Subcutaneous Injection and Brush Application of Ovalbumin–Aluminum Salt Solution Induces Dermatitis-like Changes in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Ethical Regulations, and Experimental Design
2.2. Induction of Atopic Dermatitis
2.3. Evaluation of Alloknesis and Cutaneous Alterations
2.4. Histological Evaluation
2.5. Statistical Analysis
3. Results
3.1. Response of Alloknesis, Scratching, and Skin Lesions in Balb/c and ICR-CD1 Animals Treated with OVA–Aluminum Salt Solution
3.2. Histological Changes on the Skin of the Back in Balb/c and ICR-CD1 Animals Treated with OVA–Aluminum Salt Solution
3.3. Functional and Histological Changes in ICR-CD1 Mice Treated with OVA–Aluminum Salt Solution
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 129Sv | 129 parental strain mice |
| AD | Atopic dermatitis |
| ARRIVE guides | Animal research reporting of in vivo experiments guides |
| Balb/c | Bagg albino-c mice |
| C3H/HeN | C3H/Heston mice |
| C57BL/6 | C57BLACK6 mice |
| CCL11 | C-C motif chemokine 11 |
| CCL26CGRP | C-C motif chemokine 26Calcitonine gene-related peptide |
| DPX | Dibutylphthalate polystyrene xylene |
| H&E | Hematoxylin and eosin |
| IASP | International Association for the Study of Pain |
| ICR-CD1 or CD1 | Institute of Cancer Research (ICR)-CD1 mice |
| IgE | Immunoglobulin E |
| IL13 | Interleukin-13 |
| IL25 | Interleukin-25 |
| IL31 | Interleukin-31 |
| IL33 | Interleukin-33 |
| IL4 | Interleukin-4 |
| IL5 | Interleukin-5 |
| IL6 | Interleukin-6 |
| IL9 | Interleukin-9 |
| NC/Nga | NC/Nagoya university mice |
| OVA | Ovalbumin |
| OVA-AL | Ovalbumin–aluminum salt solution |
| PBSPGP9.5 | Phosphate buffer salineProtein Gene Product 9.5 |
| SEM | Standard Error of the Mean |
| SJL/J | Swiss Jim Lambert mice |
| SKH-1/Hr | SKH1 Hairless mice |
| TGF-β | Transforming Growth Factor beta |
| Th2 | Helper type 2 lymphocyte |
| TRPTRPV1 | Transient Receptor Potential ion channel Transient receptor potential vanilloid 1 |
References
- Kellogg, C.; Smogorzewski, J. Update on Atopic Dermatitis. Adv. Pediatr. 2023, 70, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.M.; Eichenfield, L.F.; Boguniewicz, M. Atopic dermatitis (Atopic eczema). In Fitzpatrick’s Dermatology in General Medicine, 8th ed.; Goldsmith, L.A., Katz, G.I., Gilchrest, B.A., Paller, A.S., Leffell, D.A., Wolff, K., Eds.; McGrawHill Medical: New York, NY, USA, 2008; Volume 1, pp. 165–181. [Google Scholar]
- Rosa, G.; Fernandez, A.P.; Vij, A.; Sood, A.; Plesec, T.; Bergfeld, W.F.; Billings, S.D. Langerhans cell collections, but not eosinophils, are clues to a diagnosis of allergic contact dermatitis in appropriate skin biopsies. J. Cutan. Pathol. 2016, 43, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Stripling, S.; Fung, S.; Cha, A.; O’Brien, A.; Schachner, L.A. Recent Developments and Advances in Atopic Dermatitis: A Focus on Epidemiology, Pathophysiology, and Treatment in the Pediatric Setting. Paediatr. Drugs. 2022, 24, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Schuler, C.F., 4th; Billi, A.C.; Maverakis, E.; Tsoi, L.C.; Gudjonsson, J.E. Novel insights into atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1145–1154. [Google Scholar] [CrossRef]
- Savva, M.; Papadopoulos, N.G.; Gregoriou, S.; Katsarou, S.; Papapostolou, N.; Makris, M.; Xepapadaki, P. Recent Advancements in the Atopic Dermatitis Mechanism. Front. Biosci. (Landmark Ed). 2024, 29, 84. [Google Scholar] [CrossRef]
- Afshari, M.; Kolackova, M.; Rosecka, M.; Čelakovská, J.; Krejsek, J. Unraveling the skin; A comprehensive review of atopic dermatitis, current understanding, and approaches. Front. Immunol. 2024, 15, 1361005. [Google Scholar] [CrossRef]
- Liu, H. Effect of Skin Barrier on Atopic Dermatitis. Dermatitis 2025, 36, 37–45. [Google Scholar] [CrossRef]
- Pan, Y.; Hochgerner, M.; Cichoń, M.A.; Benezeder, T.; Bieber, T.; Wolf, P. Langerhans cells: Central players in the pathophysiology of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 278–289. [Google Scholar] [CrossRef]
- Huang, C.; Zhuo, F.; Guo, Y.; Wang, S.; Zhang, K.; Li, X.; Dai, W.; Dou, X.; Yu, B. Skin microbiota: Pathogenic roles and implications in atopic dermatitis. Front. Cell Infect. Microbiol. 2025, 14, 1518811. [Google Scholar] [CrossRef]
- Gilhar, A.; Reich, K.; Keren, A.; Kabashima, K.; Steinhoff, M.; Paus, R. Mouse models of atopic dermatitis: A critical reappraisal. Exp. Dermatol. 2021, 30, 319–336. [Google Scholar] [CrossRef]
