Hemi-Versus Total Hip Arthroplasty in Femoral Neck Fractures? Predicting Failure on a 10-Year Data Analysis of the German Arthroplasty Registry (EPRD)
Abstract
:1. Introduction
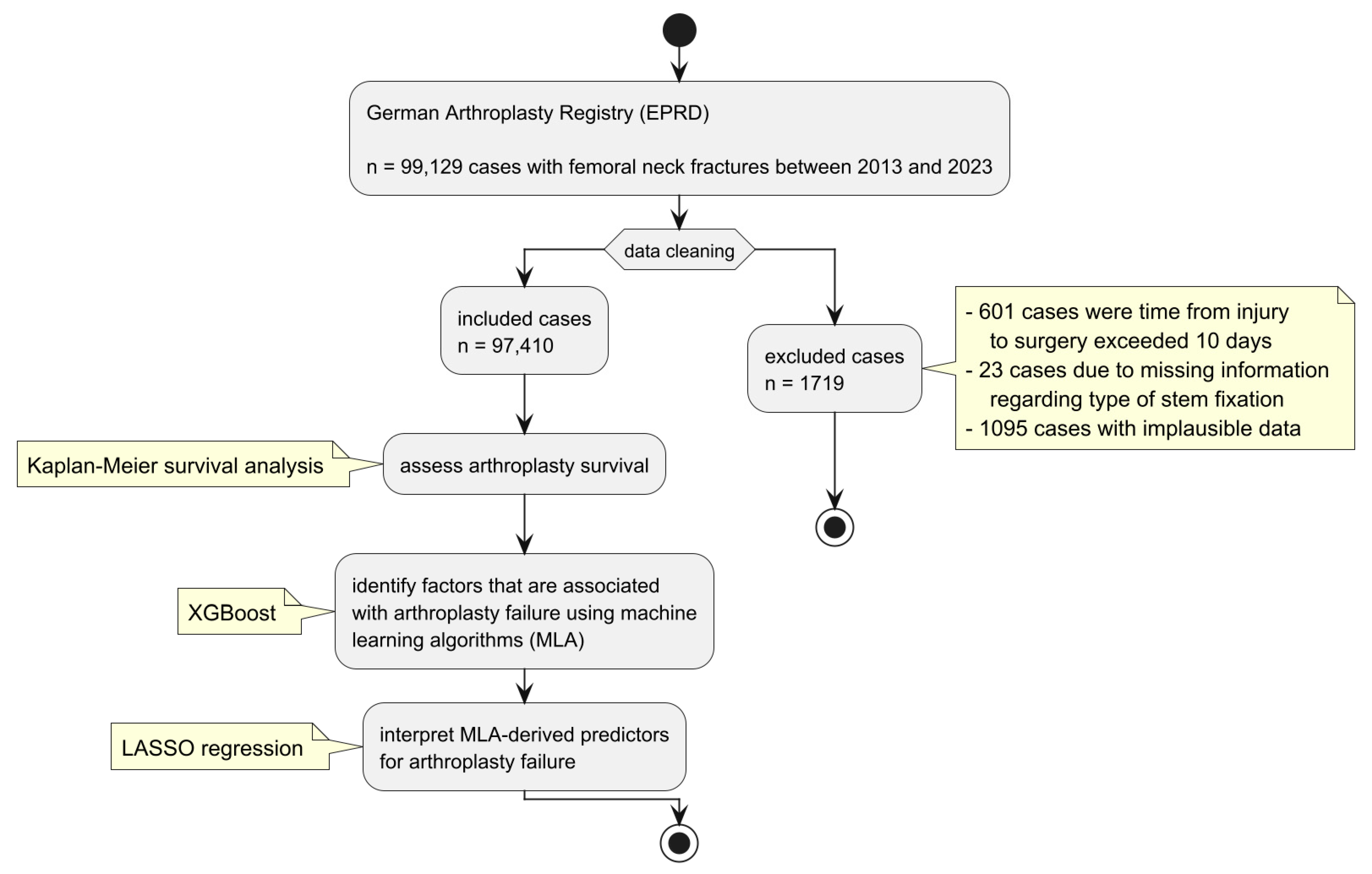
2. Materials and Methods
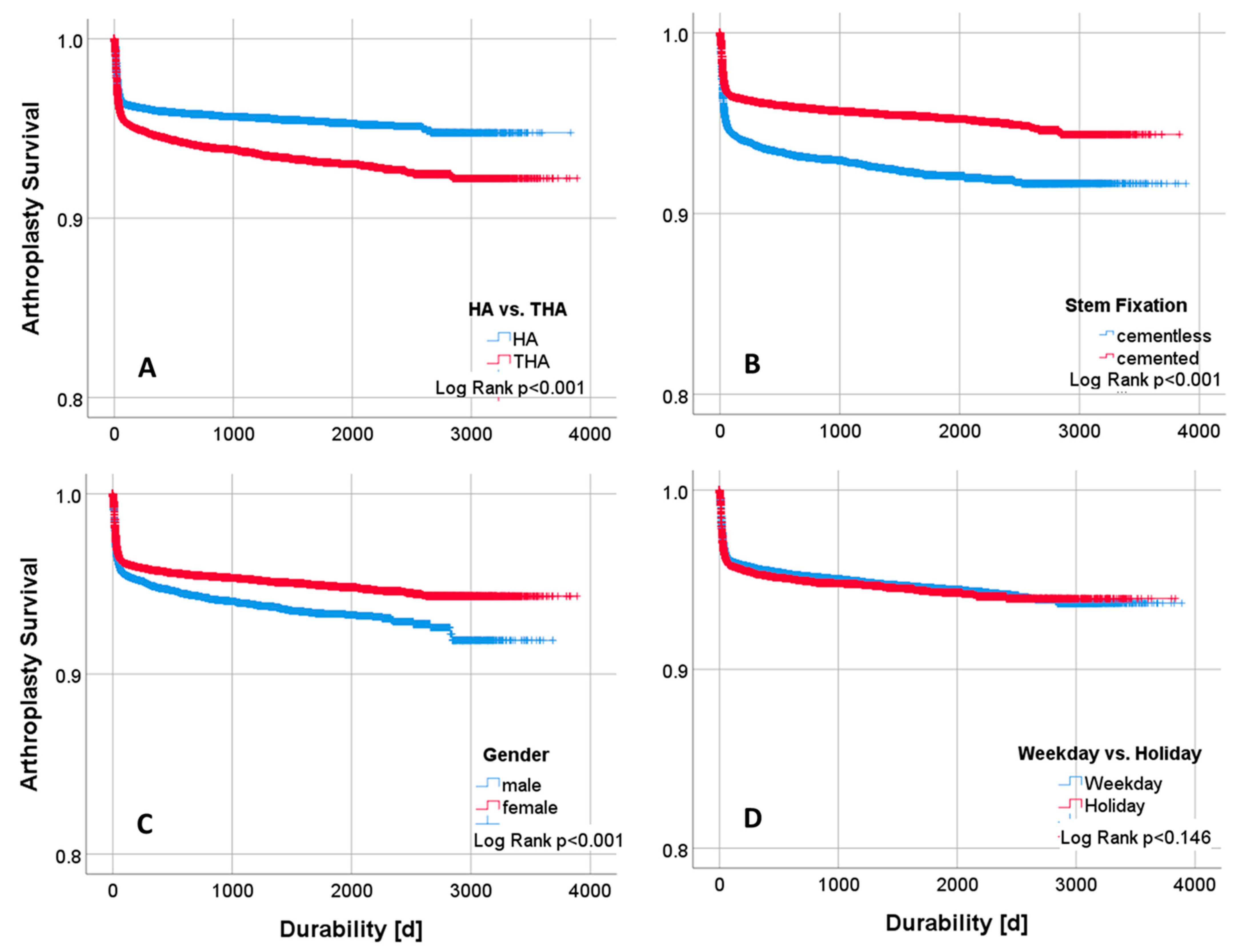
2.1. Survival Analysis
2.2. Machine Learning Algorithms (MLAs)
2.3. Regression Analysis
3. Results
3.1. Descriptive and Univariate Statistics
3.2. Machine Learning Algorithm
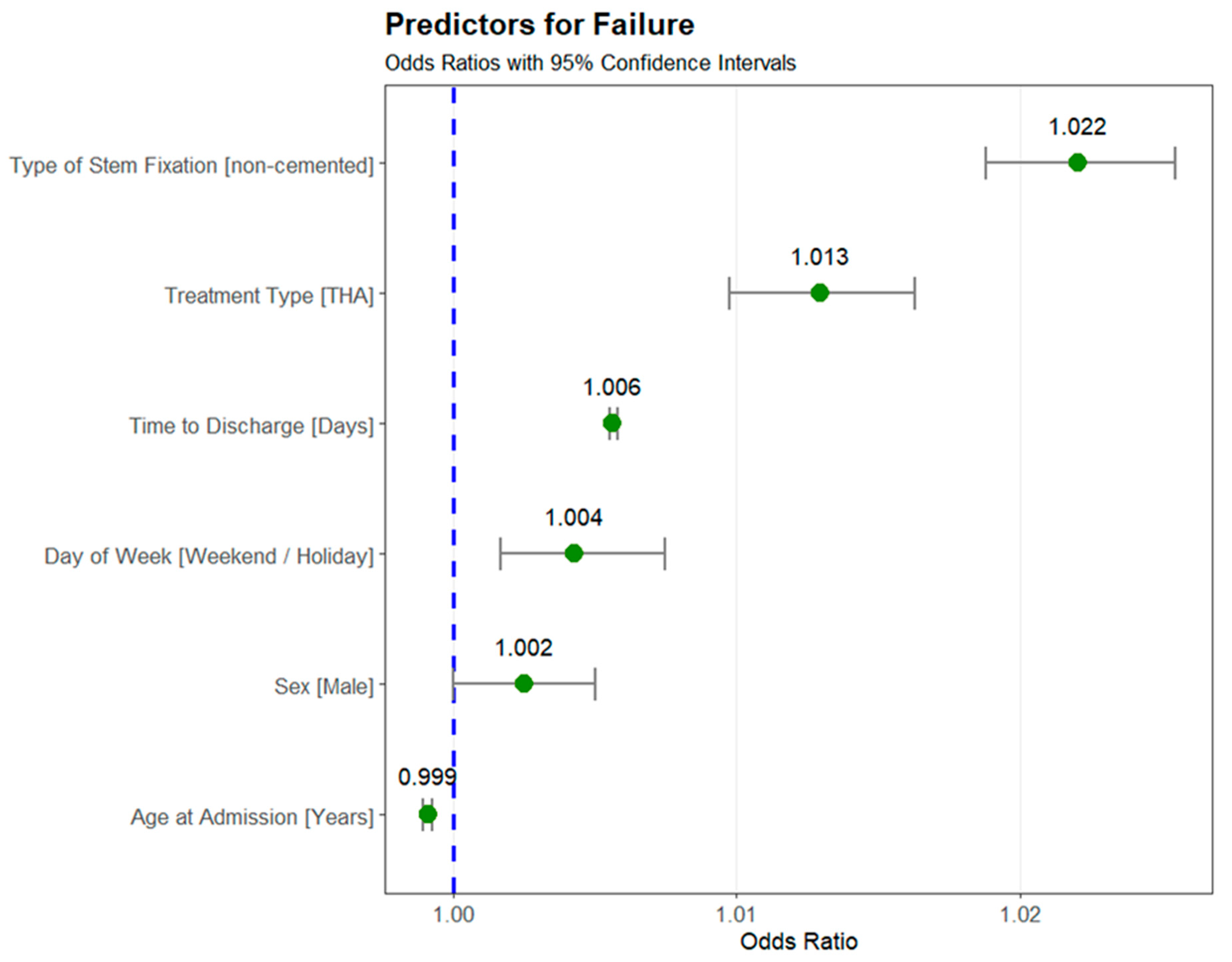
3.3. Predictors for Failure
4. Discussion
4.1. Time of Surgery or Number of Cases
4.2. Failure Sex and Age
4.3. Cemented or Cementless Technique
4.4. THA or HA?
4.5. Time of Failure
4.6. Statistics
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECS | Elixhauser Comorbidity Score |
| EPRD | German Arthroplasty Registry |
| FI | Feature Importance |
| FNF | Femoral Neck Fracture |
