Exploring the Relationship Between Brain Neurochemistry, Cervical Impairments and Pain Sensitivity in People with Migraine, Whiplash-Headache, Low Back Pain and Healthy Controls: A Secondary Analysis of a Cross-Sectional Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
Inclusion/Exclusion Criteria
2.3. Recruitment
2.4. Baseline Questionnaires
2.5. Clinical Examination
2.6. Clinical Cervical Musculoskeletal Tests
2.7. Clinical Tests of Widespread Pain Sensitivity
2.8. Clinical Classification
2.8.1. Cervical Musculoskeletal Impairment
2.8.2. Increased Cervical Pain Sensitivity
2.8.3. Clinical Evidence of Central Sensitization (Central Sensitization)
2.9. Neurochemical Measures
2.10. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Results of the Correlational Analysis
3.3. Cluster Analysis
3.4. Post Hoc Analysis
4. Discussion
5. Strengths and Limitations
6. Conclusions and Clinical Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95% CI | 95% Confidence Interval |
| ANOVA | Analysis of Variance |
| BMI | Body Mass Index |
| CCFT | Craniocervical Flexion Test |
| CFT | Cervical Flexion Test |
| CPM | Conditioned Pain Modulation |
| CPT | Cold Pain Threshold |
| CSI | Central Sensitization Inventory |
| DASS | Depression, Anxiety, and Stress Scales |
| FRT | Flexion Rotation Test |
| GABA | Gamma-Aminobutyric Acid |
| GABA+ | Gamma-Aminobutyric Acid plus macromolecules |
| Glx | Composite of Glutamate and Glutamine |
| HIT-6 | Headache Impact Test |
| IPT | Ice Pain Test |
| IQR | Interquartile Range |
| MEGA-PRESS | Mescher-Garwood Point-Resolved Spectroscopy |
| MRI | Magnetic Resonance Imaging |
| MRS | Magnetic Resonance Spectroscopy |
| MSK | Musculoskeletal |
| NDI | Neck Disability Index |
| NRS | Numeric Rating Scale |
| ODI | Oswestry Disability Index |
| PAIVM | Passive Accessory Intervertebral Movement |
| PCC | Posterior Cingulate Cortex |
| PPT | Pressure Pain Threshold |
| ROM | Range of movement |
| SD | Standard Deviation |
| WHODAS 2.0 | World Health Organization Disability Assessment Schedule 2.0 |
| WUR | Wind-Up Ratio |
References
- Henderson, L.A.; Peck, C.C.; Petersen, E.T.; Rae, C.D.; Youssef, A.M.; Reeves, J.M.; Wilcox, S.L.; Akhter, R.; Murray, G.M.; Gustin, S.M. Chronic pain: Lost inhibition? J. Neurosci. 2013, 33, 1754–1782. [Google Scholar] [CrossRef] [PubMed]
- Bathel, A.; Schweizer, L.; Stude, P.; Glaubitz, B.; Wulms, N.; Delice, S.; Schmidt-Wilcke, T. Increased thalamic glutamate/glutamine levels in migraineurs. J. Headache Pain. 2018, 19, 55. [Google Scholar] [CrossRef]
- Harris, R.E.; Sundgren, P.C.; Craig, A.D.; Kirshenbaum, E.; Sen, A.; Napadow, V.; Clauw, D.J. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009, 60, 3146–3152. [Google Scholar] [CrossRef] [PubMed]
- Enna, S.J.; McCarson, K.E. The Role of GABA in the Mediation and Perception of Pain. Adv. Pharmacol. 2006, 54, 1–27. [Google Scholar] [CrossRef]
- Rae, C.D. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 2014, 39, 1–36. [Google Scholar] [CrossRef]
- Petroff, O.A. GABA and glutamate in the human brain. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Peek, A.L.; Rebbeck, T.; Puts, N.A.; Watson, J.; Aguila, M.E.; Leaver, A.M. Brain GABA and glutamate levels across pain conditions: A systematic literature review and meta-analysis of 1H-MRS studies using the MRS-Q quality assessment tool. Neuroimage 2020, 210, 116532. [Google Scholar] [CrossRef] [PubMed]