- Saloga, J.; Renz, H.; Lack, G.; Bradley, K.L.; Greenstein, J.L.; Larsen, G.; Gelfand, E.W. Development and transfer of immediate cutaneous hypersensitivity in mice exposed to aerosolized antigen. J. Clin. Investig. 1993, 91, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Mizoguchi, E.; Brewer, J.P.; Martin, T.R.; Bhan, A.K.; Geha, R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J. Clin. Investig. 1998, 101, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Mizoguchi, E.; Oettgen, H.; Bhan, A.K.; Geha, R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Investig. 1999, 103, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Savinko, T.; Lauerma, A.; Lehtimäki, S.; Gombert, M.; Majuri, M.L.; Fyhrquist-Vanni, N.; Dieu-Nosjean, M.C.; Kemeny, L.; Wolff, H.; Homey, B.; et al. Topical superantigen exposure induces epidermal accumulation of CD8+ T cells, and mixed Th1/Th2-type dermatitis and vigorous production of IgE antibodies in the murine model of atopic dermatitis. J. Immunol. 2005, 175, 8320–8326. [Google Scholar] [CrossRef]
- Wang, G.; Savinko, T.; Wolff, H.; Dieu-Nosjean, M.C.; Kemeny, L.; Hormey, B.; Lauerma, A.I.; Alenius, H. Repeated epicutaneous exposures to ovalbumin progressively induce atopic dermatitis-like skin lesions in mice. Clin. Exp. Allergy. 2007, 37, 151–161. [Google Scholar] [CrossRef]
- Lehto, M.; Savinko, T.; Wolff, H.; Kvist, P.H.; Kemp, K.; Lauerma, A.; Alenius, H. A murine model of epicutaneous protein sensitization is useful to study efficacies of topical drug in atopic dermatitis. Int. Immunopharmacol. 2010, 10, 377–384. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J.; Kang, M.J.; Seo, J.H.; Kim, H.Y.; Jeong, S.K.; Lee, S.H.; Hong, S.J. A novel mouse model of atopic dermatitis with epicutaneous allergen sensitization and the effect of Lactobacillus rhamnosus. Exp. Dermatol. 2012, 21, 672–675. [Google Scholar] [CrossRef]
- Dahten, A.; Koch, C.; Ernst, D.; Schnöller, C.; Hartmann, S.; Worm, M. Systemic PPARgamma ligation inhibits allergic immune response in the skin. J. Investig. Dermatol. 2008, 128, 2211–2218. [Google Scholar] [CrossRef]
- Jin, H.; Kumar, L.; Mathias, C.; Zurakowski, D.; Oettgen, H.; Gorelik, L.; Geha, R. Toll-like receptor 2 as important for the T(H)1 response to cutaneous sensitization. J. Allergy Clin. Immunol. 2009, 123, 875–882. [Google Scholar] [CrossRef]
- Nakajima, S.; Igyártó, B.Z.; Honda, T.; Egawa, G.; Otsuka, A.; Hara-Chikuma, M.; Watanabe, N.; Ziegler, S.F.; Tomura, M.; Inaba, K.; et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J. Allergy Clin. Immunol. 2012, 129, 1048–1055. [Google Scholar] [CrossRef]
- Gericke, J.; Ittensohn, J.; Milhály, J.; Dubrac, S.; Rühl, R. Allegen-induced dermatitis causes alterations in cutaneous retinoid-mediated signaling in mice. PLoS ONE 2013, 8, e71244. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Jeon, M.; Sung, W.J.; Han, S.M.; Pak, S.C.; Park, K.K. Beneficial effects of melittin on ovalbumin-induced atopic dermatitis in mouse. Sci. Rep. 2017, 7, 17679. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sethi, G.S.; Naura, A.S. Curcumin ameliorates ovalbumin-induced atopic dermatitis and blocks the progression of atopic march in mice. Inflammation 2020, 43, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Manicone, A.M.; McGuire, J.K.; Wang, Y.; Parks, W.C. Systemic sensitization with the protein allergen ovalbumin augments local sensitization in atopic dermatitis. J. Inflamm. Res. 2014, 7, 29–38. [Google Scholar] [PubMed]
- Majewska-Szczepanik, M.; Askenase, P.W.; Lobo, F.M.; Maricinska, K.; Wen, L.; Szczepanik, M. Epicutaneous immunization with ovalbumin and CpG induces TH1/TH17 cytokines, which regulate IgE and IgG2a production. J. Allergy Clin. Immunol. 2016, 138, 262–273. [Google Scholar] [CrossRef]
- Tamari, M.; Orimo, K.; Motomura, K.; Arae, K.; Matsuda, A.; Nakae, S.; Saito, H.; Morita, H.; Matsumoto, K. The optimal age for epicutaneous sensitization following tape-stripping in BALB/c mice. Allegol. Int. 2018, 67, 380–387. [Google Scholar] [CrossRef]
- Fang, Y.P.; Yang, S.H.; Lee, C.H.; Aljuffali, I.A.; Kao, H.C.; Fang, J.Y. What is the discrepancy between drug permeation into/across intact and diseased skins? Atopic dermatitis as a model. Int. J. Pharm. 2016, 497, 277–286. [Google Scholar] [CrossRef]
- Shershakova, N.; Baraboshkina, E.; Andreev, S.; Purgina, D.; Struchkova, I.; Kamyshnikov, O.; Nikonova, A.; Khaitov, M. Anti-inflammatory effect of fullerene C60 in a mice model of atopic dermatitis. J. Nanobiotechnol. 2016, 14, 8. [Google Scholar] [CrossRef]
- Kao, J.K.; Hsu, T.F.; Lee, M.S.; Su, T.C.; Lee, C.H.; Hsu, C.S.; Shieh, J.J.; Wang, J.Y.; Yang, R.C. Subcutaneous injection of recombinant heat shock protein 70 ameliorates atopic dermatitis skin lesions in a mouse model. Kaohsiung J. Med. Sci. 2020, 36, 186–195. [Google Scholar] [CrossRef]
- Akiyama, T.; Nguyen, T.; Curtis, E.; Nishida, K.; Devireddy, J.; Delahanty, J.; Carstens, M.I.; Carstens, E. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain 2015, 156, 1240–1246. [Google Scholar] [CrossRef]