| HA | Hemiarthroplasty |
| MLA | Machine Learning Algorithms |
| THA | Total Hip Arthroplasty |
References
- Bundesausschuss, G. Richtlinie des Gemeinsamen Bundesausschusses über Maßnahmen zur Qualitätssicherung zur Versorgung von Patienten mit einer hüftgelenknahen Femurfraktur gemäß § 136 Absatz 1 Satz 1 Nummer 2 für nach § 108 SGB V zugelassene Krankenhäuser. 2020, BAnz AT, 30.12.2020.
- Leicht, H.; Gaertner, T.; Günster, C.; Halder, A.M.; Hoffmann, R.; Jeschke, E.; Malzahn, J.; Tempka, A.; Zacher, J. Time to Surgery and Outcome in the Treatment of Proximal Femoral Fractures. Dtsch. Arztebl. Int. 2021, 118, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.R.; Macey, R.; Stokes, J.; Cook, J.A.; Eardley, W.G.; Griffin, X.L. Surgical interventions for treating intracapsular hip fractures in older adults: A network meta-analysis. Cochrane Database Syst. Rev. 2022, 2, Cd013404. [Google Scholar] [CrossRef]
- Schmitz, P.P.; Somford, M.P.; Jameson, S.S.; Schreurs, B.W.; van Susante, J.L.C. Controversies around hip fracture treatment: Clinical evidence versus trends from national registries. Hip Int. 2024, 34, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Szymski, D.; Walter, N.; Melsheimer, O.; Grimberg, A.; Alt, V.; Steinbrück, A.; Rupp, M. Mortality After Hemiarthroplasty for Femoral Neck Fractures. Dtsch. Arztebl. Int. 2023, 120, 297–298. [Google Scholar] [CrossRef]
- Szymski, D.; Walter, N.; Krull, P.; Melsheimer, O.; Grimberg, A.; Alt, V.; Steinbrück, A.; Rupp, M. Aseptic revisions and pulmonary embolism after surgical treatment of femoral neck fractures with cemented and cementless hemiarthroplasty in Germany: An analysis from the Germany Arthroplasty Registry (EPRD). Orthopadie 2023, 52, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Alsoof, D.; McDonald, C.L.; Kuris, E.O.; Daniels, A.H. Machine Learning for the Orthopaedic Surgeon: Uses and Limitations. J. Bone Jt. Surg. Am. 2022, 104, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Hinterwimmer, F.; Lazic, I.; Suren, C.; Hirschmann, M.T.; Pohlig, F.; Rueckert, D.; Burgkart, R.; von Eisenhart-Rothe, R. Machine learning in knee arthroplasty: Specific data are key—A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Gotzler, A.; Glowalla, C.; Hinterwimmer, F.; Schneidmüller, D.; Hungerer, S. Endoprosthetic treatment of femoral neck fractures in Germany: Cumulative analysis of EPRD registry data from 2013 to 2020. Orthopadie 2024, 53, 945–954. [Google Scholar] [CrossRef]
- ICD-10-GM Version 2022 Alphabetisches Verzeichnis. 2022. Available online: https://klassifikationen.bfarm.de/icd-10-gm/kode-suche/htmlgm2022/index.htm (accessed on 28 June 2023).
