Managing Refractory Hypoxemia in Acute Respiratory Distress Syndrome Obese Patients with Veno-Venous Extra-Corporeal Membrane Oxygenation: A Narrative Review
Abstract
1. Introduction
2. Data Collection
3. Physiological Explanation of Refractory Hypoxemia in vvECMO: Inadequacy of ECMO Blood Flow and Cardiac Output (CO)
- SPAO2 is oxygen saturation in the pulmonary artery (%);
- EF is effective flow, the fraction of pump flow oxygenated by ECMO (L/min);
- SmO2 is oxygen saturation in the blood exiting the oxygenator (membrane; %);
- CO is cardiac output (L/min);
- SvO2 is oxygen saturation in mixed venous blood (%);
- PmO2 is partial pressure of oxygen in the blood exiting the oxygenator (membrane; %);
- RLF is residual lung function;
- R is recirculation rate;
- PF is pump flow.
- The term 0.01PmO2 (in %) is a numerical estimation of the increase in SaO2 due to dissolved oxygen in the blood exiting the oxygenator.
4. Clinical Management
Technical Issues

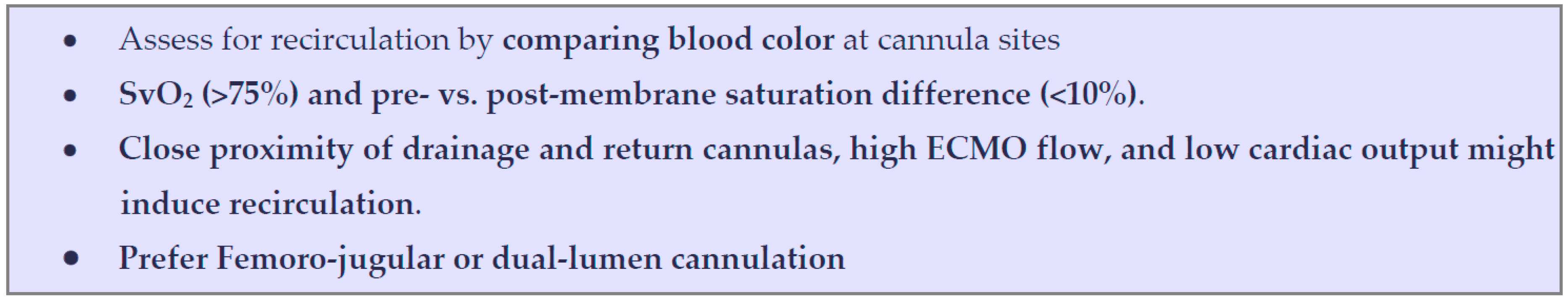

5. Standardized Protocol
6. Patient-Centered Strategy
6.1. Residual Lung Function
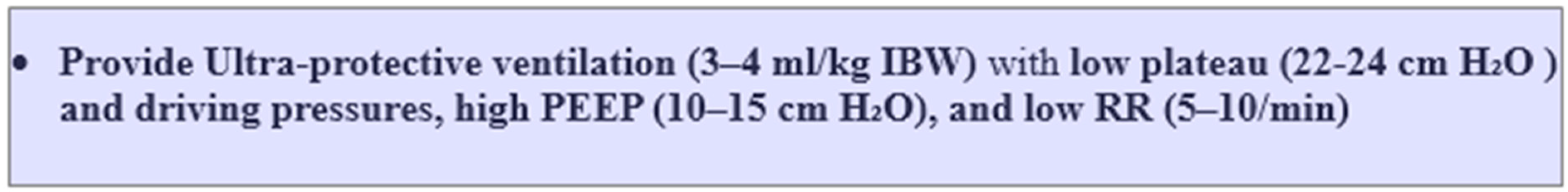




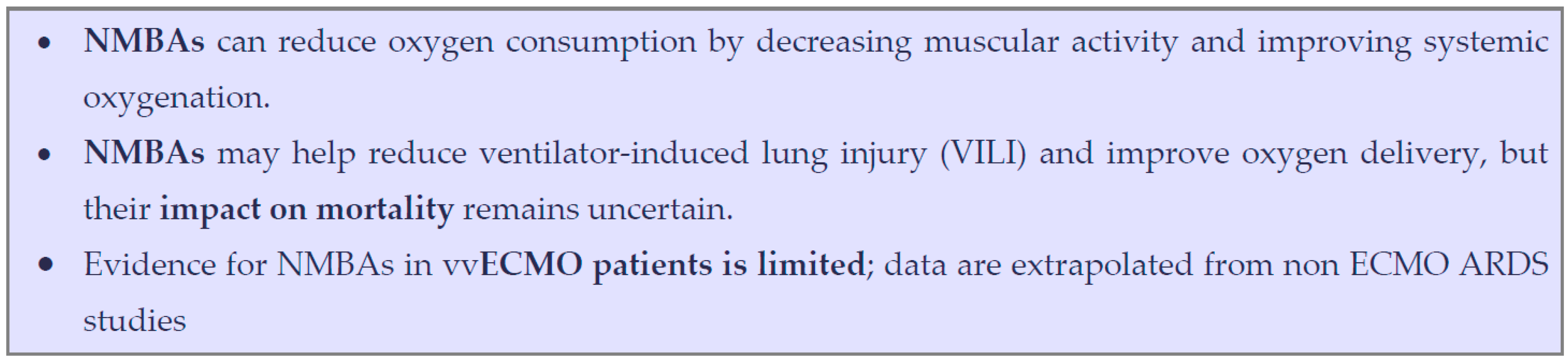
6.2. Reducing Oxygen Consumption and Carbon Dioxide Production
6.3. Optimizing Oxygen Delivery and Transfusion Strategy

6.4. Optimizing ECMO Blood Flow to Patient Blood Flow Ratio (QECMO/QCO Ratio)



7. ECMO Circuit-Centered Strategy
7.1. Enhancing QECMO/QCO by Employing Large Cannulas or Adding Additional Cannulas

7.2. Optimizing ECMO Oxygen Transfer Efficiency

7.3. The Use of Additional Circuits

7.4. Other Techniques (V-AV, VA, or VV-VA ECMO)

7.5. Resource and Cost Effectiveness
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qadir, N.; Sahetya, S.; Munshi, L.; Summers, C.; Abrams, D.; Beitler, J.; Bellani, G.; Brower, R.G.; Burry, L.; Chen, J.-T.; et al. An Update on Management of Adult Patients with Acute Respiratory Distress Syndrome: An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2024, 209, 24–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Schetz, M.; De Jong, A.; Deane, A.M.; Druml, W.; Hemelaar, P.; Pelosi, P.; Pickkers, P.; Reintam-Blaser, A.; Roberts, J.; Sakr, Y.; et al. Obesity in the critically ill: A narrative review. Intensiv. Care Med. 2019, 45, 757–769. [Google Scholar] [CrossRef]
- Tonna, J.E.; Abrams, D.; Brodie, D.; Greenwood, J.C.; Mateo-Sidron, J.A.R.; Usman, A.; Fan, E. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.L.; Stapleton, R.D.; Wang, M.; Day, A.G.; Cahill, N.E.; Dixon, A.E.; Suratt, B.T.; Heyland, D.K. Extreme Obesity and Outcomes in Critically Ill Patients. Chest 2011, 140, 1198–1206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wardell, S.; Wall, A.; Bryce, R.; Gjevre, J.A.; Laframboise, K.; Reid, J.K. The Association between Obesity and Outcomes in Critically Ill Patients. Can. Respir. J. 2015, 22, 23–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Decruyenaere, A.; Steen, J.; Colpaert, K.; Benoit, D.D.; Decruyenaere, J.; Vansteelandt, S. The obesity paradox in critically ill patients: A causal learning approach to a casual finding. Crit. Care 2020, 24, 485. [Google Scholar] [CrossRef]
- De Jong, A.; Verzilli, D.; Jaber, S. ARDS in Obese Patients: Specificities and Management. Crit. Care 2019, 23, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Javidfar, J.; Zaaqoq, A.M.; Yamashita, M.H.; Eschun, G.; Jacobs, J.P.; Heinsar, S.; Hayanga, J.W.; Peek, G.J.; Arora, R.C. Venovenous extracorporeal membrane oxygenation in obese patients. JTCVS Tech. 2021, 10, 335–348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudym, D.; Pham, T.; Rackley, C.R.; Grasselli, G.; Anderson, M.; Baldwin, M.R.; Beitler, J.; Agerstrand, C.; Serra, A.; Winston, L.A.; et al. Mortality in Patients with Obesity and Acute Respiratory Distress Syndrome Receiving Extracorporeal Membrane Oxygenation: The Multicenter ECMObesity Study. Am. J. Respir. Crit. Care Med. 2023, 208, 685–694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Swol, J.; Buchwald, D.; Dudda, M.; Strauch, J.; Schildhauer, T.A. Veno-venous extracorporeal membrane oxygenation in obese surgical patients with hypercapnic lung failure. Acta Anaesthesiol. Scand. 2014, 58, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Kon, Z.N.; Dahi, S.; Evans, C.F.; Byrnes, K.A.; Bittle, G.J.; Wehman, B.; Rector, R.P.; McCormick, B.M.; Herr, D.L.; Sanchez, P.G.; et al. Class III Obesity is Not a Contraindication to Venovenous Extracorporeal Membrane Oxygenation Support. Ann. Thorac. Surg. 2015, 100, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Stelfox, H.T.; Ahmed, S.B.; Ribeiro, R.A.; Gettings, E.M.; Pomerantsev, E.; Schmidt, U. Hemodynamic monitoring in obese patients: The impact of body mass index on cardiac output and stroke volume. Crit. Care Med. 2006, 34, 1243–1246. [Google Scholar] [CrossRef]
- Lemmens, H.J.M.; Bernstein, D.P.; Brodsky, J.B. Estimating Blood Volume in Obese and Morbidly Obese Patients. Obes. Surg. 2006, 16, 773–776. [Google Scholar] [CrossRef]
- Shashaty, M.G.S.; Stapleton, R.D. Physiological and Management Implications of Obesity in Critical Illness. Ann. Am. Thorac. Soc. 2014, 11, 1286–1297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelosi, P.; Croci, M.; Ravagnan, I.; Tredici, S.; Pedoto, A.; Lissoni, A.; Gattinoni, L. The Effects of Body Mass on Lung Volumes, Respiratory Mechanics, and Gas Exchange During General Anesthesia. Anesth. Analg. 1998, 87, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.D. Oxygen in the ICU: Too much of a good thing? JAMA 2016, 316, 1553–1554. [Google Scholar] [CrossRef]
