Left Ventricular Thrombosis in Ischemic and Non-Ischemic Cardiomyopathies: Focus on Evidence-Based Treatment
Abstract
:1. Introduction
2. Epidemiology
2.1. Ischemic Cardiopathy
2.2. Non-Ischemic Cardiomyopathy—Familial and Non-Dilated Cardiomiopathy
2.3. Non-Ischemic Cardiomyopathy—Others
3. Pathophysiology and Risk Factors
3.1. Ischemic Cardiomyopathy
3.2. Dilated Cardiomyopathy
3.3. Hypertrophic Cardiomyopathy
3.4. Cardiac Amyloidosis
3.5. Left Ventricular Non-Compaction Cardiomyopathy
4. Diagnosis
5. Therapy
5.1. Antithrombotic Agent
5.2. Antithrombotic Preventive Therapy
5.3. Duration of Anticoagulant Therapy
5.4. Which Antithrombotic Treatment to Start with
5.5. Future Perspectives
6. Conclusions
Funding
Conflicts of Interest
References
- Lee, J.M.; Park, J.J.; Jung, H.W.; Cho, Y.-S.; Oh, I.-Y.; Yoon, C.-H.; Suh, J.-W.; Chun, E.J.; Choi, S.I.; Youn, T.-J.; et al. Left ventricular thrombus and subsequent thromboembolism, comparison of anticoagulation, surgical removal, and antiplatelet agents. J. Atheroscler. Thromb. 2013, 20, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Aikawa, H.; Nakao, K.; Takagi, K.; Otsuka, F.; Kataoka, Y.; Asaumi, Y.; Sumita, Y.; Nakai, M.; Kanaoka, K.; et al. Comparison of embolic risk in left ventricular thrombus between nonischemic and ischemic cardiomyopathy: A nationwide database analysis. Int. J. Cardiol. 2024, 411, 132329. [Google Scholar] [CrossRef] [PubMed]
- Habash, F.; Vallurupalli, S. Challenges in management of left ventricular thrombus. Ther. Adv. Cardiovasc. Dis. 2017, 11, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, F.; Santoro, E.; Domenicucci, S.; Maggioni, A.; Vecchio, C. Predischarge two-dimensional echocardiographic evaluation of left ventricular thrombosis after acute myocardial infarction in the GISSI-3 study. Am. J. Cardiol. 1998, 81, 882–887. [Google Scholar] [CrossRef]
- Bulluck, H.; Chan, M.H.H.; Paradies, V.; Yellon, R.L.; Ho, H.H.; Chan, M.Y.; Chin, C.W.L.; Tan, J.W.; Hausenloy, D.J. Incidence and predictors of left ventricular thrombus by cardiovascular magnetic resonance in acute ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: A meta-analysis. J. Cardiovasc. Magn. Reson. 2018, 20, 72. [Google Scholar] [CrossRef]
- Sacoransky, E.; Ke, D.Y.J.; Dave, P.; Alexander, B.; El Sherbini, A.; Abunassar, J.; Abuzeid, W. Incidence of left ventricular thrombus following STEMI in the modern era via multimodality imaging: A systematic review and meta-analysis. IJC Heart Vasc. 2024, 52, 101396. [Google Scholar] [CrossRef]
- Choi, J.; Saravia, S.D.; Matthews, C.; Kong, D.; Gupta, S.; Gandhi, K.; Moras, E.; Mahmood, K. TCT-549 Outcomes and Predictors of Left Ventricular Thrombus in NSTEMI: A Retrospective Study. J. Am. Coll. Cardiol. 2023, 82 (Suppl. S17), B221. [Google Scholar] [CrossRef]
- Li, X.-L.; Adi, D.; Wu, Y.; Aizezi, A.; Li, Y.-P.; Kerem, M.; Wei, X.; Liu, F.; Ma, X.; Ma, Y.-T. A nomogram to predict ventricular thrombus in dilated cardiomyopathy patients. J. Thromb. Thrombolysis. 2024, 57, 29–38. [Google Scholar] [CrossRef]
- de Gregorio, C.; Grimaldi, P.; Lentini, C. Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: A systematic review. Int. J. Cardiol. 2008, 131, 18–24. [Google Scholar] [CrossRef]
- Vukomanovic, D.; Hajek, K.; Unzek, S.; Gopalan, R.; Mookadam, F. The stress clot: Mid-cavitary variant takotsubo cardiomyopathy with left ventricular thrombus. Echocardiography 2023, 40, 1280–1284. [Google Scholar] [CrossRef]
- Rowin, E.J.; Maron, B.J.; Haas, T.S.; Garberich, R.F.; Wang, W.; Link, M.S.; Maron, M.S. Hypertrophic Cardiomyopathy With Left Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. J. Am. Coll. Cardiol. 2017, 69, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Syed, I.S.; Martinez, M.; Oh, J.K.; Jaffe, A.S.; Grogan, M.; Edwards, W.D.; Gertz, M.A.; Klarich, K.W. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009, 119, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Hirono, K.; Takarada, S.; Miyao, N.; Nakaoka, H.; Ibuki, K.; Ozawa, S.; Origasa, H.; Ichida, F. Thromboembolic events in left ventricular non-compaction: Comparison between children and adults—A systematic review and meta-analysis. Open Heart 2022, 9, e001908. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Charron, P.; Eicher, J.; Giorgi, R.; Donal, E.; Laperche, T.; Boulmier, D.; Pascal, C.; Logeart, D.; Jondeau, G.; et al. Isolated left ventricular non-compaction in adults: Clinical and echocardiographic features in 105 patients. Results from a French registry. Eur. J. Heart Fail. 2011, 13, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Tichelbäcker, T.; Körber, M.I.; Mauri, V.; Iliadis, C.; Metze, C.; Adler, C.; Baldus, S.; Rudolph, V.; Halbach, M.; Pfister, R.; et al. Prevalence of left ventricular thrombus formation after mitral valve edge-to-edge repair. Sci. Rep. 2022, 12, 9096. [Google Scholar] [CrossRef]
- Wu, T.; Lulu, Z. Pulmonary valve involvement and left ventricular thrombosis in Behçet’s disease: A case report and literature review. Clin. Exp. Rheumatol. 2022, 40, 1607. [Google Scholar] [CrossRef]
- Ben Ghorbel, I.; Belfeki, N.; Houman, M.H. Intracardiac thrombus in Behçet’s disease. Reumatismo 2016, 68, 148–153. [Google Scholar] [CrossRef]
- Salanitri, G.C. Endomyocardial Fibrosis and Intracardiac Thrombus Occurring in Idiopathic Hypereosinophilic Syndrome. Am. J. Roentgenol. 2005, 184, 1432–1433. [Google Scholar] [CrossRef]
- Anzai, T.; Yoshikawa, T.; Kaneko, H.; Maekawa, Y.; Iwanaga, S.; Asakura, Y.; Ogawa, S. Association Between Serum C-Reactive Protein Elevation and Left Ventricular Thrombus Formation After First Anterior Myocardial Infarction. Chest 2004, 125, 384–389. [Google Scholar] [CrossRef]
- Brill, A.; Elinav, H.; Varon, D. Differential role of platelet granular mediators in angiogenesis. Cardiovasc. Res. 2004, 63, 226–235. [Google Scholar] [CrossRef]
- Bochenek, M.L.; Schäfer, K. Role of Endothelial Cells in Acute and Chronic Thrombosis. Hamostaseologie 2019, 39, 128–139. [Google Scholar] [CrossRef]
- Demarchi, A.; Somaschini, A.; Cornara, S.; Androulakis, E. Peripheral Artery Disease in Diabetes Mellitus: Focus on Novel Treatment Options. Curr. Pharm. Des. 2020, 26, 5953–5968. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.V.; Rapaport, S.I. Activation of factor VII bound to tissue factor: A key early step in the tissue factor pathway of blood coagulation. Proc. Natl. Acad. Sci. USA 1988, 85, 6687–6691. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Nakhaii-Nejad, M.; Phan, W.-L.; Kulak, S.; Radziwon-Balicka, A.; Jurasz, P.; Michelakis, E.; Jahroudi, N. Hypoxia Results in Upregulation and De Novo Activation of Von Willebrand Factor Expression in Lung Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1329–1338. [Google Scholar] [CrossRef]
- Mackman, N. Role of Tissue Factor in Hemostasis, Thrombosis, and Vascular Development. Arterioscler Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef]
- Ritschel, V.N.; Seljeflot, I.; Arnesen, H.; Halvorsen, S.; Weiss, T.; Eritsland, J.; Andersen, G.Ø. IL-6 signalling in patients with acute ST-elevation myocardial infarction. Results Immunol. 2014, 4, 8–13. [Google Scholar] [CrossRef]