- Grimberg, A.; Jansson, V.; Lützner, J.; Melsheimer, O.; Steinbrück, A. Endoprothesenregister Deutschland (EPRD)–Jahresbericht 2021; EPRD Deutsche Endoprothesenregister gGmbH: Berlin, Germany, 2021. [Google Scholar]
- Elixhauser, A.; Steiner, C.; Harris, D.R.; Coffey, R.M. Comorbidity measures for use with administrative data. Med. Care 1998, 36, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Smith, R.P.; Uzoigwe, C.E.; Braybrooke, J.R. The weekend effect: Short-term mortality following admission with a hip fracture. Bone Jt. J. 2014, 96, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Boutera, A.; Dybvik, E.; Hallan, G.; Gjertsen, J.E. Is there a weekend effect after hip fracture surgery? A study of 74,410 hip fractures reported to the Norwegian Hip Fracture Register. Acta Orthop. 2020, 91, 63–68. [Google Scholar] [CrossRef]
- Kristiansen, N.S.; Kristensen, P.K.; Nørgård, B.M.; Mainz, J.; Johnsen, S.P. Off-hours admission and quality of hip fracture care: A nationwide cohort study of performance measures and 30-day mortality. Int. J. Qual. Health Care 2016, 28, 324–331. [Google Scholar] [CrossRef]
- Grimberg, A.; Jansson, V.; Liebs, T.R.; Melsheimer, O.; Steinbrück, A. Endoprothesenregister Deutschland (EPRD)–Jahresbericht 2022; EPRD: Berlin, Germany, 2022. [Google Scholar]
- Sayers, A.; Steele, F.; Whitehouse, M.R.; Price, A.; Ben-Shlomo, Y.; Blom, A.W. Association between surgical volume and failure of primary total hip replacement in England and Wales: Findings from a prospective national joint replacement register. BMJ Open 2020, 10, e033045. [Google Scholar] [CrossRef]
- Kaptoge, S.; Dalzell, N.; Loveridge, N.; Beck, T.J.; Khaw, K.T.; Reeve, J. Effects of gender, anthropometric variables, and aging on the evolution of hip strength in men and women aged over 65. Bone 2003, 32, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Power, J.; Loveridge, N.; Kröger, H.; Parker, M.; Reeve, J. Femoral neck cortical bone in female and male hip fracture cases: Differential contrasts in cortical width and sub-periosteal porosity in 112 cases and controls. Bone 2018, 114, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Moerman, S.; Mathijssen, N.M.C.; Tuinebreijer, W.E.; Vochteloo, A.J.H.; Nelissen, R. Hemiarthroplasty and total hip arthroplasty in 30,830 patients with hip fractures: Data from the Dutch Arthroplasty Register on revision and risk factors for revision. Acta Orthop. 2018, 89, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Venäläinen, M.S.; Panula, V.J.; Eskelinen, A.P.; Fenstad, A.M.; Furnes, O.; Hallan, G.; Rolfson, O.; Kärrholm, J.; Hailer, N.P.; Pedersen, A.B.; et al. Prediction of Early Adverse Events After THA: A Comparison of Different Machine-Learning Strategies Based on 262,356 Observations From the Nordic Arthroplasty Register Association (NARA) Dataset. ACR Open Rheumatol. 2024, 6, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.R.; Macey, R.; Parker, M.J.; Cook, J.A.; Griffin, X.L. Arthroplasties for hip fracture in adults. Cochrane Database Syst. Rev. 2022, 2, Cd013410. [Google Scholar] [CrossRef]
- Parker, M.J.; Chatterjee, R.; Onsa, M.; Cawley, S.; Gurusamy, K. Cemented versus uncemented hemiarthroplasty for displaced intracapsular fractures of the hip. Bone Jt. J. 2023, 105, 1196–1200. [Google Scholar] [CrossRef]