- Zielman, R.; Wijnen, J.P.; Webb, A.; Onderwater, G.L.J.; Ronen, I.; Ferrari, M.D.; Kan, H.E.; Terwindt, G.M.; Kruit, M.C. Cortical glutamate in migraine. Brain A J. Neurol. 2017, 140, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Gonzales de la Aleja, J.G.; Ramos, A.; Mato-Abad, V.; Martinez-Salio, A.; Hernandez-Tamames, J.A.; Molina, J.A.; Hernandez-Gallego, J.; Alvarez-Linera, J. Higher Glutamate to Glutamine Ratios in Occipital Regions in Women With Migraine During the Interictal State. Headache 2013, 53, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.; Stokoe, M.; Khaira, A.; Webb, M.; Noel, M.; Amoozegar, F.; Harris, A.D. GABA and glutamate in pediatric migraine. Pain 2021, 162, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Foerster, B.R.; Petrou, M.; Edden, R.A.; Sundgren, P.C.; Schmidt-Wilcke, T.; Lowe, S.E.; Harte, S.E.; Clauw, D.J.; Harris, R.E. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012, 64, 579–583. [Google Scholar] [CrossRef]
- Gustin, S.; Wrigley, P.; Youssef, A.; McIndoe, L. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. PAIN 2014, 115, 1027–1036. [Google Scholar] [CrossRef]
- Peek, A.L.; Leaver, A.M.; Foster, S.; Oeltzschner, G.; Puts, N.A.; Galloway, G.; Sterling, M.; Ng, K.; Refshauge, K.; Aguila, M.-E.R.; et al. Increased GABA+ in People With Migraine, Headache, and Pain Conditions- A Potential Marker of Pain. J. Pain. 2021, 22, 1631–1645. [Google Scholar] [CrossRef] [PubMed]
- Aguila, M.R.; Rebbeck, T.; Leaver, A.M.; Lagopoulos, J.; Brennan, P.C.; Hubscher, M.; Refshauge, K.M. The Association Between Clinical Characteristics of Migraine and Brain GABA Levels: An Exploratory Study. J. Pain. 2016, 17, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.C.; Carlos-Ferreira, J.; Luz, L.L.; Safronov, B.V. Presynaptic Interactions between Trigeminal and Cervical Nociceptive Afferents Supplying Upper Cervical Lamina I Neurons. J. Neurosci. 2022, 42, 3587–3598. [Google Scholar] [CrossRef] [PubMed]
- Terumitsu, M.; Takado, Y.; Fukuda, K.-I.; Kato, E.; Tanaka, S. Neurometabolite levels and relevance to central sensitization in chronic orofacial pain patients: A magnetic resonance spectroscopy study. J. Pain. Res. 2022, 15, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N.; Govind, J. Cervicogenic headache: An assessment of the evidence on clinical diagnosis, invasive tests, and treatment. Lancet Neurol. 2009, 8, 959–968. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Do, T.P.; Melo-Carrillo, A.; Burstein, R.; Moskowitz, M.A. Migraine and the trigeminovascular system-40 years and counting. Lancet. Neurol. 2019, 18, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W. The neurobiology of central sensitization. J. Musculoskelet. Pain. 2002, 10, 23–33. [Google Scholar] [CrossRef]
- Székely, J.I.; Torok, K.; Mate, G. The role of ionotropic glutamate receptors in nociception with special regard to the AMPA binding sites. Curr. Pharm. Des. 2002, 8, 887–912. [Google Scholar] [CrossRef] [PubMed]
- International Headache Society. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain. 2019, 160, 28–37. [Google Scholar] [CrossRef]