- Neumann, F.-J.; Ott, I.; Marx, N.; Luther, T.; Kenngott, S.; Gawaz, M.; Kotzsch, M.; Schomig, A. Effect of Human Recombinant Interleukin-6 and Interleukin-8 on Monocyte Procoagulant Activity. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 3399–3405. [Google Scholar] [CrossRef]
- Cermak, J.; Key, N.S.; Bach, R.R.; Balla, J.; Jacob, H.S.; Vercellotti, G.M. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood 1993, 82, 513–520. [Google Scholar] [CrossRef]
- Demarchi, A.; Cornara, S.; Somaschini, A.; Fortuni, F.; Mandurino-Mirizzi, A.; Crimi, G.; Ferlini, M.; Gnecchi, M.; De Servi, S.; Visconti, L.O.; et al. Has hyperglycemia a different prognostic role in STEMI patients with or without diabetes? Nutr. Metab. Cardiovasc. Dis. 2021, 31, 528–531. [Google Scholar] [CrossRef]
- Lagrand, W.K.; Visser, C.A.; Hermens, W.T.; Niessen, H.W.M.; Verheugt, F.W.A.; Wolbink, G.-J.; Hack, C.E. C-Reactive Protein as a Cardiovascular Risk Factor. Circulation 1999, 100, 96–102. [Google Scholar] [CrossRef]
- van Dijk, A.; Krijnen, P.A.J.; Vermond, R.A.; Pronk, A.; Spreeuwenberg, M.; Visser, F.C.; Berney, R.; Paulus, W.J.; Hack, C.E.; van Milligen, F.J.; et al. Inhibition of type 2A secretory phospholipase A2 reduces death of cardiomyocytes in acute myocardial infarction. Apoptosis 2009, 14, 753–763. [Google Scholar] [CrossRef]
- Lechner, I.; Reindl, M.; Tiller, C.; Holzknecht, M.; Fink, P.; Plangger, J.; Mayr, A.; Klug, G.; Bauer, A.; Reinstadler, S.J.; et al. Association between inflammation and left ventricular thrombus formation following ST-elevation myocardial infarction. Int. J. Cardiol. 2022, 361, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Shacham, Y.; Leshem-Rubinow, E.; Ben Assa, E.; Rogowski, O.; Topilsky, Y.; Roth, A.; Steinvil, A. Comparison of C-reactive protein and fibrinogen levels in patients having anterior wall ST-Segment elevation myocardial infarction with versus without left ventricular thrombus (from a primary percutaneous coronary intervention cohort). Am. J. Cardiol. 2013, 112, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Cambronero-Cortinas, E.; Bonanad, C.; Monmeneu, J.V.; Lopez-Lereu, M.P.; Gavara, J.; de Dios, E.; Rios, C.; Perez, N.; Racugno, P.; Paya, A.; et al. Incidence, Outcomes, and Predictors of Ventricular Thrombus after Reperfused ST-Segment–Elevation Myocardial Infarction by Using Sequential Cardiac MR Imaging. Radiology 2017, 284, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Shacham, Y.; Leshem-Rubinow, E.; Ben Assa, E.; Rogowski, O.; Topilsky, Y.; Roth, A.; Steinvil, A. Frequency and correlates of early left ventricular thrombus formation following anterior wall acute myocardial infarction treated with primary percutaneous coronary intervention. Am. J. Cardiol. 2013, 111, 667–670. [Google Scholar] [CrossRef]
- Weinsaft, J.W.; Kim, J.; Medicherla, C.B.; Ma, C.L.; Codella, N.C.; Kukar, N.; Alaref, S.; Kim, R.J.; Devereux, R.B. Echocardiographic Algorithm for Post-Myocardial Infarction LV Thrombus: A Gatekeeper for Thrombus Evaluation by Delayed Enhancement CMR. JACC Cardiovasc. Imaging 2016, 9, 505–515. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, R.; Wu, G.; Zhu, Q.; Zhou, C.; Wang, L.; Xue, C.; Jiang, Y.; Cai, X.; Huang, W.; et al. The prevalence, predictors, and outcomes of spontaneous echocardiographic contrast or left ventricular thrombus in patients with HFrEF. ESC Heart Fail. 2021, 8, 1284–1294. [Google Scholar] [CrossRef]
- Hooks, M.; Okasha, O.; Velangi, P.S.; Nijjar, P.S.; Farzaneh-Far, A.; Shenoy, C. Left ventricular thrombus on cardiovascular magnetic resonance imaging in non-ischaemic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1425–1433. [Google Scholar] [CrossRef]
- Choi, U.L.; Park, J.-H.; Sun, B.J.; Oh, J.K.; Seong, S.W.; Lee, J.-H.; Choi, S.W.; Jeong, J.-O.; Kwon, I.S.; Seong, I.-W. Impaired left ventricular diastolic function is related to the formation of left ventricular apical thrombus in patients with acute anterior myocardial infarction. Heart Vessel. 2018, 33, 447–452. [Google Scholar] [CrossRef]
- Delemarre, B.J.; Visser, C.A.; Bot, H.; Dunning, A.J. Prediction of apical thrombus formation in acute myocardial infarction based on left ventricular spatial flow pattern. J. Am. Coll. Cardiol. 1990, 15, 355–360. [Google Scholar] [CrossRef]
- Van Dantzig, J.M.; Delemarre, B.J.; Bot, H.; Koster, R.W.; Visser, C.A. Doppler left ventricular flow pattern versus conventional predictors of left ventricular thrombus after acute myocardial infarction. J. Am. Coll. Cardiol. 1995, 25, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Günthard, J.; Stocker, F.; Bolz, D.; Jäggi, E.; Ghisla, R.; Oberhänsli, I.; Wyler, F. Dilated cardiomyopathy and thrombo-embolism. Eur J Pediatr. 1996, 156, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Günthard, J.; Stocker, F.; Bolz, D.; Jäggi, E.; Ghisla, R.; Oberhänsli, I.; Wyler, F. Risk Factors for Left Ventricular Thrombus Formation in Patients with Dilated Cardiomyopathy. Semin. Thromb. Hemost. 2022, 49, 673–678. [Google Scholar] [CrossRef]
- Pancaldi, E.; Tedino, C.; Riccardi, M. Endothelial function evaluation in idiopathic vs. ischemic dilated cardiomyopathy. Am. J. Cardiovasc. Dis. 2022, 12, 136–142. [Google Scholar] [PubMed]
- Sitges, M.; Roig, E.; Morales, M.; Azqueta, M.; Villa, F.P.; Paré, C.; Orús, J.; Heras, M.; Sanz, G. Impaired Endothelium-Dependent Forearm Vasodilation in Idiopathic Dilated Cardiomyopathy Is Related to Severe Left Ventricular Dysfunction and Elevated Serum Tumor Necrosis Factor Levels. Rev. Española De Cardiol. Engl. Ed. 2005, 58, 465–610. [Google Scholar] [CrossRef]
- Stolen, K.Q.; Kemppainen, J.; Kalliokoski, K.K.; Karanko, H.; Toikka, J.; Janatuinen, T.; Raitakari, O.T.; Airaksinen, K.; Nuutila, P.; Knuuti, J. Myocardial perfusion reserve and peripheral endothelial function in patients with idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2004, 93, 64–68. [Google Scholar] [CrossRef]
- Richard, P.; Villard, E.; Charron, P.; Isnard, R. The Genetic Bases of Cardiomyopathies. J Am Coll Cardiol. 2006, 48 (Suppl. S9), A79–A89. [Google Scholar] [CrossRef]
- Knoll, R.; Postel, R.; Wang, J.; Kratzner, R.; Hennecke, G.; Vacaru, A.M.; Vakeel, P.; Schubert, C.; Murthy, K.; Rana, B.K.; et al. Laminin-α4 and Integrin-Linked Kinase Mutations Cause Human Cardiomyopathy Via Simultaneous Defects in Cardiomyocytes and Endothelial Cells. Circulation 2007, 116, 515–525. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ikeda, U.; Furuhashi, K.; Irokawa, M.; Nakayama, T.; Shimada, K. The coagulation system is activated in idiopathic cardiomyopathy. J. Am. Coll. Cardiol. 1995, 25, 1634–1640. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, K.-Q.; Yang, Y.-K.; Liu, Y.-X.; Tian, T.; Song, L.; Jiang, X.-J.; Zhou, X.-L. Clinical Characteristics and Prognosis of End-stage Hypertrophic Cardiomyopathy. Chin. Med. J. 2015, 128, 1483–1489. [Google Scholar] [CrossRef]
