Aronow, W. Cardiotoxicity of cancer chemotherapy in clinical practice. Hosp. Pract. 2019, 47, 6–15. [Google Scholar] [CrossRef]
- Vyas, D.; Laput, G.; Vyas, A. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. OncoTargets Ther. 2014, 7, 1015–1023. [Google Scholar] [CrossRef]
- Yang, R.; Tan, C.; Najafi, M. Cardiac inflammation and fibrosis following chemo/radiation therapy: Mechanisms and therapeutic agents. Inflammopharmacology 2021, 30, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.M.F.L. Effects of radiotherapy in coronary artery disease. Curr. Atheroscler. Rep. 2019, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hufnagle, J.J.; Andersen, S.N.; Maani, E.V. Radiation-Induced Cardiac Toxicity. In StatPearls [Internet]; Updated 29 May 2023; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Lee, M.S.; Finch, W.; Mahmud, E. Cardiovascular complications of radiotherapy. Am. J. Cardiol. 2013, 112, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Parikh, R.; Neilan, T.G. Cardiotoxicity of immune therapy. Cardiol. Clin. 2019, 37, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Lobenwein, D.; Kocher, F.; Dobner, S.; Gollmann-Tepeköylü, C.; Holfeld, J. Cardiotoxic mechanisms of cancer immunotherapy—A systematic review. Int. J. Cardiol. 2021, 323, 179–187. [Google Scholar] [CrossRef]
- Quagliariello, V.; Passariello, M.; Coppola, C.; Rea, D.; Barbieri, A.; Scherillo, M.; Monti, M.; Iaffaioli, R.; De Laurentiis, M.; Ascierto, P.; et al. Cardiotoxicity and pro-inflammatory effects of the immune checkpoint inhibitor Pembrolizumab associated to Trastuzumab. Int. J. Cardiol. 2019, 292, 171–179. [Google Scholar] [CrossRef]
- Lopez-Candales, A.; Mathur, P.; Mounsey, J.P.; Veeraputhiran, M. Is heart rate in post-hematopoietic stem cell transplant patients clinically relevant? Postgrad. Med. 2022, 134, 7–10. [Google Scholar] [CrossRef]
- Trimarchi, G.; Teresi, L.; Licordari, R.; Pingitore, A.; Pizzino, F.; Grimaldi, P.; Calabrò, D.; Liotta, P.; Micari, A.; de Gregorio, C.; et al. Transient Left Ventricular Dysfunction from Cardiomyopathies to Myocardial Viability: When and Why Cardiac Function Recovers. Biomedicines 2024, 12, 1051. [Google Scholar] [CrossRef]
- Anker, M.S.; Ebner, N.; Hildebrandt, B.; Springer, J.; Sinn, M.; Riess, H.; Anker, S.D.; Landmesser, U.; Haverkamp, W.; von Haehling, S. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer: Results of a prospective cardiovascular long-term study. Eur. J. Heart Fail. 2016, 18, 1524–1534. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, S.; Lim, S.M.; Lee, M.K.; Giovannucci, E.L.; Kim, J.H.; Kim, S.I.; Jeon, J.Y. Resting heart rate as a prognostic factor for mortality in patients with breast cancer. Breast Cancer Res. Treat. 2016, 159, 375–384. [Google Scholar] [CrossRef]
- Mędrek, S.; Szmit, S. Baseline Electrocardiographic and Echocardiographic Assessment May Help Predict Survival in Lung Cancer Patients—A Prospective Cardio-Oncology Study. Cancers 2022, 14, 2010. [Google Scholar] [CrossRef] [PubMed]
- American College of Cardiology. Tachycardia in Cancer Patients May Signal Increased Mortality Risk. 25 January 2019. Available online: https://www.acc.org/about-acc/press-releases/2019/01/25/14/19/tachycardia-in-cancer-patients-may-signal-increased-mortality-risk (accessed on 1 December 2024).