- LaMotte, R.H.; Lundberg, L.E.; Torebjörk, H.E. Pain, hyperalgesia an activity in nociceptive C units in humans after intradermal injection of capsaicin. J. Physiol. 1992, 448, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Simone, D.A.; Alreja, M.; LaMotte, R.H. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens. Mot. Res. 1991, 8, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.R.; Lee, S.H.; Kim, B.; Nam, M.H.; Ahn, Y.K.; Park, Y.M.; Jeong, S.M.; Park, M.J.; Song, K.B.; Lee, S.Y.; et al. Rinococcus gnavus ameliorates atopic dermatitis by enhancing Treg cell and metabolites in BALB/c mice. Pediatr. Allergy Immunol. 2022, 33, e13678. [Google Scholar] [CrossRef] [PubMed]
- Kypriotou, M.; Rivero, D.; Haller, S.; Mariotto, A.; Huber, M.; Acha-Orbea, H.; Werner, S.; Hohl, D. Activin a inhibits antigen-induced allergy in murine epicutaneous sensitization. Front. Immunol. 2013, 4, 246. [Google Scholar] [CrossRef]
- Larsen, S.T.; Nielsen, G.D.; Thygesen, P. Investigation of the adjuvant effect of polyethylene glycol (PEG) 400 in BALB/c mice. Int. J. Pharm. 2002, 231, 51–55. [Google Scholar] [CrossRef]
- Ghafourian Boroujerdnia, M.; Azemi, M.E.; Hemmati, A.A.; Taghian, A.; Azadmehr, A. Immunomodulatory effects of Astragalus gypsicolus hydroalcoholic extract in ovalbumin-induced allergic mice model. Iran. J. Allergy Asthma Immunol. 2011, 10, 281–288. [Google Scholar]
- Durham, S.R.; Shamji, M.H. Allergen immunotherapy: Past, present and future. Nat. Rev. Immunol. 2023, 23, 317–328. [Google Scholar] [CrossRef]
- Saeed, S.; Irfan, M.; Naz, S.; Liaquat, M.; Jahan, S.; Hayat, S. Routes and barriers associated with protein and peptide drug delivery system. J. Pak. Med. Assoc. 2021, 71, 2032–2039. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Morton, D.B.; Jennings, M.; Buckwell, A.; Ewbank, R.; Godfrey, C.; Holgate, B.; Inglis, I.; James, R.; Page, C.; Sharman, I.; et al. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab. Anim. 2001, 35, 1–41. [Google Scholar]
- Choi, D.W.; Jung, S.Y.; Kim, G.D.; Lee, S.Y.; Shin, H.S. Miquelianin Inhibits Allergic Responses in Mice by Suppressing CD4+ T Cell Proliferation. Antioxidants 2021, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port. 2019, 32, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T.; Paller, A.S.; Kabashima, K.; Feely, M.; Rueda, M.J.; Ross Terres, J.A.; Wollenberg, A. Atopic dermatitis: Pathomechanisms and lessons learned from novel systemic therapeutic options. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1432–1449. [Google Scholar] [CrossRef]
- Quan, V.L.; Erickson, T.; Daftary, K.; Chovatiya, R. Atopic Dermatitis Across Shades of Skin. Am. J. Clin. Dermatol. 2023, 24, 731–751. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The translational revolution in atopic dermatitis: The paradigm shift from pathogenesis to treatment. Cell Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef]
- Kwatra, S.G.; Misery, L.; Clibborn, C.; Steinhoff, M. Molecular and cellular mechanisms of itch and pain in atopic dermatitis and implications for novel therapeutics. Clin. Transl. Immunol. 2022, 11, e1390. [Google Scholar] [CrossRef]
- Gatmaitan, J.G.; Lee, J.H. Challenges and Future Trends in Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 11380. [Google Scholar] [CrossRef]
- Bartnikas, L.M.; Gurish, M.F.; Burton, O.T.; Leisten, S.; Janssen, E.; Oettgen, H.C.; Beaupré, J.; Lewis, C.N.; Austen, K.F.; Schulte, S.; et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 451–460. [Google Scholar] [CrossRef]
- Kopecki, Z.; Stevens, N.E.; Chong, H.T.; Yang, G.N.; Cowin, A.J. Flightless I alters the inflammatory response and autoantibody profile in an OVA-induced atopic dermatitis skin-like disease. Front. Immunol. 2018, 9, 1833. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Akiyama, T.; Carstens, M.I.; Ikoma, A.; Cevikbas, F.; Steinhoff, M.; Carstens, E. Mouse model of touch-evoked itch (alloknesis). J. Investig. Dermatol. 2012, 132, 1886–1891. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Ueda, C.; Asano, Y.; Takahashi, H.; Iduhara, M.; Takaki, S.; Yasui, K.; Matsuo, Y.; Arimura, A. Contribution of itch-associated stratch behavior to the development of skin lesions in Dermatophagoides farina-induced dermatitis model in NC/Nga mice. Arch. Dermatol. Res. 2009, 301, 739–746. [Google Scholar] [CrossRef]
- Nakagawa, R.; Yoshida, H.; Asakawa, M.; Tamiya, T.; Inoue, N.; Morita, R.; Inoue, H.; Nakao, A.; Yoshimura, A. Pyridone 6, a pan-JAK inhibitor, ameliorates allergic skin inflammation of NC/Nga mice via suppression of Th2 and enhancement of Th17. J. Immunol. 2011, 187, 4611–4620. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.B.; Kang, S. Topical Application of Chrysanthemum indicum L. Attenuates the Development of Atopic Dermatitis-Like Skin Lesions by Suppressing Serum IgE Levels, IFN-γ, and IL-4 in Nc/Nga Mice. Evid. Based Complement. Alternat Med. 2012, 2012, 821967. [Google Scholar] [CrossRef]
- Feng, S.; Liu, W.; Deng, S.; Song, G.; Zhou, J.; Zheng, Z.; Song, Z. An Atopic Dermatitis-Like Mouse Model by Alternate Epicutaneous Application of Dinitrofluorobenzene and an Extract of Dermatophagoides Farinae. Front. Med. 2022, 9, 843230. [Google Scholar] [CrossRef]