- Olivotto, I.; Cecchi, F.; Poggesi, C.; Yacoub, M.H. Patterns of Disease Progression in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2012, 5, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M. Left Ventricular Thrombus in Hypertrophic Cardiomyopathy. Intern. Med. 2019, 58, 465. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Kimura, Y.; Murohara, T. Prediction of Thromboembolism in Patients with Hypertrophic Cardiomyopathy. Circ. J. 2020, 84, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, C.A.; Zegkos, T.; Karamitsos, T.D.; Rowin, E.J.; Maron, M.S.; Parcharidou, D.; Kokkinidis, D.G.; Karvounis, H.; Rimoldi, O.; Maron, B.J.; et al. Prognostic role of left ventricular apical aneurysm in hypertrophic cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiol. 2021, 332, 127–132. [Google Scholar] [CrossRef]
- Fang, L.; Ellims, A.H.; Beale, A.L.; Taylor, A.J.; Murphy Ax Dart, A.M. Systemic inflammation is associated with myocardial fibrosis, diastolic dysfunction, and cardiac hypertrophy in patients with hypertrophic cardiomyopathy. Am. J. Transl. Res. 2017, 9, 5063–5073. [Google Scholar]
- Matsumori, A.; Yamada, T.; Suzuki, H.; Matoba, Y.; Sasayama, S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br. Heart J. 1994, 72, 561. [Google Scholar] [CrossRef]
- Zen, K.; Irie, H.; Doue, T.; Takamiya, M.; Yamano, T.; Sawada, T.; Azuma, A.; Matsubara, H. Analysis of Circulating Apoptosis Mediators and Proinflammatory Cytokines in Patients With Idiopathic Hypertrophic Cardiomyopathy. Int. Heart J. 2005, 46, 231–244. [Google Scholar] [CrossRef]
- Iwasaki, J.; Nakamura, K.; Matsubara, H.; Nakamura, Y.; Nishii, N.; Banba, K.; Murakami, M.; Ohta-Ogo, K.; Kimura, H.; Toh, N.; et al. Relationship between circulating levels of monocyte chemoattractant protein-1 and systolic dysfunction in patients with hypertrophic cardiomyopathy. Cardiovasc. Pathol. 2009, 18, 317–322. [Google Scholar] [CrossRef]
- Kuusisto, J.; Kärjä, V.; Sipola, P.; Kholová, I.; Peuhkurinen, K.; Jääskeläinen, P.; Naukkarinen, A.; Ylä-Herttuala, S.; Punnonen, K.; Laakso, M. Low-grade inflammation and the phenotypic expression of myocardial fibrosis in hypertrophic cardiomyopathy. Heart 2012, 98, 1007. [Google Scholar] [CrossRef]
- Icli, A.; Aksoy, F.; Dogan, A.; Arslan, A.; Akcay, S.; Yücel, H.; Ersoy, I.; Gorgulu, O. Increased Mean Platelet Volume in Hypertrophic Cardiomyopathy. Angiology 2013, 65, 420–424. [Google Scholar] [CrossRef]
- Yarom, R.; Lewis, B.S.; Lijovetzky, G.; Havivi, Y.; Chandler, J.A. Platelet studies in patients with hypertrophic cardiomyopathy. Cardiovasc. Res. 1982, 16, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, P.; Weikert, U.; Schmidt-Lucke, C.; Skurk, C.; Meyer, A.; Steffens, D.; Schultheiss, H.P.; Rauch, U. Platelet activation and thrombus formation relates to the presence of myocardial inflammation in patients with cardiomyopathy. J. Cardiol. 2014, 63, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Hohneck, A.; Overhoff, D.; Doesch, C.; Sandberg, R.; Rudic, B.; Tueluemen, E.; Budjan, J.; Szabo, K.; Borggrefe, M.; Papavassiliu, T. Extent of Late Gadolinium Enhancement Predicts Thromboembolic Events in Patients With Hypertrophic Cardiomyopathy. Circ. J. 2020, 84, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Edwards, W.D.; Oh, J.K.; Chandrasekaran, K.; Grogan, M.; Martinez, M.W.; Syed, I.I.; Hughes, D.A.; Lust, J.A.; Jaffe, A.S.; et al. Intracardiac Thrombosis and Embolism in Patients With Cardiac Amyloidosis. Circulation 2007, 116, 2420–2426. [Google Scholar] [CrossRef]
- Tana, M.; Tana, C.; Rossi, D.; Mantini, C.; Gallina, S.; Ricci, F.; Porreca, E. Thromboembolic and bleeding risk in cardiac amyloidosis. J. Thromb. Haemost. 2024, 22, 2381–2392. [Google Scholar] [CrossRef]
- Guan, J.; Mishra, S.; Shi, J.; Plovie, E.; Qiu, Y.; Cao, X.; Gianni, D.; Jiang, B.; del Monte, F.; Connors, L.H.; et al. Stanniocalcin1 is a key mediator of amyloidogenic light chain induced cardiotoxicity. Basic Res. Cardiol. 2013, 108, 378. [Google Scholar] [CrossRef]
- Guan, J.; Mishra, S.; Qiu, Y.; Shi, J.; Trudeau, K.; Las, G.; Liesa, M.; Shirihai, O.S.; Connors, L.H.; Seldin, D.C.; et al. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol. Med. 2014, 6, 1493–1507. [Google Scholar] [CrossRef]
- Brenner, D.A.; Jain, M.; Pimentel, D.R.; Wang, B.; Connors, L.H.; Skinner, M.; Apstein, C.S.; Liao, R. Human Amyloidogenic Light Chains Directly Impair Cardiomyocyte Function Through an Increase in Cellular Oxidant Stress. Circ. Res. 2004, 94, 1008–1010. [Google Scholar] [CrossRef]
- Griffin, J.M.; Rosenthal, J.L.; Grodin, J.L.; Maurer, M.S.; Grogan, M.; Cheng, R.K. ATTR Amyloidosis: Current and Emerging Management Strategies. JACC CardioOncol. 2021, 3, 488–505. [Google Scholar] [CrossRef]
- Hausfater, P.; Costedoat-Chalumeau, N.; Amoura, Z.; Cacoub, P.; Papo, T.; Grateau, G.; Leblond, V.; Godeau, P.; Piette, J.C. AL cardiac amyloidosis and arterial thromboembolic events. Scand. J. Rheumatol. 2005, 34, 315–319. [Google Scholar] [CrossRef]
- Modesto, K.M.; Dispenzieri, A.; Gertz, M.; Cauduro, S.A.; Khandheria, B.K.; Seward, J.B.; Kyle, R.; Wood, C.M.; Bailey, K.R.; Tajik, A.J.; et al. Vascular abnormalities in primary amyloidosis. Eur. Heart J. 2007, 28, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G.; Montani, N.; Anesi, E.; Palladini, G.; Lorenzutti, F.; Perfetti, V.; Merlini, G. Abnormalities in thrombin-antithrombin pathway in AL amyloidosis. Amyloid 1999, 6, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, K.I.; Choi, M.J. Understanding Hypercoagulability with Nephrotic Syndrome: How the Clot Thickens. Clin. J. Am. Soc. Nephrol. 2023, 18, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.; Doimo, S.; Ricci, F.; Sanghvi, M.M.; Pedrosa, C.; Woodbridge, S.P.; Al-Balah, A.; Zemrak, F.; Khanji, M.Y.; Munroe, P.B.; et al. Prognostic Significance of Left Ventricular Noncompaction. Circ. Cardiovasc. Imaging 2020, 13, e009712. [Google Scholar] [CrossRef] [PubMed]
- Saric, M.; Armour, A.C.; Arnaout, M.S.; Chaudhry, F.A.; Grimm, R.A.; Kronzon, I.; Landeck, B.F.; Maganti, K.; Michelena, H.I.; Tolstrup, K. Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. J. Am. Soc. Echocardiogr. 2016, 29, 1–42. [Google Scholar] [CrossRef]
- Ali, A.A.; Sakr, E.E. Left ventricle pedunculated thrombi risks and outcomes: A case report and literature review. J. Vasc. Bras. 2024, 23, e20230124. [Google Scholar] [CrossRef]
- Delewi, R.; Nijveldt, R.; Hirsch, A.; Marcu, C.B.; Robbers, L.; Hassell, M.E.; de Bruin, R.H.; Vleugels, J.; van der Laan, A.M.; Bouma, B.J.; et al. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur. J. Radiol. 2012, 81, 3900–3904. [Google Scholar] [CrossRef]
- Srichai, M.B.; Junor, C.; Rodriguez, L.L.; Stillman, A.E.; Grimm, R.A.; Lieber, M.L.; Weaver, J.A.; Smedira, N.G.; White, R.D. Clinical, imaging, and pathological characteristics of left ventricular thrombus: A comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am. Heart J. 2006, 152, 75–84. [Google Scholar] [CrossRef]
- Camaj, A.; Fuster, V.; Giustino, G.; Bienstock, S.W.; Sternheim, D.; Mehran, R.; Dangas, G.D.; Kini, A.; Sharma, S.K.; Halperin, J.; et al. Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1010–1022. [Google Scholar] [CrossRef]
- Oh, J.K.; Park, J.-H.; Lee, J.-H.; Kim, J.; Seong, I.-W. Shape and Mobility of a Left Ventricular Thrombus Are Predictors of Thrombus Resolution. Korean Circ. J. 2019, 49, 829–837. [Google Scholar] [CrossRef]