- Ahmed, A.; Pothineni, N.V.K.; Charate, R.; Garg, J.; Elbey, M.; de Asmundis, C.; LaMeir, M.; Romeya, A.; Shivamurthy, P.; Olshansky, B.; et al. Inappropriate Sinus Tachycardia: Etiology, Pathophysiology, and Management: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 2450–2462. [Google Scholar] [CrossRef] [PubMed]
- Ruzieh, M.; Moustafa, A.; Sabbagh, E.; Karim, M.M.; Karim, S. Challenges in Treatment of Inappropriate Sinus Tachycardia. Curr. Cardiol. Rev. 2018, 14, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Martino, A.; Rebecchi, M.; Sette, A.; Cicogna, F.; Politano, A.; Sgueglia, M.; de Ruvo, E.; Volterrani, M.; Calo’, L. Ivabradine versus bisoprolol in the treatment of inappropriate sinus tachycardia: A long-term follow-up study. J. Cardiovasc. Med. 2021, 22, 892–900. [Google Scholar] [CrossRef]
- Krahn, A.D.; Yee, R.; Klein, G.J.; Morillo, C. Inappropriate sinus tachycardia: Evaluation and therapy. J. Cardiovasc. Electrophysiol. 1995, 6, 1124–1128. [Google Scholar] [CrossRef]
- Ptaszynski, P.; Kaczmarek, K.; Ruta, J.; Klingenheben, T.; Wranicz, J.K. Metoprolol succinate vs. ivabradine in the treatment of inappropriate sinus tachycardia in patients unresponsive to previous pharmacological therapy. Europace 2013, 15, 116–121. [Google Scholar] [CrossRef]
- Olshansky, B.; Sullivan, R.M. Inappropriate sinus tachycardia. J. Am. Coll.Cardiol. 2013, 61, 793–801. [Google Scholar] [CrossRef]
- Haidous, M.; Al Armashi, A.R.; Balozian, P.; Ravakhah, K. A case of severe dilated cardiomyopathy and hyperthyroidism. Cureus 2022, 14, e22968. [Google Scholar] [CrossRef]
- Hoppe, U.C.; La Rosée, K.; Larbig, R.; Erdmann, E. Selective inhibition of the pacemaker channel If improves symptoms in severe dilated cardiomyopathy. Clin. Res. Cardiol. 2007, 96, 243–246. [Google Scholar] [CrossRef]
- Hori, M.; Imamura, T.; Kinugawa, K. Implication of heart rate optimization in patients with heart failure. J. Cardiol. Cases 2021, 23, 163–165. [Google Scholar] [CrossRef]
- Inamori, T.; Kodama, K.; Tamura, Y.; Okamatsu, H.; Sashida, Y.; Horibata, Y.; Taguchi, E.; Nakao, K.; Sakamoto, T. Inappropriate sinus tachycardia-induced cardiomyopathy with severe functional mitral regurgitation and successful treatment with ivabradine. J. Cardiol. Cases 2022, 25, 6–9. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Choi, K.J.; Song, J.M.; Hwang, E.S.; Park, K.M.; Nam, G.B.; Kim, J.J.; Kim, Y.H. Diagnostic approach and treatment strategy in tachycardia-induced cardiomyopathy. Clin. Cardiol. Int. Index. Peer-Rev. J. Adv. Treat. Cardiovasc. Dis. 2008, 31, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, L.; Zhao, J.; Guo, Y.; Liu, J.; Shi, D.; Yang, J.; Liu, Y.; Lai, J.; Shen, Z. Early short-term ivabradine treatment in new-onset acute systolic heart failure and sinus tachycardia patients with inflammatory rheumatic disease. Exp. Ther. Med. 2019, 18, 305–311. [Google Scholar] [CrossRef]
- Akopyan, K.; Rackauskas, M.; Gries, C.; Emtiazjoo, A.; Saha, B.K.; Shah, A. Novel use of ivabradine for persistent sinus tachycardia in a patient on extracorporeal life support with right ventricular dysfunction. Cureus 2024, 16, e62638. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D. Non-Pharmacological and Pharmacological Management of Cardiac Dysautonomia Syndromes. J. Atr. Fibrillation 2020, 13, 2395. [Google Scholar] [CrossRef]
- De Asmundis, C.; Pannone, L.; Lakkireddy, D.; Beaver, T.M.; Brodt, C.R.; Lee, R.J.; Sorgente, A.; Gauthey, A.; Monaco, C.; Overeinder, I.; et al. Targeted treatment of inappropriate sinoatrial node tachycardia based on electrophysiological and structural mechanisms. Am. J. Cardiol. 2022, 183, 24–32. [Google Scholar] [CrossRef]
- Lai, R.C.; Xu, M.X.; Huang, W.Q.; Wang, X.D.; Zeng, W.A.; Lin, W.Q. [Beneficial effects of metoprolol on perioperative cardiac function of elderly esophageal cancer patients]. Aizheng 2006, 25, 609–613. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med6&NEWS=N&AN=16687084 (accessed on 1 December 2024).