- Anggraeni, S.; Triesayuningtyas, D.N.; Endaryanto, A.; Prakoeswa, C.R.S. Evaluation of scoring atopic dermatitis (SCORAD) and scratching behavior in BALB/c mice treated with house dust mite immunotherapy. Vet. Integr. Sci. 2024, 22, 121–129. [Google Scholar] [CrossRef]
- Leclere, M.; Desnoyers, M.; Beauchamp, G.; Lavoie, J.P. Comparison of four staining methods for detection of mast cells in equine bronchoalveolar lavage fluid. J. Vet. Intern. Med. 2006, 20, 377–381. [Google Scholar] [CrossRef]
- Mutsaddi, S.; Kotrashetti, V.S.; Nayak, R.S.; Pattanshetty, S.M. Comparison of histochemical staining techniques for detecting mast cells in oral lesions. Biotech. Histochem. 2019, 94, 459–468. [Google Scholar] [CrossRef]
- Damman, J.; Diercks, G.F.H.; van Doorn, M.B.; Pasmans, S.G.; Hermans, M.A.W. Cutaneous Lesions of Mastocytosis: Mast Cell Count, Morphology, and Immunomolecular Phenotype. Am. J. Dermatopathol. 2023, 45, 697–703. [Google Scholar] [CrossRef]
- Ozgogan, M.; Yildiz, F.; Gurer, A.; Orhun, S.; Kulacoglu, H.; Aydin, R. Changes in collagen and elastic fiber contents of the skin, rectus sheath, transversalis fascia and peritoneum in primary inguinal hernia patients. Bratisl. Lek. Listy. 2006, 107, 235–238. [Google Scholar]
- Suvik, A.; Effendy, A.W.M. The use of modified Masson’s Trichrome staining in collagen evaluation in wound healing study. Malasian J. Vet. Res. 2012, 3, 39–47. [Google Scholar]
- Manne, J.; Markova, M.; Siracusa, L.D.; Jimenez, S.A. Collagen content in skin and internal organs of the tight skin mouse: An animal model of scleroderma. Biochem. Res. Int. 2013, 2013, 436053. [Google Scholar] [CrossRef]
- Wyles, S.P.; Proffer, S.L.; Farris, P.; Randeall, L.; Hillestad, M.L.; Lupo, M.P.; Behfar, A. Effect of topical human platelet extract (HPE) for facial skin rejuvenation: A histological study of collagen and elastin. J. Drugs Dermatol. 2024, 23, 735–740. [Google Scholar] [CrossRef]
- Chen, M.; Ding, P.; Yang, L.; He, X.; Gao, C.; Yang, G.; Zhang, H. Evaluation of anti-inflammatory activities of Qingre-Qushi Recipe (QRQS) against atopic dermatitis: Potential mechanism of inhibition of IL-33/ST2 signal transduction. Evid. Based Complement. Alternat Med. 2017, 2017, 2489842. [Google Scholar] [CrossRef]
- Tan, L.; Lu, J.; Chen, M.; Xiang, Y.; Cheng, Q.; Liang, Y.; Huang, J.; Huang, J.; Chen, J.; Gao, L. Epicutaneous sensitization with ovalbumin, staphylococcal enterotoxin B and vitamin D analogue induces atopic dermatitis in mice. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2017, 42, 1023–1029. [Google Scholar]
- Kim, H.J.; Lee, S.H.; Hong, S.J. Antibiotics-induced dysbiosis of intestinal microbiota aggravates atopic dermatitis in mice by altered short-chain fatty acids. Allergy Asthma Immunol. Res. 2020, 12, 137–148. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, S. Sophoricoside from Styphnolobium japonicum improves experimental atopic dermatitis in mice. Phytomedicine. 2021, 82, 153463. [Google Scholar] [CrossRef]
- Ham, W.K.; Lee, E.J.; Jeon, M.S.; Kim, H.Y.; Agrahari, G.; An, E.J.; Bang, C.H.; Kim, D.S.; Kim, T.Y. Treatment with phosphodiester CpG-ODN ameliorates atopic dermatitis by enhancing TFG-β signaling. GMB Rep. 2021, 54, 142–147. [Google Scholar]
- Woo, Y.R.; Park, S.Y.; Choi, K.; Hong, E.S.; Kim, S.; Kim, H.S. Air pollution and atopic dermatitis: The impact of particulate matter (PM10) on and AD mouse-model. Int. J. Mol. Sci. 2020, 21, 6079. [Google Scholar] [CrossRef]
- Gu, H.; Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Han, S.M.; Leem, J.; Park, K.K. Therapeutic effects of bee venom on experimental atopic dermatitis. Mol. Med. Rep. 2018, 18, 3711–3718. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, W.H.; Wang, X.R.; Liu, Y.; Chen, M.; Xu, X.G.; Liao, W.Q.; Hu, J.H. Tryptase and protease-activated receptor-2 stimulate scratching behavior in a murine model of ovalbumin-induced atopic-like dermatitis. Int. Immunopharmacol. 2015, 28, 507–512. [Google Scholar] [CrossRef]
- Domocos, D.; Follansbee, T.; Nguyen, A.; Nguyen, T.; Carstens, M.I.; Carstens, E. Cinnamaldehyde elicits itch behavior via TRPV1 and TRPV4 but not TRPA1. Itch 2020, 5, e36. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Y.; Xiong, J.; Chen, X.; Liang, J.; Ji, W. The kinin B1 receptor mediates alloknesis in a murine model of inflammation. Neurosci. Lett. 2014, 560, 31–35. [Google Scholar] [CrossRef]
- Sakai, K.; Sanders, K.M.; Youssef, M.R.; Yanushefski, K.M.; Jensen, L.; Yosipovitch, G.; Akiyama, T. Mouse model of imiquimod-induced psoriatic itch. Pain 2016, 157, 2536–2543. [Google Scholar] [CrossRef]