- Weinsaft, J.W.; Kim, R.J.; Ross, M.; Krauser, D.; Manoushagian, S.; LaBounty, T.M.; Cham, M.D.; Min, J.K.; Healy, K.; Wang, Y.; et al. Contrast-enhanced anatomic imaging as compared to contrast-enhanced tissue characterization for detection of left ventricular thrombus. JACC Cardiovasc. Imaging 2009, 2, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Phuah, Y.; Tan, Y.X.; Zaghloul, S.; Sim, S.; Wong, J.; Usmani, S.; Snell, L.; Thavabalan, K.; García-Pérez, C.L.; Kumar, N.S.; et al. A systematic review and meta-analysis of transthoracic echocardiogram vs. cardiac magnetic resonance imaging for the detection of left ventricular thrombus. Eur. Heart J.—Imaging Methods Pract. 2023, 1, qyad041. [Google Scholar] [CrossRef] [PubMed]
- Hilberath, J.N.; Oakes, D.A.; Shernan, S.K.; Bulwer, B.E.; D’Ambra, M.N.; Eltzschig, H.K. Safety of Transesophageal Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Doherty, J.U.; Dehmer, G.J. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 Appropriate Use Criteria for Multimodality Imaging in the Assessment of Cardiac Structure and Function in Nonvalvular Heart Disease. J. Am. Soc. Echocardiogr. 2019, 32, 553–579. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, M.C.; He, Y.Q.; Liu, L.; Tong, Y.L.; Yang, P. Application of spectral computed tomography dual-substance separation technology for diagnosing left ventricular thrombus. J. Int. Med. Res. 2015, 44, 54–66. [Google Scholar] [CrossRef]
- Bittencourt, M.S.; Achenbach, S.; Marwan, M.; Seltmann, M.; Muschiol, G.; Ropers, D.; Daniel, W.G.; Pflederer, T. Left ventricular thrombus attenuation characterization in cardiac computed tomography angiography. J. Cardiovasc. Comput. Tomogr. 2012, 6, 121–126. [Google Scholar] [CrossRef]
- Sechtem, U.; Theissen, P.; Heindel, W.; Hungerberg, K.; Deutsch, H.-J.; Welslau, R.; Curtius, J.M.; Hügel, W.; Höpp, H.-W.; Schicha, H. Diagnosis of left ventricular thrombi by magnetic resonance imaging and comparison with angiocardiography, computed tomography and echocardiography. Am. J. Cardiol. 1989, 64, 1195–1199. [Google Scholar] [CrossRef]
- Nakao, Y.; Aono, J.; Namiguchi, K.; Nishimura, T.; Izutani, H.; Higashi, H.; Inaba, S.; Nishimura, K.; Inoue, K.; Ikeda, S.; et al. Usefulness of contrast computed tomography for diagnosing left ventricular thrombus before impella insertion. J. Cardiol. Cases 2020, 22, 291–293. [Google Scholar] [CrossRef]
- Ouchi, K.; Nakamura, F.; Ikutomi, M.; Oshima, T.; Ishiwata, J.; Shinohara, H.; Kouzaki, T.; Amaki, T. Usefulness of contrast computed tomography to detect left ventricular apical thrombus associated with takotsubo cardiomyopathy. Heart Vessel. 2016, 31, 822–827. [Google Scholar] [CrossRef]
- Flohr, T.; Petersilka, M.; Henning, A.; Ulzheimer, S.; Ferda, J.; Schmidt, B. Photon-counting CT review. Phys. Medica Eur. J. Med. Phys. 2020, 79, 126–136. [Google Scholar] [CrossRef]
- Mergen, V.; Sartoretti, T.; Baer-Beck, M.; Schmidt, B.; Petersilka, M.; Wildberger, J.E.; Euler, A.; Eberhard, M.; Alkadhi, H. Ultra-High-Resolution Coronary CT Angiography With Photon-Counting Detector CT: Feasibility and Image Characterization. Investig. Radiol. 2022, 57, 780–788. [Google Scholar] [CrossRef]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Kisohara, M.; Kitera, N.; Itoh, T.; Murai, K.; Hiwatashi, A.; Kawai, T. Identification of a small thrombus in the left ventricle identified on iodine maps derived from dual-source photon-counting detector CT. Radiol. Case Rep. 2024, 19, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.T.; Le, T.M.; Tran, C.C.; Nguyen, K.D.; Nguyen, A.D.Q.; Nguyen, C.D.; Nguyen, C.M.; Nguyen, T.T.; Tran, L.M.B. Left ventricular thrombus in patient with nonischemic cardiomyopathy: A case report. Radiol. Case Rep. 2024, 19, 5241–5247. [Google Scholar] [CrossRef] [PubMed]
- Vidula, M.K.; Han, Y.; Litt, H.; Bravo, P.E. Left ventricular mural thrombus appearing as a photopenic defect on myocardial viability PET imaging. J. Nucl. Cardiol. 2022, 29, 2713–2715. [Google Scholar] [CrossRef]
- Soulen, R.L.; Grollman, J.H.; Paglia, D.; Kreulen, T. Coronary neovascularity and fistula formation: A sign of mural thrombus. Circulation 1977, 56, 663–666. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Allard, L.; Bernhard, B.; Windecker, S.; Valgimigli, M.; Gräni, C. Left ventricular thrombus in ischaemic heart disease: Diagnosis, treatment, and gaps of knowledge. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 496–509. [Google Scholar] [CrossRef]
- Sürder, D.; Gisler, V.; Corti, R.; Moccetti, T.; Klersy, C.; Zuber, M.; Windecker, S.; Moschovitis, A.; Kozerke, S.; Lüscher, T.F.; et al. Thrombus formation in the left ventricle after large myocardial infarction—Assessment with cardiac magnetic resonance imaging. Swiss Med. Wkly. 2015, 145, w14122. [Google Scholar] [CrossRef]
- Roifman, I.; Connelly, K.A.; Wright, G.A.; Wijeysundera, H.C. Echocardiography vs Cardiac Magnetic Resonance Imaging for the Diagnosis of Left Ventricular Thrombus: A Systematic Review. Can. J. Cardiol. 2015, 31, 785–791. [Google Scholar] [CrossRef]
- Alhassan, D.A.; Waheed, K.B.; Sharif, M.N.; UlHassan, M.Z.; Ghaffar, F.; Saleem, K.S.; Said, E.F.M.; Altalaq, B.M.; Arulanantham, Z.J.; Qarmash, A.O. Detection of Left Ventricular Thrombi On Cardiac Magnetic Resonance Viability Studies. J. Saudi. Heart Assoc. 2020, 32, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Lattuca, B.; Bouziri, N.; Kerneis, M.; Portal, J.-J.; Zhou, J.; Hauguel-Moreau, M.; Mameri, A.; Zeitouni, M.; Guedeney, P.; Hammoudi, N.; et al. Antithrombotic Therapy for Patients With Left Ventricular Mural Thrombus. J. Am. Coll. Cardiol. 2020, 75, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and with Left Ventricular Thrombus: A Scientific Statement from the American Heart Association. Circulation 2022, 146, E205–E223. [Google Scholar] [CrossRef]
- Jugdutt, B.I.; Sivaram, C.A. Prospective Two-Dimensional Echocardiographic Evaluation of Left Ventricular Thrombus and Embolism After Acute Myocardial Infarction. JACC 1989, 13, 554–564. [Google Scholar] [CrossRef]
- Keating, E.C.; Gross, S.A.; Schlamowitz, R.A.; Glassman, J.; Mazur, J.H.; Pitt, W.A.; Miller, D. Mural thrombi in myocardial infarctions. Prospective evaluation by two-dimensional echocardiography. Am. J. Med. 1983, 74, 989–995. [Google Scholar] [CrossRef]
- Weinreich, D.J.; Burke, J.F.; Pauletto, F.J. Left ventricular mural thrombi complicating acute myocardial infarction. Long-term follow-up with serial echocardiography. Ann. Intern. Med. 1984, 100, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Vaitkus, P.T.; Barnathan, E.S. Embolic potential, prevention and management of mural thrombus complicating anterior myocardial infarction: A meta-analysis. J. Am. Coll. Cardiol. 1993, 22, 1004–1009. [Google Scholar] [CrossRef]
- O’gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e78–e140. [Google Scholar] [CrossRef]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef]