- Huddleston, J.I., 3rd; De, A.; Jaffri, H.; Barrington, J.W.; Duwelius, P.J.; Springer, B.D. Cementless Fixation Is Associated With Increased Risk of Early and All-Time Revision After Hemiarthroplasty But Not After THA for Femoral Neck Fracture: Results From the American Joint Replacement Registry. Clin. Orthop. Relat. Res. 2021, 479, 2194–2202. [Google Scholar] [CrossRef]
- Langslet, E.; Frihagen, F.; Opland, V.; Madsen, J.E.; Nordsletten, L.; Figved, W. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: 5-year followup of a randomized trial. Clin. Orthop. Relat. Res. 2014, 472, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- AOA; Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2022 Annual Report; Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR): Adelaide, Australia, 2022; p. 487. [Google Scholar]
- Duijnisveld, B.J.; Koenraadt, K.L.M.; van Steenbergen, L.N.; Bolder, S.B.T. Mortality and revision rate of cemented and uncemented hemiarthroplasty after hip fracture: An analysis of the Dutch Arthroplasty Register (LROI). Acta Orthop. 2020, 91, 408–413. [Google Scholar] [CrossRef]
- Dahl, O.E.; Pripp, A.H. Does the Risk of Death Within 48 Hours of Hip Hemiarthroplasty Differ Between Patients Treated with Cemented and Cementless Implants? A Meta-analysis of Large, National Registries. Clin. Orthop. Relat. Res. 2022, 480, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.A.; Achten, J.; Parsons, N.; Griffin, X.L.; Png, M.E.; Gould, J.; McGibbon, A.; Costa, M.L. Cemented or Uncemented Hemiarthroplasty for Intracapsular Hip Fracture. N. Engl. J. Med. 2022, 386, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Varady, N.H.; Oeding, J.F.; Gausden, E.B.; Ricci, W.M.; Chen, A.F. Cemented Hemiarthroplasty Results in Substantial Cost Savings Over Uncemented Hemiarthroplasty for the Treatment of Femoral Neck Fractures in Patients Over 60 Years Old: A Markov Analysis. J. Arthroplast. 2024. [Google Scholar] [CrossRef]
- Hansson, S.; Nemes, S.; Karrholm, J.; Rogmark, C. Reduced risk of reoperation after treatment of femoral neck fractures with total hip arthroplasty. Acta Orthop. 2017, 88, 500–504. [Google Scholar] [CrossRef]
- Picus, R.; Ziernhöld, G.; Bonetti, M. 2. Bericht Prothesenregister Hüftprothesen 2010–2015 Autonome Provinz Bozen; Autonome Provinz Bozen: Bozen, Italy, 2017. [Google Scholar]
- Grimberg, A.; Jansson, V.; Liebs, T.R.; Melsheimer, O.; Steinbrück, A. Endoprothesenregister Deutschland (EPRD)–Jahresbericht 2017; EPRD: Berlin, Germany, 2018. [Google Scholar]
- Kristensen, T.B.; Dybvik, E.; Kristoffersen, M.; Dale, H.; Engesæter, L.B.; Furnes, O.; Gjertsen, J.E. Cemented or Uncemented Hemiarthroplasty for Femoral Neck Fracture? Data from the Norwegian Hip Fracture Register. Clin. Orthop. Relat. Res. 2020, 478, 90–100. [Google Scholar] [CrossRef]
- McKie, J.; Devane, P.; Young, S.; Coleman, B. The New Zealand Joint Registry—Twenty-Five Year Report 1999–2023; New Zealand Orthopaedic Association: Wellington, New Zealand, 2024; 167p, Available online: https://www.nzoa.org.nz/sites/default/files/NZJR_Twenty%20Five%20Year%20Report_Aug2024.pdf (accessed on 28 June 2023).
- W-Dahl, A.; Kärrholm, J.; Rogmark, C.; Nåtman, J.; Bülow, E.; Arani, P.I.; Mohaddes, M.; Rolfson, O. Annual Report 2023—The Swedish Arthroplasty Registry; The Swedish Arthroplasty Register: Gothenburg, Sweden, 2023; 302p, Available online: https://registercentrum.blob.core.windows.net/sar/r/SAR_Annual-report-2023_EN-DS5gryeOB.pdf (accessed on 28 June 2023).