- Back, S.K.; Jeong, K.Y.; Li, C.; Lee, J.; Lee, S.B.; Na, H.S. Chronically relapsing pruritic dermatitis in the rats treated as neonate with capsaicin: A potential rat model of human atopic dermatitis. J. Dermatol. Sci. 2012, 67, 111–119. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Lee, H.; Jo, S.Y.; Yang, W.M. (R)-(+)-pulegone suppresses allergic and inflammation responses on 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice model. J. Dermatol. Sci. 2018, 91, 292–300. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, S.H.; Jhee, K.H.; Yang, S.A. Zizania latifolia and Its Major Compound Tricin Regulate Immune Responses in OVA-Treated Mice. Molecules 2022, 27, 3978. [Google Scholar] [CrossRef]
- Vaia, M.; Petrosino, S.; De Filippis, D.; Negro, L.; Guarino, A.; Carnuccio, R.; Di Marzo, V.; Iuvone, T. Palmitoylethanolamide reduces inflammation and itch in a mouse model of contact allergic dermatitis. Eur. J. Pharmacol. 2016, 791, 669–674. [Google Scholar] [CrossRef]
- Yamaura, K.; Doi, R.; Suwa, E.; Ueno, K. Repeated application of glucocorticoids exacerbate pruritus via inhibition of prostaglandin D2 production of mast cells in a murine model of allergic contact dermatitis. J. Toxicol. Sci. 2012, 37, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Seong, G.S.; Choung, S.Y. Fermented Morinda citrifolia (Noni) Alleviates DNCB-Induced Atopic Dermatitis in NC/Nga Mice through Modulating Immune Balance and Skin Barrier Function. Nutrients 2020, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Seong, G.S.; Kim, Y.D.; Choung, S.Y. Deacetylasperulosidic Acid Ameliorates Pruritus, Immune Imbalance, and Skin Barrier Dysfunction in 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis NC/Nga Mice. Int. J. Mol. Sci. 2021, 23, 226. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J. Allergy Clin. Immunol. 2000, 105, 860–876. [Google Scholar] [CrossRef]
- Matsui, K.; Nishikawa, A. Percutaneous application of peptidoglycan from Staphylococcus aureus induces an increase in mast cell numbers in the dermis of mice. Clin. Exp. Allergy 2005, 35, 382–387. [Google Scholar] [CrossRef]
- Simon, D.; Braathen, L.R.; Simon, H.U. Eosinophils and atopic dermatitis. Allergy 2004, 59, 561–570. [Google Scholar] [CrossRef]
- Katagiri, K.; Arakawa, S.; Hatano, Y.; Fujiwara, S. Tolerogenic antigen-presenting cells successfully inhibit atopic dermatitis-like skin lesion induced by repeated epicutaneous exposure to ovalbumin. Arch. Dermatol. Res. 2008, 300, 583–593. [Google Scholar] [CrossRef]
- Hong, M.R.; Lei, D.; Yousaf, M.; Chavda, R.; Gabriel, S.; Janmohamed, S.R.; Silverberg, J.I. Reliability and Longitudinal Course of Itch/Scratch Severity in Adults with Atopic Dermatitis. Dermatitis 2021, 32, S28–S32. [Google Scholar] [CrossRef]
- Staughton, R.C.D.; Bridgett, C.K.; Norén, P.; Goulding, J.M.R.; Affleck, A.; Walsh, S. Habitual scratching amplifies and perpetuates atopic dermatitis. Br. J. Dermatol. 2020, 183, 403. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Arai, I.; Takano, N.; Tanaka, M.; Nakaike, S. Induction of scratching behaviour and dermatitis in various strains of mice cohabiting with NC/Nga mice with chronic dermatitis. Br. J. Dermatol. 2006, 154, 28–33. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Takaoka, A.; Sugimoto, M.; Honma, Y.; Sakurai, T.; Futaki, N.; Arai, I. Itch-associated scratching contributes to the development of dermatitis and hyperimmunoglobulinaemia E in NC/Nga mice. Exp. Dermatol. 2011, 20, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Green, A.D.; Young, K.K.; Lehto, S.G.; Smith, S.B.; Mogil, J.S. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain 2006, 124, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bień, K.; Żmigrodzka, M.; Orłowski, P.; Fruba, A.; Szymański, Ł.; Stankiewicz, W.; Nowak, Z.; Malewski, T.; Krzyżowska, M. Involvement of Fas/FasL pathway in the murine model of atopic dermatitis. Inflamm. Res. 2017, 66, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Galand, C.; Leyva-Castillo, J.M.; Yoon, J.; Han, A.; Lee, M.S.; McKenzie, A.N.J.; Stassen, M.; Oyoshi, M.K.; Finkelman, F.D.; Geha, R.S. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J. Allergy Clin. Immunol. 2016, 138, 1356–1366. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, S.H. Therapeutic Effects of Korean Red Ginseng Extract in a Murine Model of Atopic Dermatitis: Anti-pruritic and Anti-inflammatory Mechanism. J. Korean Med. Sci. 2017, 32, 679–687. [Google Scholar] [CrossRef]
- Beng, H.; Su, H.; Wang, S.; Kuai, Y.; Hu, J.; Zhang, R.; Liu, F.; Tan, W. Differential effects of inhaled R- and S-terbutaline in ovalbumin-induced asthmatic mice. Int. Immunopharmacol. 2019, 73, 581–589. [Google Scholar] [CrossRef]
- Song, J.; Zhu, X.M.; Wei, Q.Y. MSCs reduce airway remodeling in the lungs of asthmatic rats through the Wnt/β-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11199–11211. [Google Scholar]
- Lei, F.; Zhu, D.; Sun, J.; Dong, Z. Effects of minimal persistent inflammation on nasal mucosa of experimental allergic rhinitis. Am. J. Rhinol. Allergy 2010, 24, e23–e28. [Google Scholar] [CrossRef]