- Kouvaras, G.; Chronopoulos, G.; Soufras, G.; Sofronas, G.; Solomos, D.; Bakirtzis, A.; Pissimissis, E.; Tzonou, A.; Cokkinos, D. The effects of long-term antithrombotic treatment on left ventricular thrombi in patients after an acute myocardial infarction. Am. Heart J. 1990, 119, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Maniwa, N.; Fujino, M.; Nakai, M.; Nishimura, K.; Miyamoto, Y.; Kataoka, Y.; Asaumi, Y.; Tahara, Y.; Nakanishi, M.; Anzai, T.; et al. Anticoagulation combined with antiplatelet therapy in patients with left ventricular thrombus after first acute myocardial infarction. Eur. Heart J. 2018, 39, 201–208. [Google Scholar] [CrossRef]
- Robinson, A.A.; Trankle, C.R.; Eubanks, G.; Schumann, C.; Thompson, P.; Wallace, R.L.; Gottiparthi, S.; Ruth, B.; Kramer, C.M.; Salerno, M.; et al. Off-label Use of Direct Oral Anticoagulants Compared With Warfarin for Left Ventricular Thrombi. JAMA Cardiol. 2020, 5, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Daher, J.; Da Costa, A.; Hilaire, C.; Ferreira, T.; Pierrard, R.; Guichard, J.B.; Romeyer, C.; Isaaz, K. Management of Left Ventricular Thrombi with Direct Oral Anticoagulants: Retrospective Comparative Study with Vitamin K Antagonists. Clin. Drug Investig. 2020, 40, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.; Straw, S.; Craven, T.P.; Stirling, K.; Wheatcroft, S.B.; Witte, K.K. Direct oral anticoagulants compared to vitamin K antagonist for the management of left ventricular thrombus. ESC Heart Fail. 2020, 7, 2032. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, D.; Zhang, Q.; Qu, M.; Yu, M.; Jiang, Z.; Li, D.; Yang, P.; Zhang, W. Rivaroxaban versus Vitamin K Antagonists (warfarin) based on the triple therapy for left ventricular thrombus after ST-Elevation myocardial infarction. Heart Vessel. 2022, 37, 374–384. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Zhou, Y.; Shen, H.; Chai, M.; Ma, X.; Han, H.; Shao, Q.; Li, Q. Efficacy and Safety of Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus After Acute Anterior Myocardial Infarction in Patients Who Underwent Percutaneous Coronary Intervention. Curr. Vasc. Pharmacol. 2022, 20, 517–526. [Google Scholar] [CrossRef]
- Guddeti, R.R.; Anwar, M.; Walters, R.W.; Apala, D.; Pajjuru, V.; Kousa, O.; Gujjula, N.R.; Alla, V.M. Treatment of Left Ventricular Thrombus With Direct Oral Anticoagulants: A Retrospective Observational Study. Am. J. Med. 2020, 133, 1488–1491. [Google Scholar] [CrossRef]
- Mansouri, P.; Jazi, Z.A.; Mansouri, M.H.; Dehghan, H.; Zavar, R.; Hashemi, S.M.; Sattar, F.; Sadeghi, M.; Amirpour, A.; Abdar, M. Evaluation of the efficacy and safety of rivaroxaban compared to warfarin in patients with left ventricular apical thrombus: A randomized clinical trial. Thromb. J. 2024, 22, 66. [Google Scholar] [CrossRef]
- Alcalai, R.; Butnaru, A.; Moravsky, G.; Yagel, O.; Rashad, R.; Ibrahimli, M.; Planer, D.; Amir, O.; Elbaz-Greener, G.; Leibowitz, D. Apixaban vs. warfarin in patients with left ventricular thrombus: A prospective multicentre randomized clinical trial‡. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 660–667. [Google Scholar] [CrossRef]
- Youssef, A.A.; Alrefae, M.A.; Khalil, H.H.; Abdullah, H.I.; Khalifa, Z.S.; Al Shaban, A.A.; Wali, H.A.; AlRajab, M.R.; Saleh, O.M.; Nashy, B.N. Apixaban in Patients With Post-Myocardial Infarction Left Ventricular Thrombus: A Randomized Clinical Trial. CJC Open 2022, 5, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, M.; Saleh, Y.; Fareed, A.; Nossikof, A.; Wang, L.; Morsi, M.; Eshak, N.; Abdelkarim, O.; Badran, H.; Almaghraby, A. Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi (No-LVT Trial). J. Am. Coll. Cardiol. 2021, 77, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Dalia, T.; Lahan, S.; Ranka, S.; Goyal, A.; Zoubek, S.; Gupta, K.; Shah, Z. Warfarin versus direct oral anticoagulants for treating left ventricular thrombus: A systematic review and meta-analysis. Thromb. J. 2021, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Wright, P.; Alizadeh, M.A.; Fhadil, S.; Rathod, K.S.; Guttmann, O.; Knight, C.; Timmis, A.; Baumbach, A.; Wragg, A.; et al. The use of novel oral anticoagulants compared to vitamin K antagonists (warfarin) in patients with left ventricular thrombus after acute myocardial infarction. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 398–404. [Google Scholar] [CrossRef]
- Gogos, C.; Anastasiou, V.; Papazoglou, A.S.; Daios, S.; Didagelos, M.; Kamperidis, N.; Moschovidis, V.; Papadopoulos, S.F.; Iatridi, F.; Sarafidis, P.; et al. Direct Oral Anticoagulants versus Vitamin K Antagonists for the Management of Left Ventricular Thrombus after Myocardial Infarction: A Meta-analysis. Am. J. Cardiol. 2024, 232, 18–25. [Google Scholar] [CrossRef]
- Cruz Rodriguez, J.B.; Okajima, K.; Greenberg, B.H. Management of left ventricular thrombus: A narrative review. Ann. Transl. Med. 2021, 9, 520. [Google Scholar] [CrossRef]
- Ding, K.J.; Cammann, V.L.; Szawan, K.A.; Stähli, B.E.; Wischnewsky, M.; Di Vece, D.; Citro, R.; Jaguszewski, M.; Seifert, B.; Sarcon, A.; et al. Intraventricular Thrombus Formation and Embolism in Takotsubo Syndrome: Insights From the International Takotsubo Registry. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 279–287. [Google Scholar] [CrossRef]
- Kido, K.; Guglin, M. Anticoagulation Therapy in Specific Cardiomyopathies: Isolated Left Ventricular Noncompaction and Peripartum Cardiomyopathy. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 31–36. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Corrado, D.; Wichter, T.; Link, M.S.; Hauer, R.; Marchlinski, F.; Anastasakis, A.; Bauce, B.; Basso, C.; Brunckhorst, C.; Tsatsopoulou, A.; et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: An international task force consensus statement. Circulation 2015, 132, 441–453. [Google Scholar] [CrossRef]
- Muchtar, E.; Blauwet, L.A.; Gertz, M.A. Restrictive cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 2017, 121, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Meurin, P.; Carreira, V.B.; Dumaine, R.; Shqueir, A.; Milleron, O.; Safar, B.; Perna, S.; Smadja, C.; Genest, M.; Garot, J.; et al. Incidence, diagnostic methods, and evolution of left ventricular thrombus in patients with anterior myocardial infarction and low left ventricular ejection fraction: A prospective multicenter study. Am. Heart J. 2015, 170, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.A.; Jain, A.; Gentry, M.; McNamara, R.L. Left ventricular thrombi after STEMI in the primary PCI era: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 221, 554–559. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.P.; Vaduganathan, M.; McCarthy, K.J.; Januzzi, J.L.; Bhatt, D.L.; McEvoy, J.W. Left Ventricular Thrombus After Acute Myocardial Infarction: Screening, Prevention, and Treatment. JAMA Cardiol. 2018, 3, 642–649. [Google Scholar] [CrossRef]
- Le May, M.R.; Acharya, S.; Wells, G.A.; Burwash, I.; Chong, A.Y.; So, D.Y.; Glover, C.A.; Froeschl, M.P.; Hibbert, B.; Marquis, J.-F.; et al. Prophylactic warfarin therapy after primary percutaneous coronary intervention for anterior ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2015, 8 Pt B, 155–162. [Google Scholar] [CrossRef]