- ARDSnet. NIH NHLBI ARDS Clinical Network Mechanical Ventilation Protocol Summary. Available online: http://www.ardsnet.org/files/ventilator_protocol_2008-07.pdf (accessed on 25 February 2025).
- Duan, E.H.; Adhikari, N.K.J.; D’aragon, F.; Cook, D.J.; Mehta, S.; Alhazzani, W.; Goligher, E.; Charbonney, E.; Arabi, Y.M.; Karachi, T.; et al. Management of Acute Respiratory Distress Syndrome and Refractory Hypoxemia. A Multicenter Observational Study. Ann. Am. Thorac. Soc. 2017, 14, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, S.; Ohshimo, S.; Takeuchi, M.; Yasuda, H.; Ichikado, K.; Tsushima, K.; Egi, M.; Hashimoto, S.; Shime, N.; Saito, O.; et al. ARDS Clinical Practice Guideline 2021. J. Intensiv. Care 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Barrot, L.; Asfar, P.; Mauny, F.; Winiszewski, H.; Montini, F.; Badie, J.; Quenot, J.-P.; Pili-Floury, S.; Bouhemad, B.; Louis, G.; et al. Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2020, 382, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Panwar, R.; Hardie, M.; Bellomo, R.; Barrot, L.; Eastwood, G.M.; Young, P.J.; Capellier, G.; Harrigan, P.W.J.; Bailey, M. Conservative versus Liberal Oxygenation Targets for Mechanically Ventilated Patients. A Pilot Multicenter Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 193, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Girardis, M.; Busani, S.; Damiani, E.; Donati, A.; Rinaldi, L.; Marudi, A.; Morelli, A.; Antonelli, M.; Singer, M. Effect of conservative vs. conventional oxygen therapy on mortality among patients in an intensive care unit: The oxygen-ICU randomized clinical trial. JAMA. 2016, 316, 1583–1589. [Google Scholar] [CrossRef]
- Nielsen, F.M.; Klitgaard, T.L.; Siegemund, M.; Laake, J.H.; Thormar, K.M.; Cole, J.M.; Aagaard, S.R.; Bunzel, A.-M.G.; Vestergaard, S.R.; Langhoff, P.K.; et al. Lower vs Higher Oxygenation Target and Days Alive Without Life Support in COVID-19: The HOT-COVID Randomized Clinical Trial. JAMA 2024, 331, 1185–1194. [Google Scholar] [CrossRef]
- Messaï, E.; Bouguerra, A.; Harmelin, G.; Di Lascio, G.; Cianchi, G.; Bonacchi, M. A new formula for determining arterial oxygen satura-tion during venovenous extracorporeal oxygenation. Intensive Care Med. 2013, 39, 327–334. [Google Scholar] [CrossRef]
- Schmidt, M.; Tachon, G.; Devilliers, C.; Muller, G.; Hekimian, G.; Bréchot, N.; Merceron, S.; Luyt, C.E.; Trouillet, J.-L.; Chastre, J.; et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensiv. Care Med. 2013, 39, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.; Melenovsky, V.; Redfield, M.M.; Nishimura, R.A.; Borlaug, B.A. High-Output Heart Failure: A 15-Year Experience. J. Am. Coll. Cardiol. 2016, 68, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Straaten, H.M.O.-V.; Jansen, P.G.M.; Velthuis, H.T.; Beenakkers, I.C.M.; Stoutenbeek, C.P.; van Deventer, S.J.H.; Sturk, A.; Eysman, L.; Wildevuur, C.R.H. Increased oxygen consumption after cardiac surgery is associated with the inflammatory response to endotoxemia. Intensiv. Care Med. 1996, 22, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chaturvedi, A. Imaging adults on extracorporeal membrane oxygenation (ECMO). Insights Into Imaging 2014, 5, 731–742. [Google Scholar] [CrossRef]
- Gajkowski, E.F.; Herrera, G.; Hatton, L.; Antonini, M.V.; Vercaemst, L.; Cooley, E. ELSO Guidelines for Adult and Pediatric Extracorporeal Membrane Oxygenation Circuits. ASAIO J. 2022, 68, 133–152. [Google Scholar] [CrossRef]
- Gehron, J.; Bandorski, D.; Mayer, K.; Böning, A. The Impact of Recirculation on Extracorporeal Gas Exchange and Patient Oxygenation during Veno-Venous Extracorporeal Membrane Oxygenation—Results of an Observational Clinical Trial. J. Clin. Med. 2023, 12, 416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pooth, J.; Förster, J.K.; Benk, C.; Diel, P.; Brixius, S.J.; Maier, S.; Supady, A.; Wengenmayer, T.; Staudacher, D.L.; Haimerl, G.; et al. Impact of Cannulation Strategy and Extracorporeal Blood Flow on Recirculation During Veno-Venous Extracorporeal Membrane Oxygenation. Artif. Organs, 2025; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.P.; Marcial, A.S.; Brismar, T.B.; Broman, L.M.; Wittberg, L.P. Hemodynamic and recirculation performance of dual lumen cannulas for venovenous extracorporeal membrane oxygenation. Sci. Rep. 2023, 13, 7472. [Google Scholar] [CrossRef]
- Schoeberl, A.-K.; Staudacher, D.; Kawashima, M.; Fischer, C.; Cypel, M.; Buchtele, N.; Staudinger, T.; Aigner, C.; Hoetzenecker, K.; Schweiger, T. Al-ternative venous access sites for dual-lumen extracorporeal membrane oxygenation cannulation. Interdiscip. Cardio-Vasc. Thorac. Surg. 2024, 38, ivae060. [Google Scholar]
- Morgan, M.; Hopwood, M.J.; Dunbar, J.A. Shared guidelines and protocols to achieve better health outcomes for people living with serious mental illness. Med. J. Aust. 2022, 217 (Suppl. S7), S34–S35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Holets, S.R.; Li, M.; Meyer, T.J.; Latuche, L.J.R.; Oeckler, R.A.; Bohman, J.K. The Impact of a Standardized Refractory Hypoxemia Protocol on Outcome of Subjects Receiving Venovenous Extracorporeal Membrane Oxygenation. Respir. Care 2021, 66, 837–844. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, A.G.; Holets, S.R.; Tescher, A.N.; Elmer, J.; Arteaga, G.M.; Schears, G.; Patch, R.K.; Bohman, J.K.; Oeckler, R.A. The Clinical Effect of an Early, Protocolized Approach to Mechanical Ventilation for Severe and Refractory Hypoxemia. Respir. Care 2020, 65, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Calfee, C.S.; Camporota, L.; Poole, D.; Amato, M.B.P.; Antonelli, M.; Arabi, Y.M.; Baroncelli, F.; Beitler, J.R.; Bellani, G.; et al. ESICM guidelines on acute respiratory distress syndrome: Definition, phenotyping and respiratory support strategies. Intensiv. Care Med. 2023, 49, 727–759. [Google Scholar] [CrossRef] [PubMed]