- Neblett, R.; Cohen, H.; Choi, Y.; Hartzell, M.M.; Williams, M.; Mayer, T.G.; Gatchel, R.J. The Central Sensitization Inventory (CSI): Establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J. Pain. Off. J. Am. Pain. Soc. 2013, 14, 438–445. [Google Scholar] [CrossRef]
- Castien, R.F.; Blankenstein, A.H.; Windt, D.A.; Dekker, J. Minimal clinically important change on the Headache Impact Test-6 questionnaire in patients with chronic tension-type headache. Cephalalgia 2012, 32, 710–714. [Google Scholar] [CrossRef]
- Vernon, H.; Mior, S. The Neck Disability Index: A study of reliability and validity. J. Manip. Physiol. Ther. 1991, 14, 409–415. [Google Scholar]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952; discussion 2952. [Google Scholar] [CrossRef]
- Baron, M.; Schieir, O.; Hudson, M.; Steele, R.; Kolahi, S.; Berkson, L.; Couture, F.; Fitzcharles, M.A.; Gagne, M.; Garfield, B.; et al. The clinimetric properties of the World Health Organization Disability Assessment Schedule II in early inflammatory arthritis. Arthritis Rheum. 2008, 59, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- Hall, T.; Robinson, K. The flexion-rotation test and active cervical mobility--a comparative measurement study in cervicogenic headache. Man. Ther. 2004, 9, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Hengeveld, E.; Banks, K. Maitland’s Vertebral Manipulation: Management of Neuromusculoskeletal Disorders-Volume 1; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; Volume 1. [Google Scholar]
- Jull, G.A.; O’Leary, S.P.; Falla, D.L. Clinical assessment of the deep cervical flexor muscles: The craniocervical flexion test. J. Manip. Physiol. Ther. 2008, 31, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Bronfort, G.; Evans, R.; Nelson, B.; Aker, P.D.; Goldsmith, C.H.; Vernon, H. A randomized clinical trial of exercise and spinal manipulation for patients with chronic neck pain. Spine 2001, 26, 788–797. [Google Scholar] [CrossRef]
- Dumas, J.P.; Arsenault, A.B.; Boudreau, G.; Magnoux, E.; Lepage, Y.; Bellavance, A.; Loisel, P. Physical impairments in cervicogenic headache: Traumatic vs. nontraumatic onset. Cephalalgia 2001, 21, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Edmondston, S.J.; Wallumrod, M.E.; Macleid, F.; Kvamme, L.S.; Joebges, S.; Brabham, G.C. Reliability of isometric muscle endurance tests in subjects with postural neck pain. J. Manip. Physiol. Ther. 2008, 31, 348–354. [Google Scholar] [CrossRef]
- Liang, Z.; Thomas, L.; Jull, G.; Treleaven, J. Cervical musculoskeletal impairments in migraine. Arch. Physiother. 2021, 11, 27. [Google Scholar] [CrossRef]
- Rebbeck, T.; Moloney, N.; Azoory, R.; Hübscher, M.; Waller, R.; Gibbons, R.; Beales, D. Clinical Ratings of Pain Sensitivity Correlate With Quantitative Measures in People With Chronic Neck Pain and Healthy Controls: Cross-Sectional Study. Phys. Ther. 2015, 95, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Rolke, R.; Baron, R.; Maier, C.a.; Tölle, T.; Treede, R.-D.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Tilley, P.; Bisset, L. The Reliability and Validity of Using Ice to Measure Cold Pain Threshold. BioMed Res. Int. 2017, 2017, 7640649. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Wrigley, P.J.; Dean, C.M.; Graham, P.L.; Hush, J.M. From acute to persistent low back pain: A longitudinal investigation of somatosensory changes using quantitative sensory testing—An exploratory study. Pain. Rep. 2018, 3, e641. [Google Scholar] [CrossRef] [PubMed]