- Shavadia, J.S.; Youngson, E.; Bainey, K.R.; Bakal, J.; Welsh, R.C. Outcomes and Prognostic Impact of Prophylactic Oral Anticoagulation in Anterior ST-Segment Elevation Myocardial Infarction Patients with Left Ventricular Dysfunction. J. Am. Heart Assoc. 2017, 6, e006054. [Google Scholar] [CrossRef]
- Lamberts, M.; Olesen, J.B.; Ruwald, M.H.; Hansen, C.M.; Karasoy, D.; Kristensen, S.L.; Køber, L.; Torp-Pedersen, C.; Gislason, G.H.; Hansen, M.L. Bleeding After Initiation of Multiple Antithrombotic Drugs, Including Triple Therapy, in Atrial Fibrillation Patients Following Myocardial Infarction and Coronary Intervention A Nationwide Cohort Study. Circulation 2012, 126, 10. [Google Scholar] [CrossRef]
- Andrade, J.G.; Deyell, M.W.; Khoo, C.; Lee, M.; Humphries, K.; Cairns, J.A. Risk of Bleeding on Triple Antithrombotic Therapy After Percutaneous Coronary Intervention/Stenting: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2013, 29, 204–212. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Roffi, M.; Patrono, C.; Collet, J.-P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, D.; Zhang, Q.; Jin, L.; Zheng, H.; Qu, M.; Yu, M.; Jiang, Z.; Li, D.; Li, S.; et al. Prophylactic Rivaroxaban Therapy for Left Ventricular Thrombus After Anterior ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2022, 15, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.; Enriquez, A.; Suarez-Fuster, L.; Baranchuk, A. Atrial fibrillation in patients with inherited cardiomyopathies. Europace 2019, 21, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Grünig, E.; Tasman, J.A.; Kücherer, H.; Franz, W.; Kübler, W.; Katus, H.A. Frequency and Phenotypes of Familial Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 1998, 1, 186–194. [Google Scholar] [CrossRef]
- Losi, M.-A.; Betocchi, S.; Aversa, M.; Lombardi, R.; Miranda, M.; D’Alessandro, G.; Cacace, A.; Tocchetti, C.-G.; Barbati, G.; Chiariello, M. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2004, 94, 895–900. [Google Scholar] [CrossRef]
- Maron, B.J.; Olivotto, I.; Bellone, P.; Conte, M.R.; Cecchi, F.; Flygenring, B.P.; Casey, S.A.; Gohman, T.E.; Bongioanni, S.; Spirito, P. Clinical Profile of Stroke in 900 Patients with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2002, 2, 301–307. [Google Scholar] [CrossRef]
- Mizia-Stec, K.; Caforio, A.L.; Charron, P.; Gimeno, J.R.; Elliott, P.; Kaski, J.P.; Maggioni, A.P.; Tavazzi, L.; Rigopoulos, A.G.; Laroche, C.; et al. Atrial fibrillation, anticoagulation management and risk of stroke in the Cardiomyopathy/Myocarditis registry of the EURObservational Research Programme of the European Society of Cardiology. ESC Heart Fail. 2020, 7, 3601–3609. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L.; On behalf of the American Heart Association Heart Failure; et al. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, E7–E22. [Google Scholar] [CrossRef]
- El-Am, E.A.; Dispenzieri, A.; Melduni, R.M.; Ammash, N.M.; White, R.D.; Hodge, D.O.; Noseworthy, P.A.; Lin, G.; Pislaru, S.V.; Egbe, A.C.; et al. Direct Current Cardioversion of Atrial Arrhythmias in Adults with Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2019, 73, 589–597. [Google Scholar] [CrossRef]
- Fazio, G.; Corrado, G.; Zachara, E.; Rapezzi, C.; Sulafa, A.K.; Sutera, L.; Stollberger, C.; Sormani, L.; Finsterer, J.; Benatar, A.; et al. Anticoagulant drugs in noncompaction: A mandatory therapy? J. Cardiovasc. Med. 2008, 9, 1095–1097. [Google Scholar] [CrossRef]
- Stöllberger, C.; Finsterer, J. Left ventricular hypertrabeculation/noncompaction and stroke or embolism. Cardiology 2005, 103, 68–72. [Google Scholar] [CrossRef]
- Vilches, S.; Fontana, M.; Gonzalez-Lopez, E.; Mitrani, L.; Saturi, G.; Renju, M.; Griffin, J.M.; Caponetti, A.; Gnanasampanthan, S.; Santos, J.D.L.; et al. Systemic embolism in amyloid transthyretin cardiomyopathy. Eur. J. Heart Fail. 2022, 24, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Yang, P.-S.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Pak, H.-N.; Lee, M.-H.; Joung, B.; et al. Effectiveness and Safety of Non-Vitamin K Antagonist Oral Anticoagulants in Patients With Atrial Fibrillation With Hypertrophic Cardiomyopathy: A Nationwide Cohort Study. Chest 2019, 155, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Lau, Y.C.; Senoo, K.; Lane, D.A.; Hong, K.; Lip, G.Y. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: A systemic review and meta-analysis of randomized trials. Eur. J. Heart Fail. 2015, 17, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, P.A.; Yao, X.; Shah, N.D.; Gersh, B.J. Stroke and Bleeding Risks in NOAC- and Warfarin-Treated Patients With Hypertrophic Cardiomyopathy and Atrial Fibrillation. J. Am. Coll. Cardiol. 2016, 67, 3020–3021. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, H.-K.; Jung, J.-H.; Han, K.-D.; Lee, H.; Park, J.-B.; Kim, H.M.; Kim, Y.-J.; Ommen, S.R. Novel Oral Anticoagulants for Primary Stroke Prevention in Hypertrophic Cardiomyopathy Patients with Atrial Fibrillation. Stroke 2019, 50, 2582–2586. [Google Scholar] [CrossRef]
- Lin, Y.; Xiong, H.; Su, J.; Lin, J.; Zhou, Q.; Lin, M.; Zhao, W.; Peng, F. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with hypertrophic cardiomyopathy with non-valvular atrial fibrillation. Heart Vessel. 2022, 37, 1224–1231. [Google Scholar] [CrossRef]
- Peters, F.; Khandheria, B.K.; Botha, F.; Libhaber, E.; Matioda, H.; dos Santos, C.; Govender, S.; Meel, R.; Essop, M.R. Clinical outcomes in patients with isolated left ventricular noncompaction and heart failure. J. Card. Fail. 2014, 20, 709–715. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef]
- Johannessen, K.A.; Nordrehaug, J.E.; Von Der Lippe, G. Left ventricular thrombi after short-term high-dose anticoagulants in acute myocardial infarction. Eur. Heart J. 1987, 8, 975–980. [Google Scholar] [CrossRef]
- Yang, Q.; Quan, X.; Wang, C.; Yu, L.; Yang, Y.; Zhu, J.; Liang, Y. A prediction model for left ventricular thrombus persistence/recurrence: Based on a prospective study and a retrospective study. Thromb. J. 2023, 21, 50. [Google Scholar] [CrossRef]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef]
- Treatment of Post-STEMI Left Ventricular Thrombus with Optimized Anticoagulant (EARLYmyo-LVT Trial). NCT03764241. Available online: https://clinicaltrials.gov/study/NCT03764241 (accessed on 16 February 2025).
- Anti-CoagulaTion on Left Ventricular Thrombus After ST Segment Elevation Myocardial Infarction (ACTonLVT Trial). NCT05892042. Available online: https://clinicaltrials.gov/study/NCT05892042 (accessed on 16 February 2025).
- Anticoagulation in Post MI LV Thrombus Trial in Nepal (WaRMIN Trial). NCT05794399. Available online: https://clinicaltrials.gov/study/NCT05794399 (accessed on 16 February 2025).
- Treatment With Apixaban Versus Warfarin in Patients with Left Ventricular Thrombus After Acute Myocardial Infarction (RESOLVE-AMI Trial). NCT06515730. Available online: https://clinicaltrials.gov/study/NCT06515730 (accessed on 16 February 2025).
- Direct Oral Anticoagulants for Prevention of lEft ventRIcular Thrombus After Anterior Acute Myocardial InFarction—APERITIF (APERITIF). NCT05077683. Available online: https://clinicaltrials.gov/study/NCT05077683 (accessed on 16 February 2025).