- El Safoury, O.S.; Fawzy, M.M.; El Maadawa, Z.M.; Mohamed, D.H. Quantitation of mast cells and collagen fibers in skin tags. Indian J. Dermatol. 2009, 54, 319–322. [Google Scholar] [CrossRef]
- Riekki, R.; Harvima, I.T.; Jukkola, A.; Risteli, J.; Oikarinen, A. The production of collagen and the activity of mast-cell chymase increase in human skin after irradiation therapy. Exp. Dermatol. 2004, 13, 364–371. [Google Scholar] [CrossRef]
- Gordon, J.R.; Galli, S.J. Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the Fc epsilon RI. Role for mast cell-derived transforming growth factor beta and tumor necrosis factor alpha. J. Exp. Med. 1994, 180, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, E.; Nagler, A.; Pickholtz, D.; Gillery, P.; Reich, R.; Maquart, F.X.; Levi-Schaffer, F. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: A direct role for mast cells in skin fibrosis. Clin. Exp. Allergy 2002, 32, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, K.M.; Kim, H.O.; Jang, Y.C.; Kwak, I.S. Clinical and histological correlation in post-burn hypertrophic scar for pain and itching sensation. Ann. Dermatol. 2013, 25, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.M.; Bakus, A.D.; Garden, B.C.; Lai, O.; Jones, V.A.; Garden, J.M. Treatment of Pain in Keloids Using Only a Long-Pulsed 1064 nm Nd:YAG Laser. Lasers Surg Med. 2021, 53, 66–69. [Google Scholar] [CrossRef]
- Thomas, E.; George, A.; Deodhar, D.; John, M. Scleromyxedema: An Atypical Case. Indian J. Dermatol. 2015, 60, 323. [Google Scholar]
- Zhu, X.; Jiang, L.; Zhong, Q.; Kong, X.; Zhang, R.; Zhu, L.; Liu, Q.; Wu, W.; Tan, Y.; Wang, J.; et al. Abnormal expression of interleukin-6 is associated with epidermal alternations in localized scleroderma. Clin. Rheumatol. 2022, 41, 2179–2187. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Q.; Wang, L.; Tu, W.; Chu, H.; Ding, W.; Jiang, S.; Ma, Y.; Shi, X.; Pu, W.; et al. Increased expression of latent TGF-β-binding protein 4 affects the fibrotic process in scleroderma by TGF-β/SMAD signaling. Lab. Investig. 2017, 97, 591–601. [Google Scholar] [CrossRef]
- Xu, J.; Zanvit, P.; Hu, L.; Tseng, P.Y.; Liu, N.; Wang, F.; Liu, O.; Zhang, D.; Jin, W.; Guo, N.; et al. The Cytokine TGF-β Induces Interleukin-31 Expression from Dermal Dendritic Cells to Activate Sensory Neurons and Stimulate Wound Itching. Immunity 2020, 53, 371–383. [Google Scholar] [CrossRef]
- Keshari, S.; Sipayung, A.D.; Hsieh, C.C.; Su, L.J.; Chiang, Y.R.; Chang, H.C.; Yang, W.C.; Chuang, T.H.; Chen, C.L.; Huang, C.M. IL-6/p-BTK/p-ERK signaling mediates calcium phosphate-induced pruritus. FASEB J. 2019, 33, 12036–12046. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.Y.; Kim, M.H.; Han, J.M.; Lee, J.E.; Kim, E.H.; Hong, J.; Kim, J.; Yang, W.M. Topical Application of Angelica sinensis Improves Pruritus and Skin Inflammation in Mice with Atopic Dermatitis-Like Symptoms. J. Med. Food. 2016, 19, 98–105. [Google Scholar] [CrossRef]
- Olivry, T.; Mayhew, D.; Paps, J.S.; Linder, K.E.; Peredo, C.; Rajpal, D.; Hofland, H.; Cote-Sierra, J. Early Activation of Th2/Th22 Inflammatory and Pruritogenic Pathways in Acute Canine Atopic Dermatitis Skin Lesions. J. Investig. Dermatol. 2016, 136, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Ryuk, J.A.; Ko, B.S.; Moon, N.R.; Park, S. Pinus koraiensis needle or cone extracts alleviate atopic dermatitis symptoms by regulating immunity and suppressing inflammation in HaCaT cells and Nc/Nga mice. J. Food Biochem. 2022, 46, e14135. [Google Scholar] [CrossRef] [PubMed]
- Escoubet, L.; Rey, A.; Wong, A.; Bernad, J.; Lepert, J.C.; Orfila, C.; Pipy, B. Increased cyclooxygenase-2 and 5-lipoxygenase activating protein expression in peritoneal macrophages during ovalbumin immunization of mice and cytosolic phospholipase A(2) activation after antigen challenge. Biochim. Biophys. Acta 2000, 1487, 92–105. [Google Scholar] [CrossRef]
- de Siqueira, A.L.; Russo, M.; Steil, A.A.; Facincone, S.; Mariano, M.; Jancar, S. A new murine model of pulmonary eosinophilic hypersensitivity: Contribution to experimental asthma. J. Allergy Clin. Immunol. 1997, 100, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Manzolli, S.; Macedo-Soares, M.F.; Vianna, E.O.; Sannomiya, P. Allergic airway inflammation in hypothyroid rats. J. Allergy Clin. Immunol. 1999, 104, 595–600. [Google Scholar] [CrossRef]
- Sonar, S.S.; Ehmke, M.; Marsh, L.M.; Dietze, J.; Dudda, J.C.; Conrad, M.L.; Renz, H.; Nockher, W.A. Clara cells drive eosinophil accumulation in allergic asthma. Eur. Respir. J. 2012, 39, 429–438. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Jeffery, H.; Davis, S.S. Long-term antibody responses in mice following subcutaneous immunization with ovalbumin entrapped in biodegradable microparticles. Vaccine 1993, 11, 965–969. [Google Scholar] [CrossRef]