- Valencia, C.; Kindler, L.L.; Fillingim, R.B.; George, S.Z. Stability of conditioned pain modulation in two musculoskeletal pain models: Investigating the influence of shoulder pain intensity and gender. BMC Musculoskelet. Disord. 2013, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Beales, D.; Mitchell, T.; Moloney, N.; Rabey, M.; Ng, W.; Rebbeck, T. Masterclass: A pragmatic approach to pain sensitivity in people with musculoskeletal disorders and implications for clinical management for musculoskeletal clinicians. Musculoskelet. Sci. Pract. 2021, 51, 102221. [Google Scholar] [CrossRef] [PubMed]
- Apti, A.; Kuru Çolak, T.; Akçay, B. Normative Values for Cervical and Lumbar Range of Motion in Healthy Young Adults. J. Turk. Spinal Surg. 2023, 34, 113–117. [Google Scholar] [CrossRef]
- Jull, G.; Amiri, M.; Bullock-Saxton, J.; Darnell, R.; Lander, C. Cervical Musculoskeletal Impairment in Frequent Intermittent Headache. Part 1: Subjects With Single Headaches. Cephalalgia 2007, 27, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Jull, G.; Sterling, M.; Falla, D.; Treleaven, J.; O’Leary, S. Chapter 4—Alterations in Cervical Muscle Function in Neck Pain. In Whiplash, Headache, and Neck Pain; Jull, G., Sterling, M., Falla, D., Treleaven, J., O’Leary, S., Eds.; Churchill Livingstone: Edinburgh, UK, 2008. [Google Scholar] [CrossRef]
- O’Leary, S.; Falla, D.; Elliott, J.M.; Jull, G. Muscle Dysfunction in Cervical Spine Pain: Implications for Assessment and Management. J. Orthop. Sports Phys. Ther. 2009, 39, 324–333. [Google Scholar] [CrossRef]
- Liang, Z.; Thomas, L.; Jull, G.; Minto, J.; Zareie, H.; Treleaven, J. Neck pain associated with migraine does not necessarily reflect cervical musculoskeletal dysfunction. Headache J. Head. Face Pain. 2021, 61, 882–894. [Google Scholar] [CrossRef]
- Curatolo, M. Personalized medicine: Somatosensory phenotyping in musculoskeletal pain conditions. Eur. J. Pain. 2023, 27, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Peek, A.; Rebbeck, T.; Leaver, A.; Puts, N.; Foster, S.; Refshauge, K.; Oeltzschner, G.; Panel, M.E. A Comprehensive Guide to MEGA-PRESS for GABA Measurement. Anal. Biochem. 2021, 669, 115113. [Google Scholar] [CrossRef]
- van Veenendaal, T.M.; Backes, W.H.; van Bussel, F.C.; Edden, R.A.; Puts, N.A.; Aldenkamp, A.P.; Jansen, J.F. Glutamate quantification by PRESS or MEGA-PRESS: Validation, repeatability, and concordance. Magn. Reson. Imaging 2018, 48, 107–114. [Google Scholar] [CrossRef]
- Hubbard, C.; Khan, S.; Keaser, M.; Goyal, M.; Seminowicz, D. (214) Gray matter abnormalities in migraine patients associated with disease chronicity, attack frequency, and intensity ofmigraine pain. J. Pain. 2014, 15, S29. [Google Scholar] [CrossRef]
- Coppola, G.; Di Renzo, A.; Tinelli, E.; Di Lorenzo, C.; Scapeccia, M.; Parisi, V.; Serrao, M.; Evangelista, M.; Ambrosini, A.; Colonnese, C.; et al. Resting state connectivity between default mode network and insula encodes acute migraine headache. Cephalalgia 2018, 38, 846–854. [Google Scholar] [CrossRef]