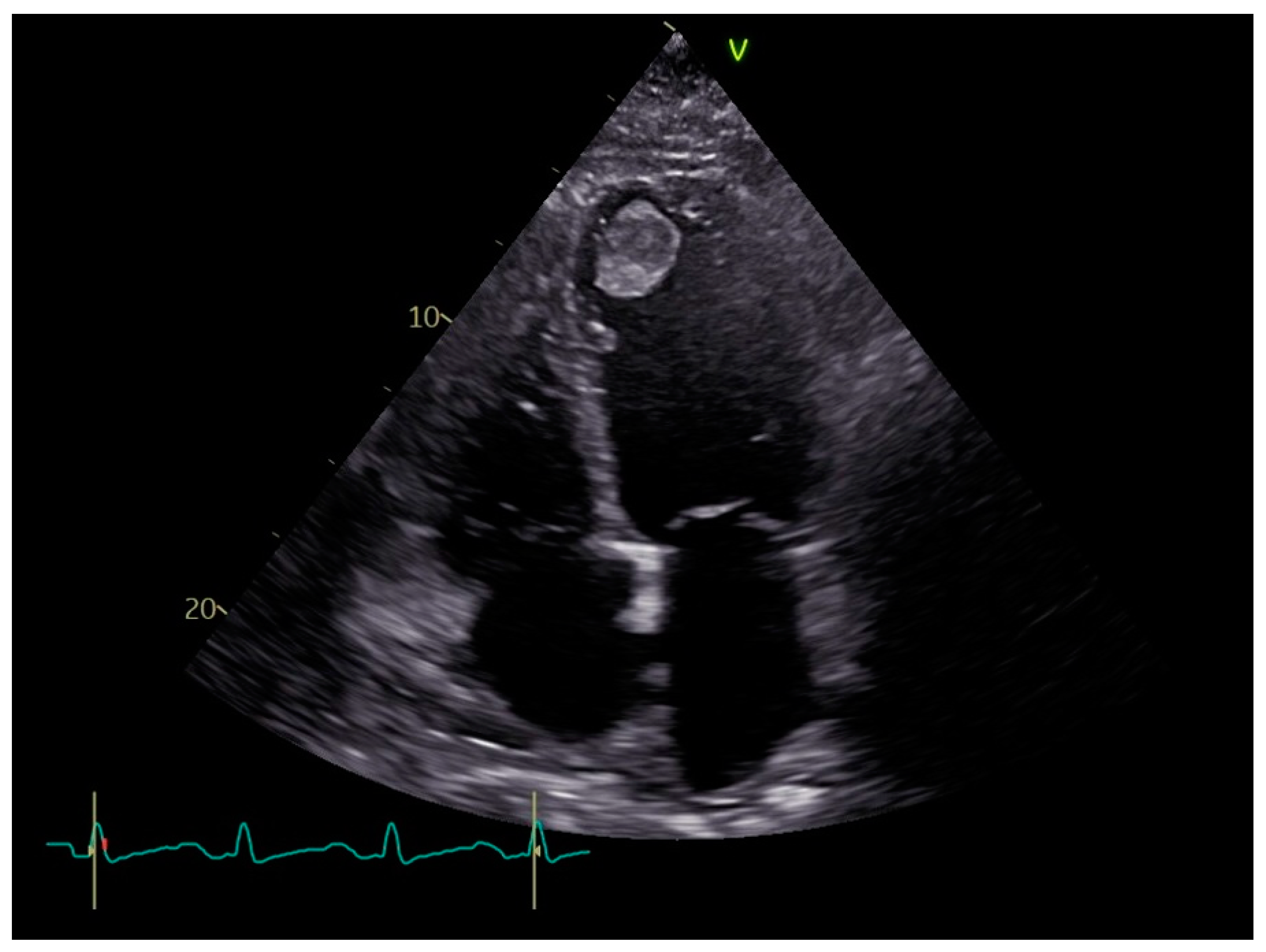
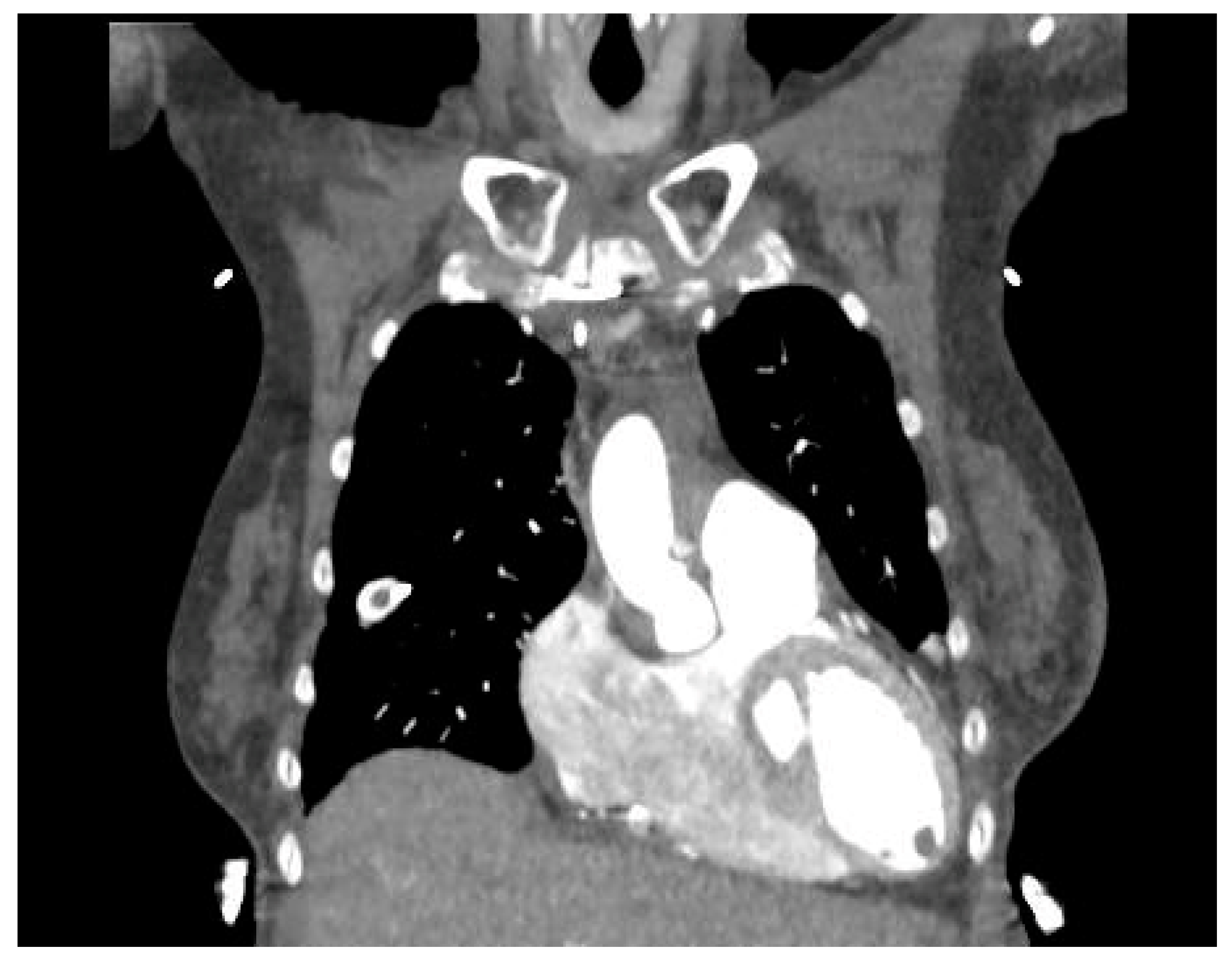

| Pros | Cons | Findings | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| TTE | Easily available, cheap, safe | Impaired acoustic window, operator depending, difficult detection of mural and small thrombi | Echo-dense mass with well-defined margins, separated from the endocardium | 21–35%
| 86–88% |
| TEE | Cheap, safe | Cardiac apex not always assessable | Echo-dense mass with well-defined margins, separated from the endocardium | 58% | 98% |
| CCT | High precision | Limited tissue characterization of the masses, contrast-based exam | Cavity-filling defect | 91%
| 97% |
| Contrast ventriculography | High specificity | Low spatial resolution, invasive, contrast-based exam, acute phase exam, not repeatable | Cavity-filling defect | 30% | 85–90% |
| CMR | High spatial and temporal resolution, high soft-tissue contrast | Costly, time consuming, difficult to perform in acutely ill patients | Low-signal intensity intraventricular defect |
|
|
| Reference | Study Design | Patients (n) | Outcomes | Key Results | Limitations |
|---|---|---|---|---|---|
| Maniwa N. et al. (2018) [112] | Retrospective cohort study | 84 | Warfarin with TTR ≥ 50% vs. warfarin with TTR < 50% in patients with first MI and LVT. Evaluation of systemic embolism. | Longer TTR was associated with a lower risk of systemic embolism (TTR ≥ 50%: 2.9% vs. TTR < 50%: 19%; p = 0.036). | Small sample size. Retrospective design. |
| Robinson A.A. et al. (2020) [113] | Retrospective cohort study | 514 | DOACs vs. warfarin in patients with LVT. Evaluation of stroke and embolic events. | DOACs were associated with a higher risk of stroke and systemic embolism (HR: 2.64 [95%CI: 1.28–5.43]; p = 0.01). | No safety outcomes. Retrospective design. |
| Daher J. et al. (2020) [114] | Retrospective cohort study | 59 | VKAs vs. DOACs in patients with LVT. Evaluation of thrombus resolution and embolic events. | No difference was found for thrombus resolution (VKAs: 71.4%; DOACs: 70.6%; p = 0.9) and embolic events (VKAs: 9.5%; DOACs: 11.8%; p = 0.8). Patients without thrombus resolution on DOAC were switched to VKA (INR: 3–4) with subsequent complete thrombus clearance. | Small sample size. No safety outcomes. Retrospective design. |
| Iqbal H. et al. (2020) [115] | Retrospective cohort study | 84 | VKAs vs. DOACs in patients with LVT. Evaluation of thrombus resolution, embolic events, bleeding, and all-cause death. | No difference was found for thrombus resolution (VKAs: 76%; DOACs: 65%; p = 0.33), stroke (VKAs: 2%; DOACs: 0%; p = 0.55), bleedings (VKAs: 10%; DOACs: 0%; p = 0.13), and all-cause death (VKAs: 10%; DOACs: 14%; p = 0.61). | Small sample size. No standardized follow-up imaging. Retrospective design. |
| Kouvaras G. et al. (1990) [111] | RCT | 60 | Warfarin vs. aspirin (650 mg daily) vs. placebo in patients with MI and LVT. Evaluation of thrombus resolution, embolic events, and bleeding. | Thrombus resolution was observed in 60% of the patients in the warfarin group, 45% in the aspirin group and 10% in the placebo group. Three patients in the placebo group had an embolic event. Three patients in the warfarin group had a bleeding event. | Small sample size. Diagnostic limitations due to old echocardiographic technology. No bleeding definition. |
| Abdelnabi M. et al. (2021) [122] | RCT | 79 | Warfarin vs. rivaroxaban (20 mg daily) in patients with LVT. Evaluation of thrombus resolution, stroke, systemic embolism, and major bleeding (ISTH criteria). | No difference was found for thrombus resolution (warfarin: 80%; rivaroxaban: 87.2%; p = 0.39), stroke (warfarin: 10%; rivaroxaban: 0%; p = 0.08), systemic embolism (warfarin: 5%; rivaroxaban: 0%; p = 0.25), and major bleedings (warfarin: 15%; rivaroxaban: 5.1%; p = 0.11). | Small sample size. Unblinded. |
| Alcalai R. et al. (2022) [120] | RCT | 35 | Warfarin vs. apixaban (5 mg daily, according to label) in patients with MI and LVT. Evaluation of thrombus resolution, major bleeding (ISTH criteria), and stroke/systemic embolism. | No difference was found for thrombus resolution (warfarin: 93%; apixaban: 94%; p = 1). Due to the low event rate, the study was underpowered to assess significant differences for major bleedings (warfarin: 13.3%; apixaban: 0%; p = N/A) and stroke/systemic embolism (warfarin: 6.6%; apixaban: 0%; p = N/A). | Small sample size. Unblinded. Underpowered for key outcomes. |
| Youssef A.A. et al. (2022) [121] | RCT | 50 | Warfarin vs. apixaban (5 mg daily) in patients with anterior MI and LVT. Evaluation of thrombus resolution and major adverse cardiovascular events: all-cause death/ischemic stroke or TIA/MI or acute peripheral artery emboli/clinically relevant bleedings (BARC criteria). | No difference was found for thrombus resolution (warfarin: 80%; apixaban: 76%; p = 0.3). No major adverse cardiovascular event was reported. | Small sample size. Unblinded. |
| Mansouri P. et al. (2024) [119] | RCT | 52 | Warfarin vs. rivaroxaban (20 mg daily) on top of DAPT (aspirin + clopidogrel) in patients with MI undergoing PCI and LVT. Evaluation of thrombus resolution and bleeding. | No difference was found for thrombus resolution (warfarin: 69.2%; rivaroxaban: 76.9%; p = 0.53) and bleeding (warfarin: 1%; rivaroxaban: 1%; p = 1). | Small sample size. Single-center. Unblinded. No bleeding definition. |
| Vaitkus P.T. et al. (1993) [108] | Meta-analysis | 270 | Warfarin vs. placebo in patients with anterior MI and LVT. | Anticoagulation with warfarin reduced the embolic risk compared to no anticoagulation (OR 0.14 [95%CI: 0.04–0.52]). | Small sample size of the included studies. Diagnostic limitations due to old echocardiographic technologies. No safety outcomes. |