- Vissers, J.L.; van Esch, B.C.; Hofman, G.A.; Kapsenberg, M.L.; Weller, F.R.; van Oosterhout, A.J. Allergen immunotherapy induces a suppressive memory response mediated by IL-10 in a mouse asthma model. J. Allergy Clin. Immunol. 2004, 113, 1204–1210. [Google Scholar] [CrossRef]
- Lehtola, T.; Nummenmaa, E.; Nieminen, R.; Hämäläinen, M.; Vuolteenaho, K.; Moilanen, E. The glucocorticoid dexamethasone alleviates allergic inflammation through a mitogen-activated protein kinase phosphatase-1-dependent mechanism in mice. Basic. Clin. Pharmacol. Toxicol. 2024, 134, 686–694. [Google Scholar] [CrossRef]
- Verri, W.A., Jr.; Guerrero, A.T.; Fukada, S.Y.; Valerio, D.A.; Cunha, T.M.; Xu, D.; Ferreira, S.H.; Liew, F.Y.; Cunha, F.Q. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 2723–2728. [Google Scholar] [CrossRef]
- Gil, T.Y.; Jin, B.R.; An, H.J. Ovalbumin Induces Atopic Dermatitis-like Skin Lesions in Different Species of mice: Pilot study. J. Conver. Korean Med. 2021, 2, 13–22. [Google Scholar]
- Dahlberg, P.E.; Schartner, J.M.; Timmel, A.; Seroogy, C.M. Daily subcutaneous injections of peptide induce CD4+ CD25+ T regulatory cells. Clin. Exp. Immunol. 2007, 149, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, H.S.; Hwang, Y.H.; Kim, J.J.; Kang, K.Y.; Kim, S.J.; Kim, H.K.; Kim, J.D.; Jeong, D.H.; Paik, M.J.; et al. Enhanced anti-tumor immunotherapy by dissolving microneedle patch loaded ovalbumin. PLoS ONE 2019, 14, e0220382. [Google Scholar] [CrossRef] [PubMed]
- Hirobe, S.; Kawakita, T.; Yamasaki, T.; Ito, S.; Tachibana, M.; Okada, N. Adjuvant Activity of CpG-Oligonucleotide Administered Transcutaneously in Combination with Vaccination Using a Self-Dissolving Microneedle Patch in Mice. Vaccines 2021, 9, 1480. [Google Scholar] [CrossRef]
- Chen, M.C.; Lai, K.Y.; Ling, M.H.; Lin, C.W. Enhancing immunogenicity of antigens through sustained intradermal delivery using chitosan microneedles with a patch-dissolvable design. Acta Biomater. 2018, 65, 66–75. [Google Scholar] [CrossRef]
- Pamornpathomkul, B.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. Enhancement of Skin Permeation and Skin Immunization of Ovalbumin Antigen via Microneedles. AAPS PharmSciTech 2017, 18, 2418–2426. [Google Scholar] [CrossRef]
- Casaro, M.; Souza, V.R.; Oliveira, F.A.; Ferreira, C.M. OVA-Induced Allergic Airway Inflammation Mouse Model. Methods Mol. Biol. 2019, 1916, 297–301. [Google Scholar]
- Ko, M.T.; Huang, S.C.; Kang, H.Y. Establishment and characterization of an experimental mouse model of allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2015, 272, 1149–1155. [Google Scholar] [CrossRef]
- Liu, C.; Pang, C.; Chen, D.S.; Wang, J.; Yi, W.Q.; Yu, N.; Chen, L. In vivo visualization and analysis of ciliary motion in allergic rhinitis models induced by ovalbumin. Exp. Biol. Med. 2022, 247, 1287–1297. [Google Scholar] [CrossRef]
- Wegmann, M.; Fehrenbach, H.; Fehrenbach, A.; Held, T.; Schramm, C.; Garn, H.; Renz, H. Involvement of distal airways in a chronic model of experimental asthma. Clin. Exp. Allergy 2005, 35, 1263–1671. [Google Scholar] [CrossRef]
- Kumar, R.K.; Herbert, C.; Foster, P.S. Expression of growth factors by airway epithelial cells in a model of chronic asthma: Regulation and relationship to subepithelial fibrosis. Clin. Exp. Allergy 2004, 34, 567–675. [Google Scholar] [CrossRef]
- Kucharewicz, I.; Bodzenta-Łukaszyk, A.; Buczko, W. Experimental asthma in rats. Pharmacol. Rep. 2008, 60, 783–788. [Google Scholar]
- Buday, T.; Gavliakova, S.; Mokry, J.; Medvedova, I.; Kavalcikova-Bogdanova, N.; Plevkova, J. The Guinea Pig Sensitized by House Dust Mite: A Model of Experimental Cough Studies. Adv. Exp. Med. Biol. 2016, 905, 87–95. [Google Scholar]
- Dill, J.; Landrigan, P.; Ghose, T. A study of the effects of systemic administration of adrenal glucocorticoids in an experimental model of hypersensitivity pneumonitis. Clin. Allergy 1977, 7, 83–92. [Google Scholar] [CrossRef]
- Butler, J.E.; Swanson, P.A.; Richerson, H.B.; Ratajczak, H.V.; Richards, D.W.; Suelzer, M.T. The local and systemic IgA and IgG antibody responses of rabbits to a soluble inhaled antigen: Measurement of responses in a model of acute hypersensitivity pneumonitis. Am. Rev. Respir. Dis. 1982, 126, 80–85. [Google Scholar]
- Yukawa, T.; Makino, S.; Fukuda, T.; Kamikawa, Y. Experimental model of anaphylaxis-induced beta-adrenergic blockade in the airways. Ann. Allergy 1986, 57, 219–224. [Google Scholar]
- Al-Salam, S.; Aburawi, E.H.; Al-Hammadi, S.; Dhanasekaran, S.; Shafiuallah, M.; Yasin, J.; Sudhadevi, M.; Awwad, A.; Alper, S.L.; Kazzam, E.E.; et al. Cellular and Immunohistochemical Changes in Anaphylactic Shock Induced in the Ovalbumin-Sensitized Wistar Rat Model. Biomolecules 2019, 9, 101. [Google Scholar] [CrossRef]
- Thakur, V.R.; Mehta, A.A. Ovalbumin/lipopolysaccharide induced vasculitis in rats: A new predictive model. J. Basic. Clin. Physiol. Pharmacol. 2021, 33, 445–455. [Google Scholar] [CrossRef]