- Edden, R.A.; Puts, N.A.; Harris, A.D.; Barker, P.B.; Evans, C.J. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 2014, 40, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Puts, N.A.J.; Edden, R.A.E. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 2015, 42, 1431–1440. [Google Scholar] [CrossRef]
- Aguila, M.E.; Lagopoulos, J.; Leaver, A.M.; Rebbeck, T.; Hubscher, M.; Brennan, P.C.; Refshauge, K.M. Elevated levels of GABA+ in migraine detected using (1) H-MRS. NMR Biomed. 2015, 28, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, K.L.; Kaube, H.; Goadsby, P.J. Sumatriptan can inhibit trigeminal afferents by an exclusively neural mechanism. Brain 1996, 119 Pt 5, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Fernández, I.; Ríos-León, M.; Deelchand, D.K.; Garrido, L.; Torres-Llacsa, M.; García-García, F.; Vidorreta, M.; Ip, I.B.; Bridge, H.; Taylor, J.; et al. Chronic neuropathic pain components in whiplash-associated disorders correlate with metabolite concentrations in the anterior cingulate and dorsolateral prefrontal cortex: A consensus-driven MRS re-examination. Front. Med. 2024, 11, 1404939. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, X.; Bai, X.; Zhang, Y.; Yuan, Z.; Tang, H.; Li, Z.; Hu, Z.; Zhang, Y.; Yu, X.; et al. Gamma-aminobutyric acid and glutamate/glutamine levels in the dentate nucleus and periaqueductal gray with episodic and chronic migraine: A proton magnetic resonance spectroscopy study. J. Headache Pain. 2022, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.; Sundgren, P.; Hubbard, J.; Craig, A.D.; Clauw, D. Bilateral anterior insular glutamate (Glu) is asymmetrically associated with experimental pain in individuals with fibromyalgia and pain-free controls. Arthritis Rheum. 2009, 10, 1994. [Google Scholar]
- Harte, S.E.; Clauw, D.J.; Hayes, J.M.; Feldman, E.L.; St Charles, I.C.; Watson, C.J. Reduced intraepidermal nerve fiber density after a sustained increase in insular glutamate: A proof-of-concept study examining the pathogenesis of small fiber pathology in fibromyalgia. Pain. Rep. 2017, 2, e590. [Google Scholar] [CrossRef]
- Montemurro, N.; Trilli, I.; Bordea, I.R.; Ferrara, E.; Francesco, M.D.; Caccamo, F.; Malcangi, G.; Rapone, B. Are Whiplash-Associated Disorders and Temporomandibular Disorders in a Trauma Related Cause and Effect Relationship? A Review. Medicina 2023, 59, 1482. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Grimes, D.A. Unequal group sizes in randomised trials: Guarding against guessing. Lancet 2002, 359, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Sanaei Nezhad, F.; Anton, A.; Michou, E.; Jung, J.; Parkes, L.M.; Williams, S.R. Quantification of GABA, glutamate and glutamine in a single measurement at 3 T using GABA-edited MEGA-PRESS. NMR Biomed. 2018, 31, e3847. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.E.; Lauriat, T.L.; Shanahan, M.; Renshaw, P.F.; Jensen, J.E. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: A phantom study at 4 Tesla. J. Magn. Reson. 2011, 208, 210–218. [Google Scholar] [CrossRef]
- Bell, T.; Boudes, E.S.; Loo, R.S.; Barker, G.J.; Lythgoe, D.J.; Edden, R.A.E.; Lebel, R.M.; Wilson, M.; Harris, A.D. In vivo Glx and Glu measurements from GABA-edited MRS at 3 T. NMR Biomed. 2021, 34, e4245. [Google Scholar] [CrossRef]

|
Migraine (n = 20) |
Whiplash-Headache (n = 20) |
Low Back Pain (n = 20) |
Healthy Controls (n = 21) | p-Value |
Post Hoc Adj.