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 4. [Google Scholar] [CrossRef]
- Lazic, I.; Hinterwimmer, F.; Langer, S.; Pohlig, F.; Suren, C.; Seidl, F.; Rückert, D.; Burgkart, R.; von Eisenhart-Rothe, R. Prediction of Complications and Surgery Duration in Primary Total Hip Arthroplasty Using Machine Learning: The Necessity of Modified Algorithms and Specific Data. J. Clin. Med. 2022, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Prokopetz, J.J.; Losina, E.; Bliss, R.L.; Wright, J.; Baron, J.A.; Katz, J.N. Risk factors for revision of primary total hip arthroplasty: A systematic review. BMC Musculoskelet. Disord. 2012, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wahafu, T.; Cheng, M.; Cheng, T.; Zhang, Y.; Zhang, X. The influence of obesity on primary total hip arthroplasty outcomes: A meta-analysis of prospective cohort studies. Orthop. Traumatol. Surg. Res. 2015, 101, 289–296. [Google Scholar] [CrossRef]
- Haynes, J.; Nam, D.; Barrack, R.L. Obesity in total hip arthroplasty: Does it make a difference? Bone Jt. J. 2017, 99, 31–36. [Google Scholar] [CrossRef]
- Abraham, V.M.; Booth, G.; Geiger, P.; Balazs, G.C.; Goldman, A. Machine-learning Models Predict 30-Day Mortality, Cardiovascular Complications, and Respiratory Complications After Aseptic Revision Total Joint Arthroplasty. Clin. Orthop. Relat. Res. 2022, 480, 2137–2145. [Google Scholar] [CrossRef]
- Khosravi, B.; Rouzrokh, P.; Maradit Kremers, H.; Larson, D.R.; Johnson, Q.J.; Faghani, S.; Kremers, W.K.; Erickson, B.J.; Sierra, R.J.; Taunton, M.J.; et al. Patient-specific Hip Arthroplasty Dislocation Risk Calculator: An Explainable Multimodal Machine Learning-based Approach. Radiol. Artif. Intell. 2022, 4, e220067. [Google Scholar] [CrossRef]
- Dmitrienko, A.; D’Agostino, R.B., Sr. Multiplicity Considerations in Clinical Trials. N. Engl. J. Med. 2018, 378, 2115–2122. [Google Scholar] [CrossRef]
- Pocock, S.J.; Clayton, T.C.; Altman, D.G. Survival plots of time-to-event outcomes in clinical trials: Good practice and pitfalls. Lancet 2002, 359, 1686–1689. [Google Scholar] [CrossRef]
- Hinterwimmer, F.; Lazic, I.; Langer, S.; Suren, C.; Charitou, F.; Hirschmann, M.T.; Matziolis, G.; Seidl, F.; Pohlig, F.; Rueckert, D.; et al. Prediction of complications and surgery duration in primary TKA with high accuracy using machine learning with arthroplasty-specific data. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 1323–1333. [Google Scholar] [CrossRef]


| Intact | Failure | Overall | p-Value | |
|---|---|---|---|---|
| (n = 92,982) | (n = 4428) | (n = 97,410) | ||
| Age at Admission (Years) | ||||
| Mean (SD) | 81.0 (9.31) | 78.9 (9.95) | 80.9 (9.35) | <0.001 |
| Median [Min, Max] | 82.0 [24.0, 111] | 81.0 [33.0, 103] | 82.0 [24.0, 111] | |
| Sex | ||||
| female | 66,111 (71.1%) | 2987 (67.5%) | 69,098 (70.9%) | <0.001 |
| male | 26,871 (28.9%) | 1441 (32.5%) | 28,312 (29.1%) | |
| Time between Injury and Surgery (Days) | ||||
| Mean (SD) | 0.988 (1.11) | 0.999 (1.17) | 0.988 (1.11) | 0.545 |