- Guervilly, C.; Fournier, T.; Chommeloux, J.; Arnaud, L.; Pinglis, C.; Baumstarck, K.; Boucekine, M.; Valera, S.; Sanz, C.; Adda, M.; et al. Ultra-lung-protective ventilation and biotrauma in severe ARDS patients on veno-venous extracorporeal membrane oxygenation: A randomized controlled study. Crit. Care 2022, 26, 383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rozencwajg, S.; Guihot, A.; Franchineau, G.; Lescroat, M.; Bréchot, N.; Hékimian, G.; Lebreton, G.; Autran, B.; Luyt, C.-E.; Combes, A.; et al. Ultra-Protective Ventilation Reduces Biotrauma in Patients on Venovenous Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, C.; Hu, D.; Sun, F.; Zhang, G.; Zhang, Z.; Dong, Y.; Lv, J.; Mei, Y.; Chen, X. Randomized controlled trial of ultra-protective vs. protective ventilation strategy in veno-arterial extracorporeal membrane oxygenation patients with refractory cardiogenic shock: A study protocol for the ultra-ECMO trial. Front. Cardiovasc. Med. 2023, 10, 1092653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishikimi, M.; Ohshimo, S.; Hamaguchi, J.; Fujizuka, K.; Hagiwara, Y.; Anzai, T.; Ishii, J.; Ogata, Y.; Aokage, T.; Ikeda, T.; et al. High versus low positive end-expiratory pressure setting in patients receiving veno-venous extracorporeal membrane oxygenation support for severe acute respiratory distress syndrome: Study protocol for the multicentre, randomised ExPress SAVER Trial. BMJ Open 2023, 13, e072680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pensier, J.; de Jong, A.; Hajjej, Z.; Molinari, N.; Carr, J.; Belafia, F.; Chanques, G.; Futier, E.; Azoulay, E.; Jaber, S. Effect of lung recruitment maneuver on oxygenation, physiological parameters and mortality in acute respiratory distress syndrome patients: A systematic review and meta-analysis. Intensiv. Care Med. 2019, 45, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Dianti, J.; Tisminetzky, M.; Ferreyro, B.L.; Englesakis, M.; Del Sorbo, L.; Sud, S.; Talmor, D.; Ball, L.; Meade, M.; Hodgson, C.; et al. Association of Positive End-Expiratory Pressure and Lung Recruitment Selection Strategies with Mortality in Acute Respiratory Distress Syndrome: A Systematic Review and Network Meta-analysis. Am. J. Respir. Crit. Care Med. 2022, 205, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs. low PEEP on mortality in patients with acute respiratory distress syndrome: A randomized clinical trial. JAMA 2017, 318, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.-M.; Paquet, J.; Wysocki, M.; Demory, D.; Donati, S.; Granier, I.; Corno, G.; Durand-Gasselin, J. Optimal duration of a sustained inflation recruitment maneuver in ARDS patients. Intensiv. Care Med. 2011, 37, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Riva, D.; Contador, R.; Baez-Garcia, C.; Xisto, D.; Cagido, V.; Martini, S.; Morales, M.; Rocco, P.; Faffe, D.; Zin, W. Recruitment maneuver: RAMP versus CPAP pressure profile in a model of acute lung injury. Respir. Physiol. Neurobiol. 2009, 169, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.; Goligher, E.C.; Young, M.E.; Keating, J.L.; Holland, A.E.; Romero, L.; Bradley, S.J.; Tuxen, D. Recruitment manoeuvres for adults with acute respiratory distress syndrome receiving mechanical ventilation. Cochrane Database Syst. Rev. 2016, 2018, CD006667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, Y.; Cao, R.; Wang, Y.; Li, G. Lung Recruitment Maneuvers for ARDS Patients: A Systematic Review and Meta-Analysis. Respiration 2019, 99, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.L.; Cooper, D.J.; Arabi, Y.; King, V.; Bersten, A.; Bihari, S.; Brickell, K.; Davies, A.; Fahey, C.; Fraser, J.; et al. Maximal Recruitment Open Lung Ventilation in Acute Respiratory Distress Syndrome (PHARLAP). A Phase II, Multicenter Randomized Controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2019, 200, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.D.S.; Droghi, M.T.; Fumagalli, J.; Marrazzo, F.; Florio, G.; Grassi, L.G.; Gomes, S.; Morais, C.C.A.; Ramos, O.P.S.; Bottiroli, M.; et al. High Pleural Pressure Prevents Alveolar Overdistension and Hemodynamic Collapse in Acute Respiratory Distress Syndrome with Class III Obesity. A Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 575–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amato, M.B.P.; Meade, M.O.; Slutsky, A.S.; Brochard, L.; Costa, E.L.V.; Schoenfeld, D.A.; Stewart, T.E.; Briel, M.; Talmor, D.S.; Mercat, A.; et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2015, 372, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Deniel, G.; Dhelft, F.; Lancelot, S.; Orkisz, M.; Roux, E.; Mouton, W.; Benzerdjeb, N.; Richard, J.