- Henmi, H.; Imamura, T. Implication of ivabradine in patients with acute heart failure and sinus tachycardia following allogenic hematopoietic cell transplantation. Intern. Med. 2023, 62, 813. [Google Scholar] [CrossRef]
- Scagliola, R.; Brunelli, C. More on ivabradine in tachycardia with paraganglioma. New Engl. J. Med. 2019, 380, 2590. [Google Scholar] [CrossRef]
- Harada, Y.; Shimada, K.; Kubota, Y.; Yoshimoto, T. Ivabradine for Chemotherapy-Related Cardiac Dysfunction in Breast Cancer. Cureus 2021, 13, e18731. [Google Scholar] [CrossRef]
- Lewis, R.; Niazi-Ali, S.; McIvor, A.; Kanj, S.S.; Maertens, J.; Bassetti, M.; Levine, D.; Groll, A.H.; Denning, D.W. Triazole antifungal drug interactions—Practical considerations for excellent prescribing. J. Antimicrob. Chemother. 2024, 79, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Kourek, C.; Touloupaki, M.; Rempakos, A.; Loritis, K.; Tsougkos, E.; Paraskevaidis, I.; Briasoulis, A. Cardioprotective Strategies from Cardiotoxicity in Cancer Patients: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2022, 9, 259. [Google Scholar] [CrossRef]
- Pituskin, E.; Mackey, J.R.; Koshman, S.; Jassal, D.; Pitz, M.; Haykowsky, M.J.; Pagano, J.J.; Chow, K.; Thompson, R.B.; Vos, L.J.; et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101–Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 870–877. [Google Scholar] [CrossRef]
- Dent, S.F.; Kikuchi, R.; Kondapalli, L.; Ismail-Khan, R.; Brezden-Masley, C.; Barac, A.; Fradley, M. Optimizing Cardiovascular Health in Patients With Cancer: A Practical Review of Risk Assessment, Monitoring, and Prevention of Cancer Treatment–Related Cardiovascular Toxicity. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 501–515. [Google Scholar] [CrossRef]
- Ascensão, A.; Magalhães, J.; Soares, J.M.C.; Ferreira, R.; Neuparth, M.J.; Marques, F.; Oliveira, P.J.; Duarte, J.A. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am. J. Physiol. Circ. Physiol. 2005, 289, H722–H731. [Google Scholar] [CrossRef]
- Van Dalen, E.C.; van der Pal, H.J.; Kremer, L.C. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst. Rev. 2016, 2020, CD005008. [Google Scholar] [CrossRef]
- Armenian, S.; Bhatia, S. Predicting and Preventing Anthracycline-Related Cardiotoxicity. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 3–12. [Google Scholar] [CrossRef]
- Johnson, M.; Keyes, D. Anthracycline Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Reinbolt, R.E.; Patel, R.; Pan, X.; Timmers, C.D.; Pilarski, R.; Shapiro, C.L.; Lustberg, M.B. Risk factors for anthracycline-associated cardiotoxicity. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2015, 24, 2173–2180. [Google Scholar] [CrossRef][Green Version]
- Safra, T. Cardiac safety of liposomal anthracyclines. Oncologist 2003, 8 (Suppl. S2), 17–24. [Google Scholar] [CrossRef]
- El Moheb, M.; Nicolas, J.; Khamis, A.M.; Iskandarani, G.; Akl, E.A.; Refaat, M. Implantable cardiac defibrillators for people with non-ischaemic cardiomyopathy. Cochrane Database Syst. Rev. 2018, 2018, CD012738. [Google Scholar] [CrossRef]