Significance < 0.05 | |
|---|---|---|---|---|---|---|
| Age (years) | 39.7 ± 10 | 42.3 ± 11.5 | 40 ± 13.8 | 38.2 ± 10.6 | - | |
| Sex (female n, %) | 16, 80 | 14, 70 | 15, 75 | 16, 76.2 | - | |
| BMI | 27 ± 6.5 | 28.4 ± 8.3 | 25.5 ± 6.2 | 25.1 ± 3.7 | - | |
| Educational level (University n, %) | 12, 60 | 7, 41 | 8, 42.1 | 16, 72.7 | - | |
| Pain Characteristics | ||||||
| Duration-years | 19.7 ± 11.3 | 2.65 ± 2.1 | 7 ± 6.6 | N/A | <0.001 | W-M; LBP-M |
| Average pain intensity in last week (NRS 0–100) | 66.1 ± 22.9 | 58.8 ± 21.4 | 55.6 ± 21.9 | N/A | - | - |
| Pain Sensitivity | ||||||
| Pain intensity at time of scan (NRS 0–100) | 36.6 ± 30.2 | 40 ± 23.4 | 33.1 ± 22.7 | 4.3 ± 9.3 | <0.001 | LBP-C; W-C; M-C |
| CSI | 48.6 ± 16.5 | 52.4 ± 18.7 | 31.3 ± 14.3 | 9.3 ± 9.6 | <0.001 | LBP-C; W-C; M-C; LBP-W |
| Disability | ||||||
| WHODAS 2.0 (IQR) | 26.9 ± 18.4 | 32.6 ± 19 | 20.5 ± 11.1 | 0.7 ± 1.6 | <0.001 | LBP-C; W-C; M-C |
| HIT-6 | 66.35 ± 6.6 | 63.71 ± 8.62 | 41.21 ± 5.42 | 32.27 ± 15.5 | <0.001 | W-C; M-C; W-LBP; LBP-M |
| Psychological Status | ||||||
| DASS Total (IQR) | 25.7 ± 20.6 | 42.3 ± 29.7 | 19.5 ± 15.7 | 4.1 ± 6.9 | <0.001 | LBP-C; W-C; M-C |
| Neurochemical Level (IU) | ||||||
| GABA+ mean (SD) | 4.87 (0.62) | 4.74 (0.43) | 4.84 (0.47) | 4.68 (0.43) | - | |
| Glx mean (SD) | 12.79 (1.80) | 12.00 (0.82) | 12.23 (0.82) | 12.81 (1.58) | - | |
| GABA+/Glx mean (SD) | 0.38 (0.05) | 0.40 (0.04) | 0.40 (0.04) | 0.37 (0.05) | - | |
| Clinical Classification * | ||||||
| Cervical MSK impairment (n, %) | 10 (50%) | 18 (90%) | 0 (0%) | 0 (0%) | - | |
| Increased cervical pain sensitivity (n, %) | 8 (40%) | 12 (60%) | 4 (20%) | 0 (0%) | - | |
| Central Sensitization (n, %) | 4 (20%) | 9 (45%) | 2 (11.1%) | 0 (0%) | - |
| Neurochemicals | ||||
|---|---|---|---|---|
| GABA+ 1 (IU) | Glx (IU) | GABA/Glx (IU) | ||
| Clinical Classification | Cervical MSK impairment | 0.03 | −0.09 | 0.10 |