-C.; Bitker, L. Pulmonary inflammation decreases with ultra-protective ventilation in experimental ARDS under VV-ECMO: A positron emission tomography study. Front. Med. 2024, 11, 1338602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hermann, M.; König, S.; Laxar, D.; Krall, C.; Kraft, F.; Krenn, K.; Baumgartner, C.; Tretter, V.; Maleczek, M.; Hermann, A.; et al. Low-Frequency Ventilation May Facilitate Weaning in Acute Respiratory Distress Syndrome Treated with Extracorporeal Membrane Oxygenation: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 5094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pirrone, M.; Fisher, D.; Chipman, D.; Imber, D.A.E.; Corona, J.; Mietto, C.; Kacmarek, R.M.; Berra, L. Recruitment Maneuvers and Positive End-Expiratory Pressure Titration in Morbidly Obese ICU Patients. Crit. Care Med. 2016, 44, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mezidi, M.; Daviet, F.; Chabert, P.; Hraiech, S.; Bitker, L.; Forel, J.-M.; Yonis, H.; Gragueb, I.; Dhelft, F.; Papazian, L.; et al. Transpulmonary pressures in obese and non-obese COVID-19 ARDS. Ann. Intensiv. Care 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bime, C.; Fiero, M.; Lu, Z.; Oren, E.; Berry, C.E.; Parthasarathy, S.; Garcia, J.G. High Positive End-Expiratory Pressure Is Associated with Improved Survival in Obese Patients with Acute Respiratory Distress Syndrome. Am. J. Med. 2016, 130, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Spina, S.; Mantz, L.; Xin, Y.; Moscho, D.C.; Santiago, R.R.D.S.; Grassi, L.; Nova, A.; Gerard, S.E.; Bittner, E.A.; Fintelmann, F.J.; et al. The pleural gradient does not reflect the superimposed pressure in patients with class III obesity. Crit. Care 2024, 28, 306. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Cossic, J.; Verzilli, D.; Monet, C.; Carr, J.; Conseil, M.; Monnin, M.; Cisse, M.; Belafia, F.; Molinari, N.; et al. Impact of the driving pressure on mortality in obese and non-obese ARDS patients: A retrospective study of 362 cases. Intensiv. Care Med. 2018, 44, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Beitler, J.R.; Sarge, T.; Banner-Goodspeed, V.M.; Gong, M.N.; Cook, D.; Novack, V.; Loring, S.H.; Talmor, D. Effect of Titrating Positive End-Expiratory Pressure (PEEP) With an Esophageal Pressure–Guided Strategy vs an Empirical High PEEP-Fio2 Strategy on Death and Days Free from Mechanical Ventilation Among Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA 2019, 321, 846–857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarge, T.; Baedorf-Kassis, E.; Banner-Goodspeed, V.; Novack, V.; Loring, S.H.; Gong, M.N.; Cook, D.; Talmor, D.; Beitler, J.R.; EPVent-2 Study Group. Effect of Esophageal Pressure–guided Positive End-Expiratory Pressure on Survival from Acute Respiratory Distress Syndrome: A Risk-based and Mechanistic Reanalysis of the EPVent-2 Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 1153–1163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terry, C.M.; Brinton, D.; Simpson, A.N.; Kirchoff, K.; Files, D.C.; Carter, G.; Ford, D.W.M.; Goodwin, A.J.M. Elevated Driving Pressure and Elastance Does Not Increase In-Hospital Mortality Among Obese and Severely Obese Patients with Ventilator Dependent Respiratory Failure. Crit. Care Explor. 2022, 4, e0811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- De Jong, A.; Molinari, N.; Sebbane, M.; Prades, A.; Fellow, N.; Futier, E.; Jung, B.; Chanques, G.; Jaber, S. Feasibility and Effectiveness of Prone Position in Morbidly Obese Patients With ARDS: A case-control clinical study. Chest 2013, 143, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Petit, M.; Fetita, C.; Gaudemer, A.; Treluyer, L.; Lebreton, G.; Franchineau, G.; Hekimian, G.; Chommeloux, J.; de Chambrun, M.P.; Brechot, N.; et al. Prone-Positioning for Severe Acute Respiratory Distress Syndrome Requiring Extracorporeal Membrane Oxygenation. Crit. Care Med. 2021, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Rilinger, J.; Zotzmann, V.; Bemtgen, X.; Schumacher, C.; Biever, P.M.; Duerschmied, D.; Kaier, K.; Stachon, P.; Mühlen, C.v.Z.; Zehender, M.; et al. Prone positioning in severe ARDS requiring extracorporeal membrane oxygenation. Crit. Care 2020, 24, 397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, H.; Pan, F.; Zhang, X.; Jia, S.; Vashisht, R.; Chen, K.; Wang, Q. Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A prospective multicenter randomized controlled study. J. Thorac. Dis. 2024, 16, 1368–1377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, R.; Tang, X.; Li, X.; Li, Y.; Liu, Y.; Li, T.; Zhao, Y.; Wang, L.; Li, H.; Li, M.; et al. Early reapplication of prone position during venovenous ECMO for acute respiratory distress syndrome: A prospective observational study and propensity-matched analysis. Ann. Intensiv. Care 2024, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Giani, M.; Rezoagli, E.; Guervilly, C.; Rilinger, J.; Duburcq, T.; Petit, M.; Textoris, L.; Garcia, B.; Wengenmayer, T.; Grasselli, G.; et al. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A pooled individual patient data analysis. Crit. Care 2022, 26, 8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Dres, M.; Guervilly, C.; Richard, J.C.; Sonneville, R.; Winiszewski, H.; Muller, G.; Beduneau, G.; et al. Prone Positioning During Extracorporeal Membrane Oxygenation in Patients with Severe ARDS: The PRONECMO Randomized Clinical Trial. JAMA 2023, 330, 2343–2353, Erratum in JAMA 2024, 331, 446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afshari, A.; Brok, J.; Møller, A.M.; Wetterslev, J. Inhaled Nitric Oxide for Acute Respiratory Distress Syndrome and Acute Lung Injury in Adults and Children: A systematic review with meta-analysis and trial sequential analysis. Anesth. Analg. 