- Refaat, M.M.; Gharios, C.; Moorthy, M.V.; Abdulhai, F.; Blumenthal, R.S.; Jaffa, M.A.; Mora, S. Exercise-Induced Ventricular Ectopy and Cardiovascular Mortality in Asymptomatic Individuals. J. Am. Coll. Cardiol. 2021, 78, 2267–2277. [Google Scholar] [CrossRef]
| Study | Study Type | Objective | Cancer Type | Participants Number | Follow Up Period | Key Findings | Year |
|---|---|---|---|---|---|---|---|
| Hemu et al. [13] | Prospective case–control | Association between resting sinus tachycardia and adverse cardiovascular outcomes and mortality in cancer patients | Cancer patients | 622 | 2008–2016 | Resting sinus tachycardia associated with adverse cardiovascular outcomes and increased mortality | 2021 |
| Anker et al. [25] | Prospective observational study | Impact of increased resting heart rate on prognosis in treatment-naïve cancer patients | Treatment-naïve unselected cancer patients | 548 | 2011–2013 | Increased resting heart rate correlates with worse prognosis | 2020 |
| Lopez-Candales et al. [38] | Editorial | Clinical relevance of heart rate in post-hematopoietic stem cell transplant patients | Post-hematopoietic stem cell transplant patients | Heart rate may have clinical significance in post-hematopoietic stem cell transplant patients | 2022 | ||
| Anker et al. [40] | Prospective case–control | Predictive value of resting heart rate on death in cancer patients | Colorectal, pancreatic, and non-small-cell lung cancer patients | 204 | 2005–2010 | Resting heart rate is an independent predictor of death | 2016 |
| Lee et al. [41] | Retrospective observational study | Resting heart rate as a prognostic factor for mortality in breast cancer patients | Breast cancer patients | 4786 | 5.0 ± 2.5 years | Higher resting heart rate associated with increased mortality | 2016 |
| Medrek & Szmit [42] | Prospective observational study | Baseline ECG and echocardiographic assessments for predicting survival in lung cancer patients | Lung cancer patients | 104 | 3 years | ECG, echocardiographic, and RHR assessments may help predict survival | 2022 |
| Katie Glen [43] | Retrospective cohort study | Association between sinus tachycardia and mortality in cancer | Lung cancer, leukemia, lymphoma or multiple myeloma | 622 | 2008–2016 | Higher mortality rate in cancer patients with tachycardia | 2019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakih, Y.; Al Sakan, M.; El Ghazawi, A.; Khoury, M.; Refaat, M.M. Exploring Resting Sinus Tachycardia in Cancer Care: A Comprehensive Review. J. Clin. Med. 2025, 14, 985. https://doi.org/10.3390/jcm14030985
Fakih Y, Al Sakan M, El Ghazawi A, Khoury M, Refaat MM. Exploring Resting Sinus Tachycardia in Cancer Care: A Comprehensive Review. Journal of Clinical Medicine. 2025; 14(3):985. https://doi.org/10.3390/jcm14030985
Chicago/Turabian StyleFakih, Yeva, Moied Al Sakan, Alaaeddine El Ghazawi, Maurice Khoury, and Marwan M. Refaat. 2025. "Exploring Resting Sinus Tachycardia in Cancer Care: A Comprehensive Review" Journal of Clinical Medicine 14, no. 3: 985. https://doi.org/10.3390/jcm14030985
APA StyleFakih, Y., Al Sakan, M., El Ghazawi, A., Khoury, M., & Refaat, M. M. (2025). Exploring Resting Sinus Tachycardia in Cancer Care: A Comprehensive Review. Journal of Clinical Medicine, 14(3), 985. https://doi.org/10.3390/jcm14030985