| Central sensitization | 0.10 | −0.04 | 0.09 | |
| Inc. Cervical pain sensitivity | 0.31 ** | −0.02 | 0.21 | |
| MSK imp. and Inc. cervical pain sensitivity | 0.18 | −0.03 | 0.14 | |
| All classifications | 0.17 | 0.02 | 0.10 | |
| None | −0.17 | −0.07 | −0.04 | |
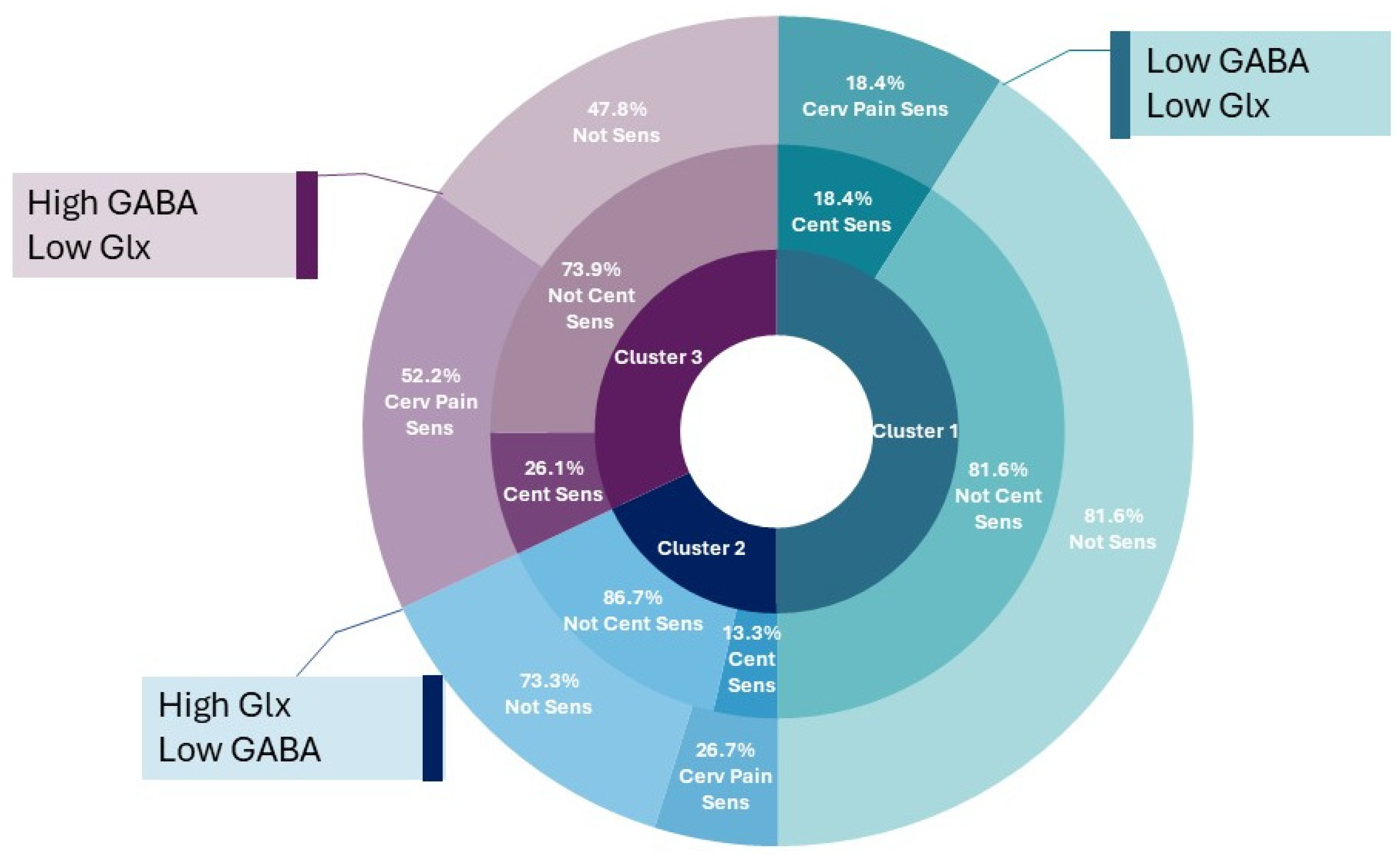
| Cluster 1 (n = 38) Lowest GABA Low Glx | Cluster 2 (n = 15) Highest Glx Low GABA | Cluster 3 (n = 23) Highest GABA Low Glx | Post Hoc | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 37.8 [34.0- 41.5] | 39.4 [33.0–45.8] | 43.0 [37.9–48.0] | - | |
| Sex (female n, %) | 31 [81.6] | 10 [66.7] | 16 [69.6] | - | |
| BMI | 26.0 [23.7–28.2] | 26.2 [23.9–29.0] | 27.7 [25.4–30.1] | - | |
| Duration (years) | 7.6 [4.0–11.3] | 6.2 [1.4–13.0] | 7.7 [2.1–11.6] | - | |
| Pain condition | |||||
| Migraine (n, [%]) | 9/38 [23.7%] | 3/15 [20%] | 5/23 [21.7%] | - | |
| Whiplash-Headache (n, [%]) | 8/38 [21.1%] | 3/15 [20%] | 7/23 [30.4%] | - | |
| Low back pain (n, [%]) | 10/38 [26.3%] | 3/15 [20%] | 7/23 [30.4%] | - | |
| Healthy control (n, [%]) | 11/38 [28.9%] | 6/15 [40%] | 3/23 [12.1%] | - | |
| Brain neurochemistry | |||||
| GABA+ levels (IU) | 4.5 [4.4–4.6] | 4.8 [4.7–4.9] | 5.1 [5.0–5.3] | 1–2; 1–3; 2–3 | |
| Glx levels (IU) | 12 [11.8–12.3] | 14.8 [13.8–14.9] | 11.9 [11.5–12.1] | 1–2; 2–3 | |
| GABA/Glx ratio | 0.38 [0.36–0.39] | 0.34 [0.32–0.35] | 0.43 [0.42–0.44] | 1–2; 2–3; 1–3 | |
| Patient-reported outcome measures | |||||
| Pain (NRS 0–100) | 41.8 [30.8–53.5] | 36.3 [17.4–55.3] | 55.0 [40.6–68.2] | - | |
| DASS total (0–42) | 15.0 [8.7–21.8] | 11.3 [4.9–18.5] | 19.3 [13.1–25.5] | - | |
| CSI (0–100) | 36.7 [28.5–44.9] | 26.5 [15.2–37.9] | 37.6 [28.8–46.4] | - | |
| WHODAS (0–100) | 19.8 [12.5–26.8] | 15.9 [7.6–25.6] | 23.2 [15.2–31.1] | - | |
| Clinical classification ^ | Total (n = 76) | Post hoc | |||