2011, 112, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Troncy, E.; Collet, J.-P.; Shapiro, S.; Guimond, J.-G.; Blair, L.; Ducruet, T.; Francoeur, M.; Charbonneau, M.; Blaise, G. Inhaled Nitric Oxide in Acute Respiratory Distress Syndrome: A pilot randomized controlled study. Am. J. Respir. Crit. Care Med. 1998, 157, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Zimmerman, J.L.; Dellinger, R.P.; Straube, R.C.; Criner, G.J.; Davis, J.K.; Kelly, K.M.; Smith, T.C.; Small, R.J. Low-Dose Inhaled Nitric Oxide in Patients with Acute Lung InjuryA Randomized Controlled Trial. JAMA 2004, 291, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Narula, P.; Xu, J.; Kazzaz, J.A.; Robbins, C.G.; Davis, J.M.; Horowitz, S.; Sparkman, L.; Boggaram, V.; Salinas, D.; Berhane, K.; et al. Synergistic cytotoxicity from nitric oxide and hyperoxia in cultured lung cells. Am. J. Physiol. Cell. Mol. Physiol. 1998, 274, L411–L416. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.E.; Böttiger, B.W.; Dusse, F.; Leister, N.; Leupold, T.; Menzel, C.; Overbeek, R.; Mathes, A. Impact of Inhaled Nitric Oxide (iNO) on the Outcome of COVID-19 Associated ARDS. J. Clin. Med. 2024, 13, 5981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Isha, S.; Balasubramanian, P.; Hanson, A.J.; Jonna, S.; Raavi, L.; Khadka, S.; Vasudhar, A.; De Frias, J.S.; Jenkins, A.; Balavenkataraman, A.; et al. Impact of low dose inhaled nitric oxide treatment in spontaneously breathing and intubated COVID-19 patients: A retrospective propensity-matched study. Crit. Care 2024, 28, 344. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, J.S.; Aldhahir, A.M.; Al Ghamdi, S.S.; AlBahrani, S.; AlDraiwiesh, I.A.; Alqarni, A.A.; Latief, K.; Raya, R.P.; Oyelade, T. Inhaled Nitric Oxide for Clinical Management of COVID-19: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dessap, A.M.; Papazian, L.; Schaller, M.; Nseir, S.; Megarbane, B.; Haudebourg, L.; Timsit, J.-F.; Teboul, J.-L.; Kuteifan, K.; Gainnier, M.; et al. Inhaled nitric oxide in patients with acute respiratory distress syndrome caused by COVID-19: Treatment modalities, clinical response, and outcomes. Ann. Intensiv. Care 2023, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Di Fenza, R.; Shetty, N.S.; Gianni, S.; Parcha, V.; Giammatteo, V.; Fakhr, B.S.; Tornberg, D.; Wall, O.; Harbut, P.; Lai, P.S.; et al. High-Dose Inhaled Nitric Oxide in Acute Hypoxemic Respiratory Failure Due to COVID-19: A Multicenter Phase II Trial. Am. J. Respir. Crit. Care Med. 2023, 208, 1293–1304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gebistorf, F.; Karam, O.; Wetterslev, J.; Afshari, A. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst. Rev. 2016, 2016, CD002787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muenster, S.; Nadal, J.; Schewe, J.-C.; Ehrentraut, H.; Kreyer, S.; Putensen, C.; Ehrentraut, S.F. Analysis of Patients with Severe ARDS on VV ECMO Treated with Inhaled NO: A Retrospective Observational Study. J. Clin. Med. 2024, 13, 1555. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crow, J.; Lindsley, J.; Cho, S.-M.; Wang, J.; Lantry, J.H.I.; Kim, B.S.; Tahsili-Fahadan, P. Analgosedation in Critically Ill Adults Receiving Extracorporeal Membrane Oxygenation Support. ASAIO J. 2022, 68, 1419–1427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marik, P.E.; Kaufman, D. The effects of neuromuscular paralysis on systemic and splanchnic oxygen utilization in mechanically ventilated patients. Chest 1996, 109, 1038–1042. [Google Scholar] [CrossRef]
- DeGrado, J.R.; Hohlfelder, B.; Ritchie, B.M.; Anger, K.E.; Reardon, D.P.; Weinhouse, G.L. Evaluation of sedatives, analgesics, and neuromuscular blocking agents in adults receiving extracorporeal membrane oxygenation. J. Crit. Care 2017, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kressin, C.; Bastin, M.T.; Ather, A.; Gopinath, A.; Srour, H.; Schadler, A.; Pandya, K. Cisatracurium Continuous Infusion Versus No Neuromuscular Blockade for Acute Respiratory Distress Syndrome on Venovenous Extracorporeal Membrane Oxygenation. J. Clin. Pharmacol. 2021, 61, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss, M.; Huang, D.T.; Brower, R.G.; Ferguson, N.D.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Gundel, S.; Hayden, D.; et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papazian, L.; Forel, J.-M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.-M.; Perez, D.; Seghboyan, J.-M.; et al. Neuromuscular Blockers in Early Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Savoie-White, F.H.; Tremblay, L.; Menier, C.A.; Duval, C.; Bergeron, F.; Tadrous, M.; Tougas, J.; Guertin, J.R.; Ugalde, P.A. The use of early neuromuscular blockage in acute respiratory distress syndrome: A systematic review and meta-analyses of randomized clinical trials. Heart Lung 2022, 57, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Hunsicker, O.; Materne, L.; Bünger, V.; Krannich, A.; Balzer, F.; Spies, C.; Francis, R.C.; Weber-Carstens, S.; Menk, M.; Graw, J.A. Lower versus higher hemoglobin threshold for transfusion in ARDS patients with and without ECMO. Crit. Care 2020, 24, 697. [Google Scholar] [CrossRef] [PubMed]