| Gogos C. et al. (2024) [125] | Meta-analysis | 605 | VKAs vs. DOACs in patients with MI with evidence of LVT. Evaluation of thrombus resolution, systemic embolism, and bleeding. | DOACs were associated with higher rates of thrombus resolution (OR 1.95 [95%CI: 1.25–3.04]; p = 0.003) and lower risk of systemic embolism (OR 0.30 [95%CI: 0.12–0.75]; p = 0.01) and bleedings (OR 0.46 [95%CI: 0.26–0.84]; p = 0.01), compared to VKAs. | Observational and randomized studies pooled together. Subgroup analyses are prone to confounding due to the small sample of the included studies. No univocal definition of bleeding across the included studies. |
| Reference | Study Design | Patients (n) | Outcomes | Key Results | Limitations |
|---|---|---|---|---|---|
| Le May M.R. et al. (2015) [135] | Retrospective cohort study | 460 | Warfarin vs. no anticoagulation, on top of DAPT (aspirin + clopidogrel) in patients with anterior MI undergoing PCI. Evaluation of NACE (all-cause death, stroke, reinfarction, and major bleeding). | Patients treated with warfarin had a higher rate of NACE (14.7% vs. 4.6%; p = 0.001). Rates of death (5.4% vs. 1.5%; p = 0.04) and stroke were also higher (3.1% vs. 0.3%; p = 0.02) in the warfarin group. | No data on INR at the time of bleeding. Retrospective design. |
| Shavadia J.S. et al. (2017) [136] | Retrospective cohort study | 2032 | Warfarin vs. no anticoagulation in patients with MI. Evaluation of composite of stroke/TIA/systemic embolism/all-cause death and bleeding requiring hospitalization in high-risk STEMI. | No difference was found for the composite outcome (warfarin: 23.3% vs. no anticoagulation: 25.3%; OR: 0.96 [95%CI: 0.60–1.55]) and bleedings (warfarin: 2.5% vs. no anticoagulation: 1.2; OR: 2.17 [95%CI: 0.43–10–96]). | Unable to exclude patients developing AF during the observation period. Retrospective design. |
| El-Am E.A. et al. (2019) [148] | Retrospective cohort study | 172 | Patients with atrial arrhythmias and cardiac amyloidosis vs. the control group with atrial arrhythmias and no cardiac amyloidosis. Evaluation of direct current cardioversion cancellation rate due to intracardiac thrombus at TEE. | Patients with cardiac amyloidosis had higher rates of intracardiac thrombus compared to the control group (cardiac amyloidosis: 81%; control group: 25%; p = 0.02). | Small sample size. Retrospective design. |
| Peters F. et al. (2014) [157] | Prospective cohort study | 55 | Warfarin vs. no anticoagulation in patients with LVNC. | 1 out of 16 patients had a systemic thromboembolic event in the warfarin group. Non-major or minor bleeding occurred in the warfarin group. | Small sample size. No bleeding definition. |
| Zhang Z. et al. (2022) [141] | RCT | 279 | Low-dose rivaroxaban (2.5 mg twice daily) vs. no anticoagulation, on top of DAPT (aspirin + clopidogrel/ticagrelor) in patients with anterior MI undergoing PCI. Evaluation of LVT formation (within 30 days), NACE (all-cause death, LVT, systemic embolism, rehospitalization, and bleedings), and bleedings (ISTH criteria). | Patients treated with rivaroxaban showed a lower rate of LVT formation (rivaroxaban: 0.7% vs. no anticoagulation: 8.6%; HR 0.08 [95%CI: 0.01–0.62]) and NACE (rivaroxaban: 6.5% vs. no anticoagulation: 16.4%; HR: 0.37 [95%CI: 0.17–0.80]), without increase in bleedings (rivaroxaban 3.6% vs. no anticoagulation: 1.7%; HR: 2.08 [95%CI: 0.38–11.33]). | Small sample size. High dropout rate. Short follow-up. |
| Reference | Study Design | Patients (n) | Duration of Anticoagulation, Days | Limitations |
|---|---|---|---|---|
| Lattuca B, et al. (2020) [102] | Retrospective cohort study | 159 | 508 (15–986) * | Diagnosis and follow-up with TTE. Retrospective design. |
| Robinson A. A., et al. (2020) [113] | Retrospective cohort study | 514 | All anticoagulants: 207 (57–491.4) * DOACs: 95.5 (69–373) * Warfarin: 241 (47–579.5) * | Diagnosis and follow-up with TTE. Retrospective design. |
| Iqbal H. et al. (2020) [115] | Retrospective cohort study | 84 | All anticoagulants: 677 ± 568 ° DOACs: 545 ± 368 ° Warfarin: 724 ± 619 ° | Small sample size. Diagnosis and follow-up with TTE. Retrospective design. |
| Abdelnabi M, et al. (2021) [122] | RCT | 79 | 180 | Small sample size. Unblinded. |
| Alcalai R. et al. (2022) [120] | RCT | 35 | 89 (85–94) * | Small sample size. Unblinded. Underpowered for key outcomes. |
| Youssef A.A. et al. (2022) [121] | RCT | 50 | 180 | Small sample size. Unblinded. |
| Mansouri P. et al. (2024) [119] | RCT | 52 | 90 (average) | Small sample size. Single-center. Unblinded. No bleeding definition. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catalani, F.; Sarzilla, S.; Will, M.; Pedrazzini, G.; Demarchi, A. Left Ventricular Thrombosis in Ischemic and Non-Ischemic Cardiomyopathies: Focus on Evidence-Based Treatment. J. Clin. Med. 2025, 14, 1615. https://doi.org/10.3390/jcm14051615
Catalani F, Sarzilla S, Will M, Pedrazzini G, Demarchi A. Left Ventricular Thrombosis in Ischemic and Non-Ischemic Cardiomyopathies: Focus on Evidence-Based Treatment. Journal of Clinical Medicine. 2025; 14(5):1615. https://doi.org/10.3390/jcm14051615
Chicago/Turabian StyleCatalani, Filippo, Simone Sarzilla, Massimiliano Will, Giovanni Pedrazzini, and Andrea Demarchi. 2025. "Left Ventricular Thrombosis in Ischemic and Non-Ischemic Cardiomyopathies: Focus on Evidence-Based Treatment" Journal of Clinical Medicine 14, no. 5: 1615. https://doi.org/10.3390/jcm14051615
APA StyleCatalani, F., Sarzilla, S., Will, M., Pedrazzini, G., & Demarchi, A. (2025). Left Ventricular Thrombosis in Ischemic and Non-Ischemic Cardiomyopathies: Focus on Evidence-Based Treatment. Journal of Clinical Medicine, 14(5), 1615. https://doi.org/10.3390/jcm14051615