| Median [Min, Max] | 1.00 [0, 10.0] | 1.00 [0, 10.0] | 1.00 [0, 10.0] | |
| Time to Discharge (Days) | ||||
| Mean (SD) | 13.2 (7.92) | 23.0 (17.7) | 13.6 (8.85) | <0.001 |
| Median [Min, Max] | 11.0 [0, 136] | 18.0 [0, 152] | 11.0 [0, 152] | |
| Treatment Type | ||||
| Hemiarthroplasty (HA) | 63,055 (67.8%) | 2525 (57.0%) | 65,580 (67.3%) | <0.001 |
| Total Hip Arthroplasty (THA) | 29,927 (32.2%) | 1903 (43.0%) | 31,830 (32.7%) | |
| Type of Stem Fixation | ||||
| non-cemented | 21,274 (22.9%) | 1547 (34.9%) | 22,821 (23.4%) | <0.001 |
| cemented | 71,708 (77.1%) | 2881 (65.1%) | 74,589 (76.6%) | |
| Durability (Days) | ||||
| Mean (SD) | 833 (751) | 124 (316) | 833 (751) | <0.001 |
| Median [Min, Max] | 632 [0, 3888] | 24 [0, 2843] | 590 [0, 3890] | |
| Day of Week—Surgery | ||||
| weekend/holiday | 21,225 (22.8%) | 1044 (23.6%) | 22,269 (22.9%) | 0.253 |
| weekday | 71,757 (77.2%) | 3384 (76.4%) | 75,141 (77.1%) | |
| Number of Cases per Year | ||||
| 1–99 | 38,947 (41.9%) | 1597 (36.1%) | 40,544 (41.6%) | <0.001 |
| 100–200 | 23,486 (25.3%) | 913 (20.6%) | 24,399 (25.0%) | |
| more than 200 | 89 (0.1%) | 1 (0.0%) | 90 (0.1%) | |
| not assessed | 30,460 (32.8%) | 1917 (43.3%) | 32,377 (33.2%) | |
| Elixhauser Comorbidity Score | ||||
| Mean (SD) | 7.2 (7.5) | 8.1 (8.0) | 7.3 (7.6) | <0.001 |
| Median [Min, Max] | 5.0 [−14.0, 56.0] | 6.0 [−11.0, 44.0] | 5.0 [−14.0, 56.0] | |
| Geriatric Complex Treatment | ||||
| no | 71,632 (77.0%) | 3474 (78.5%) | 75,106 (77.1%) | 0.030 |
| yes | 21,350 (23.0%) | 954 (21.5%) | 22,304 (22.9%) | |
| Vital Status | ||||
| alive | 49,810 (53.6%) | 2235 (50.5%) | 52,045 (53.4%) | <0.001 |
| deceased | 43,172 (46.4%) | 2193 (49.5%) | 45,365 (46.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hungerer, S.; Hinterwimmer, F.; Leister, I.; Langer, S.; Gotzler, A.; Glowalla, C. Hemi-Versus Total Hip Arthroplasty in Femoral Neck Fractures? Predicting Failure on a 10-Year Data Analysis of the German Arthroplasty Registry (EPRD). J. Clin. Med. 2025, 14, 1457. https://doi.org/10.3390/jcm14051457
Hungerer S, Hinterwimmer F, Leister I, Langer S, Gotzler A, Glowalla C. Hemi-Versus Total Hip Arthroplasty in Femoral Neck Fractures? Predicting Failure on a 10-Year Data Analysis of the German Arthroplasty Registry (EPRD). Journal of Clinical Medicine. 2025; 14(5):1457. https://doi.org/10.3390/jcm14051457
Chicago/Turabian StyleHungerer, Sven, Florian Hinterwimmer, Iris Leister, Severin Langer, Alexander Gotzler, and Claudio Glowalla. 2025. "Hemi-Versus Total Hip Arthroplasty in Femoral Neck Fractures? Predicting Failure on a 10-Year Data Analysis of the German Arthroplasty Registry (EPRD)" Journal of Clinical Medicine 14, no. 5: 1457. https://doi.org/10.3390/jcm14051457
APA StyleHungerer, S., Hinterwimmer, F., Leister, I., Langer, S., Gotzler, A., & Glowalla, C. (2025). Hemi-Versus Total Hip Arthroplasty in Femoral Neck Fractures? Predicting Failure on a 10-Year Data Analysis of the German Arthroplasty Registry (EPRD). Journal of Clinical Medicine, 14(5), 1457. https://doi.org/10.3390/jcm14051457