- Voelker, M.T.; Busch, T.; Bercker, S.; Fichtner, F.; Kaisers, U.X.; Laudi, S. Restrictive Transfusion Practice During Extracorporeal Membrane Oxygenation Therapy for Severe Acute Respiratory Distress Syndrome. Artif. Organs 2014, 39, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Agerstrand, C.L.; Burkart, K.M.; Abrams, D.C.; Bacchetta, M.D.; Brodie, D. Blood conservation in extracorporeal membrane oxygenation for acute res-piratory distress syndrome. Ann. Thorac. Surg. 2015, 99, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.J.M.; Richardson, C.M.; Sanderson, B.B.; Wong, K.M.; Wyncoll, D.M.; Camporota, L.; Barrett, N.A.M.; Hunt, B.J.; Retter, A.M. Restrictive Transfusion Practice in Adults Receiving Venovenous Extracorporeal Membrane Oxygenation: A Single-Center Experience. Crit. Care Explor. 2020, 2, e0077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martucci, G.; Schmidt, M.; Agerstrand, C.; Tabatabai, A.; Tuzzolino, F.; Giani, M.; Ramanan, R.; Grasselli, G.; Schellongowski, P.; Riera, J.; et al. Transfusion practice in patients receiving VV ECMO (PROTECMO): A prospective, multicentre, observational study. Lancet Respir. Med. 2022, 11, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Jamadarkhana, S.; Gopal, S. Clonidine in adults as a sedative agent in the intensive care unit. J. Anaesthesiol. Clin. Pharmacol. 2010, 26, 439–445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McRaven, D.R.; Kroetz, F.W.; Kioschos, J.; Kirkendall, W.M. The effect of clonidine on hemodynamics in hypertensive patients. Am. Heart J. 1971, 81, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, D.L.; Wengenmayer, T.; Schmidt, M. Beta-blockers in refractory hypoxemia on venovenous extracorporeal membrane oxygenation: A double-edged sword. Crit. Care 2023, 27, 360. [Google Scholar] [CrossRef]
- Bommiasamy, A.K.; Zakhary, B.; Ran, R. Beta-blockade in V-V ECMO. Crit. Care 2024, 28, 139. [Google Scholar] [CrossRef]
- Pappalardo, F.; Zangrillo, A.; Pieri, M.; Landoni, G.; Morelli, A.; Stefani, M.; Guarracino, F. Esmolol administration in patients with VV ECMO: Why not? J. Cardiothorac. Vasc. Anesth. 2013, 27, e40. [Google Scholar] [CrossRef] [PubMed]
- Bunge, J.J.; Diaby, S.; Valle, A.L.; Bakker, J.; Gommers, D.; Vincent, J.-L.; Creteur, J.; Taccone, F.S.; Miranda, D.R. Safety and efficacy of beta-blockers to improve oxygenation in patients on veno-venous ECMO. J. Crit. Care 2019, 53, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Guarracino, F.; Zangrillo, A.; Ruggeri, L.; Pieri, M.; Calabrò, M.G.; Landoni, G.; Stefani, M.; Doroni, L.; Pappalardo, F. β-Blockers to Optimize Peripheral Oxygenation During Extracorporeal Membrane Oxygenation: A Case Series. J. Cardiothorac. Vasc. Anesth. 2012, 26, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Keller, S.P.; Dodd-O, J.M. A case series evaluating the effect of esmolol therapy to treat hypoxemia in COVID-19 patients on VV-ECMO. Int. J. Artif. Organs 2023, 46, 381–383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krupnik, A.; Dhaliwal, J.; Hatton, K.; Harris, A.; White, A.; Schadler, A.; Ather, A. 121: Safety and Efficacy of Metoprolol Use to Improve Oxygenation in COVID-19 Patients on Venovenous Extracorporeal Membrane Oxygenation. ASAIO J. 2023, 69 (Suppl. S3), 31. [Google Scholar] [CrossRef]
- Wood, T.; Thoresen, M. Physiological responses to hypothermia. Semin. Fetal Neonatal Med. 2015, 20, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Moreau, A.; Levy, B.; Annoni, F.; Lorusso, R.; Su, F.; Belliato, M.; Taccone, F.S. The use of induced hypothermia in extracorporeal membrane oxygenation: A narrative review. Resusc. Plus 2023, 13, 100360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Napp, L.C.; Kühn, C.; Hoeper, M.M.; Vogel-Claussen, J.; Haverich, A.; Schäfer, A.; Bauersachs, J. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin. Res. Cardiol. 2015, 105, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Bojic, A.; Steiner, I.; Gamper, J.; Schellongowski, P.; Lamm, W.; Hermann, A.; Riss, K.; Robak, O.; Staudinger, T. Supraclavicular Approach to the Subclavian Vein as an Alternative Venous Access Site for ECMO Cannulae? A Retrospective Comparison. ASAIO J. 2017, 63, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Gothner, M.; Buchwald, D.; Strauch, J.T.; Schildhauer, T.A.; Swol, J. The use of double lumen cannula for veno-venous ECMO in trauma patients with ARDS. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banfi, C.; Pozzi, M.; Siegenthaler, N.; Brunner, M.-E.; Tassaux, D.; Obadia, J.-F.; Bendjelid, K.; Giraud, R. Veno-venous extracorporeal membrane oxygenation: Cannulation techniques. J. Thorac. Dis. 2016, 8, 3762–3773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mauri, T.; Spinelli, E.; Ibrahim, Q.; Rochwerg, B.; Lorusso, R.; Tonna, J.E.; Price, S.; MacLaren, G.; Pesenti, A.; Slutsky, A.S.; et al. Impact of Drainage Cannula Size and Blood Flow Rate on the Outcome of Patients Receiving Venovenous Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome: An ELSO Registry Analysis. Am. J. Respir. Crit. Care Med. 2023, 208, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, E.; Giani, M.; Slobod, D.; Pavlovsky, B.; di Pierro, M.; Crotti, S.; Lissoni, A.; Foti, G.; Grasselli, G.; Mauri, T. Physiologic Effects of Extracorporeal Membrane Oxygenation in Patients with Severe Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2024, 210, 629–638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szułdrzyński, K.; Kowalewski, M.; Jankowski, M.; Staromłyński, J.; Prokop, J.; Pasierski, M.; Chudziński, K.; Drobiński, D.; Martucci, G.; Lorusso, R.; et al. Effects of adding the second drainage cannula in severely hypoxemic patients supported with VV ECMO due to COVID-19-associated ARDS. Artif. Organs 2023, 47, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.B.; Leiendecker, E.; Creel-Bulos, C.; Miller, C.F.; Boorman, D.W.; Javidfar, J.; Attia, T.; Daneshmand, M.; Jabaley, C.S.; Caridi-Schieble, M. Outcomes following additional drainage during veno-venous extracorporeal membrane oxygenation: A single-center retrospective study. Perfusion 2024, 02676591241249609. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, A.; Kondo, Y.; Seki, K.; Kumakawa, Y.; Ishihara, T.; Sueyoshi, K.; Okamoto, K.; Tanaka, H. Use of two parallel oxygenators during veno-venous extracorporeal membrane oxygenation in a patient with severe obesity and COVID-19 pneumonia. Perfusion 2022, 38, 1738–1741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hurtado, S.; Sepulveda, V.; Godoy, C.; Bahamondes, R.; Kattan, E.; Mendez, M.; Besa, S. Parallel oxygenators in the same circuit for refractory hypoxemia on veno-venous extracorporeal membrane oxygenation. A 3-patient series. Perfusion 2023, 39, 1715–1721. [Google Scholar] [CrossRef]
- Leloup, G.; Rozé, H.; Calderon, J.; Ouattara, A. Use of two oxygenators during extracorporeal membrane oxygenator for a patient with acute respiratory distress syndrome, high-pressure ventilation, hypercapnia, and traumatic brain injury. Br. J. Anaesth. 2017, 107, 1014–1015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seadler, B.; Alagoa, K.; Zelten, J.; Ubert, H.A.; Durham, L. 1510: Dual oxygenators for refractory hypercapnia and hypoxia on ecmo: An underutilized therapy? Crit. Care Med. 2023, 52, S726. [Google Scholar] [CrossRef]
- Melro, L.M.G.; Santos, Y.d.A.P.d.; Júnior, L.C.M.C.; Besen, B.A.M.P.; Zigaib, R.; Forte, D.N.; Mendes, P.V.; Park, M. Exploring the association of two oxygenators in parallel or in series during respiratory support using extracorporeal membrane oxygenation. Rev. Bras. Ter. Intensiv. 2022, 34, 402–409. [Google Scholar] [CrossRef]
- Omlor, A.J.; Caspari, S.; Omlor, L.S.; Jungmann, A.M.; Krawczyk, M.; Schmoll, N.; Mang, S.; Seiler, F.; Muellenbach, R.M.; Bals, R.; et al. Comparison of Serial and Parallel Connections of Membrane Lungs against Refractory Hypoxemia in a Mock Circuit. Membranes 2023, 13, 809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madhani, S.P.; May, A.G.; Frankowski, B.J.; Burgreen, G.W.; Federspiel, W.J. Blood Recirculation Enhances Oxygenation Ef-ficiency of Artificial Lungs. ASAIO J. 2020, 66, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Kim, J.W.; Kim, S.H.; Moon, S.H.; Yang, J.H.; Jung, J.J.; Cha, H.J.; Choi, J.Y.; Lee, C.E.; Heo, W.; et al. The serial connection of two extracorporeal membrane oxygenators for patient with refractory hypoxemia. Heart Lung 2021, 50, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.J.; Rojo, M.; Dobaria, V.; Tedesco, J.; Schwartz, J.P. The utility of parallel venovenous extracorporeal membrane oxygenation circuits for refractory hypoxemia in severely burned patients: A case report. JTCVS Tech. 2022, 15, 126–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, A.; Shears, L.L.; Zubkus, D.; Kaczorowski, D.J. Parallel circuits for refractory hypoxemia on venovenous extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2016, 153, e49–e51. [Google Scholar] [CrossRef]
- Shoni, M.; Lazar, S.; Jackson, A.; Tonetti, M.K.; Horak, J.; Gutsche, J.; Augoustides, J.G.; Marchant, B.E.; Fernando, R.J.; Jelly, C.A.; et al. Parallel Venovenous Extracorporeal Membrane Oxygenation Circuits for Refractory Hypoxemia in a Super-Super-Obese Patient. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.J.; Stokes, J.W.; Gannon, W.D.; Francois, S.A.; Wu, W.K.; Rice, T.W.; Bacchetta, M. Extracorporeal Membrane Oxygenation Circuits in Parallel for Refractory Hypoxemia in COVID-19: A Case Series. ASAIO J. 2022, 68, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.J.; Gannon, W.D.; Francois, S.A.; Stokes, J.W.; Tipograf, Y.; Landsperger, J.S.; Semler, M.W.; Casey, J.D.; Rice, T.W.; Bacchetta, M. Extracorporeal membrane oxygenation circuits in parallel for refractory hypoxemia in patients with COVID-19. J. Thorac. Cardiovasc. Surg. 2022, 167, 746–754.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aubron, C.; Cheng, A.C.; Pilcher, D.; Leong, T.; Magrin, G.; Cooper, D.J.; Scheinkestel, C.; Pellegrino, V. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: A 5-year cohort study. Crit. Care 2013, 17, R73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falk, L.; Sallisalmi, M.; Lindholm, J.A.; Lindfors, M.; Frenckner, B.; Broomé, M.; Broman, L.M. Differential hypoxemia during venoarterial extracorporeal membrane oxygenation. Perfusion 2019, 34 (Suppl. S1), 22–29. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Curran, J.; Ahmad, D.; Nasher, N.; Miyamoto, T.; Brailovsky, E.; Shah, M.K.; Rajapreyar, I.N.; Rame, J.E.; Loforte, A.; et al. Utilization and outcomes of V-AV ECMO: A systematic review and meta-analysis. Artif. Organs 2023, 47, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Kim, J.W.; Kang, D.H.; Moon, S.H.; Kim, S.H.; Jung, J.J.; Yang, J.H.; Byun, J.H. Conversion to Veno-arteriovenous Extracorporeal Membrane Oxygenation for Differential Hypoxia. J. Chest Surg. 2023, 56, 274–281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Navas-Blanco, J.R.; Lifgren, S.A.; Dudaryk, R.; Scott, J.; Loebe, M.; Ghodsizad, A. Parallel veno-venous and veno-arterial extracorporeal membrane circuits for coexisting refractory hypoxemia and cardiovascular failure: A case report. BMC Anesthesiol. 