| Cervical MSK Impair | 9 [23.7%] | 6 [40%] | 11 [47.8%] | 26 [34.2%] | - |
| Increased cervical pain sens. | 7 [18.4%] | 4 [26.7%] | 12 [52.2%] | 23 [30.2%] | - |
| Central Sensitization | 7 [18.4%] | 2 [13.3%] | 6 [26.1%] | 15 [19.7%] | - |
| No classification | 21 [55.3%] | 5 [33.3%] | 8 [34.8%] | 34 [44.7%] | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peek, A.L.; Liang, Z.; Treleaven, J.; Rebbeck, T. Exploring the Relationship Between Brain Neurochemistry, Cervical Impairments and Pain Sensitivity in People with Migraine, Whiplash-Headache, Low Back Pain and Healthy Controls: A Secondary Analysis of a Cross-Sectional Case-Control Study. J. Clin. Med. 2025, 14, 1510. https://doi.org/10.3390/jcm14051510
Peek AL, Liang Z, Treleaven J, Rebbeck T. Exploring the Relationship Between Brain Neurochemistry, Cervical Impairments and Pain Sensitivity in People with Migraine, Whiplash-Headache, Low Back Pain and Healthy Controls: A Secondary Analysis of a Cross-Sectional Case-Control Study. Journal of Clinical Medicine. 2025; 14(5):1510. https://doi.org/10.3390/jcm14051510
Chicago/Turabian StylePeek, Aimie L., Zhiqi Liang, Julia Treleaven, and Trudy Rebbeck. 2025. "Exploring the Relationship Between Brain Neurochemistry, Cervical Impairments and Pain Sensitivity in People with Migraine, Whiplash-Headache, Low Back Pain and Healthy Controls: A Secondary Analysis of a Cross-Sectional Case-Control Study" Journal of Clinical Medicine 14, no. 5: 1510. https://doi.org/10.3390/jcm14051510
APA StylePeek, A. L., Liang, Z., Treleaven, J., & Rebbeck, T. (2025). Exploring the Relationship Between Brain Neurochemistry, Cervical Impairments and Pain Sensitivity in People with Migraine, Whiplash-Headache, Low Back Pain and Healthy Controls: A Secondary Analysis of a Cross-Sectional Case-Control Study. Journal of Clinical Medicine, 14(5), 1510. https://doi.org/10.3390/jcm14051510