2021, 21, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ka, E.S.; Lee, J.; Ahn, S.; Kim, Y.H. Parallel Venovenous and Venoarterial Extracorporeal Membrane Oxygenation for Respiratory Failure and Cardiac Dysfunction in a Patient with Coronavirus Disease 2019: A Case Report. J. Chest Surg. 2024, 57, 225–229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kluis, A.; Rawitscher, D.; Afzal, A.M.; DiMaio, J.M.; George, T.J. Impella 5.5 and venovenous extracorporeal membrane oxygenation as a bridge to ventricular assist device in cardiopulmonary failure. Bayl. Univ. Med. Cent. Proc. 2023, 36, 208–210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Encarnación-Martínez, U.; Torres-Pulido, A.; Lazcano-Díaz, E.A.; Manzur-Sandoval, D.; Baeza-Herrera, L.A.; González-Ruiz, F.J.; Jiménez-Rodríguez, G.M.; Rojas-Velasco, G. Circulatory support with triple cannulation V-PaA ECMO in a patient with acute right ventricular failure and refractory hypoxemia secondary to diffuse alveolar hemorrhage: A case report. Respir. Med. Case Rep. 2024, 50, 102064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucchini, A.; Elli, S.; De Felippis, C.; Greco, C.; Mulas, A.; Ricucci, P.; Fumagalli, R.; Foti, G. The evaluation of nursing workload within an Italian ECMO Centre: A retrospective observational study. Intensiv. Crit. Care Nurs. 2019, 55, 102749. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Brodie, D.; Bartlett, R.; Brochard, L.; Brower, R.; Conrad, S.; De Backer, D.; Fan, E.; Ferguson, N.; Fortenberry, J.; et al. Position Paper for the Organization of Extracorporeal Membrane Oxygenation Programs for Acute Respiratory Failure in Adult Patients. Am. J. Respir. Crit. Care Med. 2014, 190, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Sheldrake, J.; Ilic, D.; Watterson, J.; Berkovic, D.; Pilcher, D.; Udy, A.; Hodgson, C.L. An exploration of intensive care nurses’ perceptions of workload in providing extracorporeal membrane oxygenation (ECMO) support: A descriptive qualitative study. Aust. Crit. Care 2024, 37, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.L.; Hamilton, P.; Murry, N. Unfinished nursing care, missed care, and implicitly rationed care: State of the science review. Int. J. Nurs. Stud. 2015, 52, 1121–1137. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M.J.; Gaies, M.G.; Prosser, L.A. US and International In-Hospital Costs of Extracorporeal Membrane Oxygenation: A Systematic Review. Appl. Health Econ. Health Policy 2015, 13, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Svennevig, J.L.; Bugge, J.F.; Andresen, S.; Mathisen, A.; Karlsen, H.; Khushi, I.; Hagen, T.P. Cost of extracorporeal membrane oxygenation: Evidence from the Rikshospitalet University Hospital, Oslo, Norway. Eur. J. Cardio-Thorac. Surg. 2009, 37, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Uwumiro, F.; Otabor, N.; Okpujie, V.; Osiogo, E.O.; Osemwota, O.F.; Abesin, O.; Utibe, M.A.; Ekeh, N.; Onyekwe, A.E.; Fasoranti-Sowemimo, O.F.; et al. Rates, Outcomes, and Resource Burden of Extracorporeal Membrane Oxygenation Use in Hospitalizations in the United States During the Pandemic. Cureus 2024, 16, e54081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| EOLIA Criteria | Contraindications |
|---|---|
| Old age |
| Significant central nervous system pathology | |
| Active bleeding | |
| Contraindication for anticoagulation | |
Use of:
| End-stage lung disease (except if vvECMo is used as a bridge to lung transplant) |
| Severe immunosuppression | |
| Mechanical ventilation with high FiO2 (>0.8) for more than seven days |
| Ventilation Strategy | Protective Ventilation | Ultra (or Super) Protective Ventilation |
|---|---|---|
| Volume | 6–8 mL/kg of ideal body weight | 3–4 mL/kg of ideal body weight |
| Positive end expiratory pressure (PEEP) | >5 cm H2O | 10–15 cm H2O |
| Inspired oxygen fraction (FiO2) | Titrated to obtain optimal tissue oxygenation | Optimal FiO2 of 0.3 |
| Max respiratory rate | 35 cycles/min | 5 to 10 cycles/min |
| Max targeted driving pressure | <15 cm H2O | <15 cm H2O |
| Max targeted plateau pressure | 28–30 cm H2O | 22–24 cm H2O |
| Permissive hypercapnia and acidosis | PH > 7.30–7.25 and PaCO2 < 60 mmHg | Optimal sweeping gas in vvECMO allows normal PaCO2 value |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robert, A.; Honoré, P.M.; Bulpa, P.; Michaux, I. Managing Refractory Hypoxemia in Acute Respiratory Distress Syndrome Obese Patients with Veno-Venous Extra-Corporeal Membrane Oxygenation: A Narrative Review. J. Clin. Med. 2025, 14, 1653. https://doi.org/10.3390/jcm14051653
Robert A, Honoré PM, Bulpa P, Michaux I. Managing Refractory Hypoxemia in Acute Respiratory Distress Syndrome Obese Patients with Veno-Venous Extra-Corporeal Membrane Oxygenation: A Narrative Review. Journal of Clinical Medicine. 2025; 14(5):1653. https://doi.org/10.3390/jcm14051653
Chicago/Turabian StyleRobert, Arnaud, Patrick M. Honoré, Pierre Bulpa, and Isabelle Michaux. 2025. "Managing Refractory Hypoxemia in Acute Respiratory Distress Syndrome Obese Patients with Veno-Venous Extra-Corporeal Membrane Oxygenation: A Narrative Review" Journal of Clinical Medicine 14, no. 5: 1653. https://doi.org/10.3390/jcm14051653
APA StyleRobert, A., Honoré, P. M., Bulpa, P., & Michaux, I. (2025). Managing Refractory Hypoxemia in Acute Respiratory Distress Syndrome Obese Patients with Veno-Venous Extra-Corporeal Membrane Oxygenation: A Narrative Review. Journal of Clinical Medicine, 14(5), 1653. https://doi.org/10.3390/jcm14051653