- Hembry, R.M.; Playfair, J.; Watson, P.G.; Dingle, J.T. Experimental model for scleritis. Arch. Ophthalmol. 1979, 97, 1337–1340. [Google Scholar] [CrossRef]
- Carreras, I.; Carreras, B.; McGrath, L.; Rice, A.; Easty, D.L. Activated T cells in an animal model of allergic conjunctivitis. Br. J. Ophthalmol. 1993, 77, 509–514. [Google Scholar] [CrossRef]
- Nitzan, Y.; Boldur, I.; Afgin, Y.; Barishak, Y.R.; Malik, Z.; Sompolinsky, D. The dynamics of inflammation of the anterior eye in a novel experimental model for hypersensitivity. Cytobios 1996, 88, 105–117. [Google Scholar]
- Saldanha, J.C.; Gargiulo, D.L.; Silva, S.S.; Carmo-Pinto, F.H.; Andrade, M.C.; Alvarez-Leite, J.I.; Teixeira, M.M.; Cara, D.C. A model of chronic IgE-mediated food allergy in ovalbumin-sensitized mice. Braz. J. Med. Biol. Res. 2004, 37, 809–816. [Google Scholar] [CrossRef]
- Fontanella, G.; Bassan, N.; Vinuesa, M. Sensitization increases esterase-positive macrophage number in appendix from an animal model of food Allergy Allergol. Immunopathol. 2005, 33, 277–281. [Google Scholar] [CrossRef]
- Watanabe, H.; Toda, M.; Sekido, H.; Wellner, A.; Fujii, T.; Henle, T.; Hachimura, S.; Nakajima-Adachi, H. Heat treatment of egg white controls allergic symptoms and induces oral tolerance to ovalbumin in a murine model of food Allergy. Mol. Nutr. Food Res. 2014, 58, 394–404. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Kobayashi, K.; Murata, T. Behavioral changes of food allergic model mice during light and dark period. J. Pharmacol. Sci. 2023, 153, 113–118. [Google Scholar] [CrossRef]
- Gautam, A.M.; Pearson, C.I.; Sinha, A.A.; Smilek, D.E.; Steinman, L.; McDevitt, H.O. Inhibition of experimental autoimmune encephalomyelitis by a nonimmunogenic non-self peptide that binds to I-Au. J. Immunol. 1992, 148, 3049–3054. [Google Scholar] [CrossRef]
- Cao, Y.; Toben, C.; Na, S.Y.; Stark, K.; Nitschke, L.; Peterson, A.; Gold, R.; Schimpl, A.; Hünig, T. Induction of experimental autoimmune encephalomyelitis in transgenic mice expressing ovalbumin in oligodendrocytes. Eur. J. Immunol. 2006, 36, 207–215. [Google Scholar] [CrossRef]
- Matsubara, A.; Nishizawa, H.; Kurose, A.; Nakagawa, T.; Takahata, J.; Sasaki, A. An experimental study of inner ear injury in an animal model of eosinophilic otitis media. Acta Otolaryngol. 2014, 134, 227–232. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, C.; Chen, P.; Zhao, S. Morphological and pathological changes of Eustachian tube mucosa in an animal model of eosinophilic otitis media. Braz. J. Otorhinolaryngol. 2022, 88, 701–707. [Google Scholar] [CrossRef]
- Howson, P.; Shepard, N.; Mitchell, N. The antigen induced arthritis model: The relevance of the method of induction to its use as a model of human disease. J. Rheumatol. 1986, 13, 379–390. [Google Scholar]
- O’Byrne, E.M.; Paul, P.K.; Roberts, E.D.; Blancuzzi, V.; Wilson, D.; Goldberg, R.L.; DiPasquale, G. Comparison of magnetic resonance imaging (MRI) and histopathology in rabbit models of osteoarthritis and immune arthritis. Agents Actions 1993, 39, 157–159. [Google Scholar] [CrossRef]
- Thomé, R.; Fernandes, L.G.; Mineiro, M.F.; Simioni, P.U.; Joazeiro, P.P.; Tamashiro, W.M. Oral tolerance and OVA-induced tolerogenic dendritic cells reduce the severity of collagen/ovalbumin-induced arthritis in mice. Cell Immunol. 2012, 280, 113–123. [Google Scholar] [CrossRef]
- Teplov, A.Y.; Grishin, S.N.; Mukhamedyarov, M.A.; Ziganshin, A.U.; Zefirov, A.L.; Palotás, A. Ovalbumin-induced sensitization affects non-quantal acetylcholine release from motor nerve terminals and alters contractility of skeletal muscles in mice. Exp. Physiol. 2009, 94, 264–268. [Google Scholar] [CrossRef]
- Alenius, H.; Laouini, D.; Woodward, A.; Mizoguchi, E.; Bhan, A.K.; Castigli, E.; Oettgen, H.C.; Geha, R.S. Mast cells regulate IFN-gamma expression in the skin and circulating IgE levels in allergen-induced skin inflammation. J. Allergy Clin. Immunol. 2002, 109, 106–113. [Google Scholar] [CrossRef]
- Bonam, S.R.; Partidos, C.D.; Halmuthur, S.K.M.; Muller, S. An Overview of Novel Adjuvants Designed for Improving Vaccine Efficacy. Trends Pharmacol. Sci. 2017, 38, 771–793. [Google Scholar] [CrossRef]
- Azman, S.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Wahidin, S.; Lum, P.T.; Dhadde, S.B. Traditional Medicinal Plants Conferring Protection Against Ovalbumin-Induced Asthma in Experimental Animals: A Review. J. Asthma Allergy 2021, 14, 641–662. [Google Scholar] [CrossRef]
- European Parliament. Directive 2010/63/eu of the European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L 276/33–L 276/79. [Google Scholar]
- Real Decreto 53/2013, de 1 de febrero, por el que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia. Boletín Of. Del Estado (BOE) 2013, 34, 11370–11421.
- Yosipovitch, G.; Kim, B.; Luger, T.; Lerner, E.; Metz, M.; Adiri, R.; Canosa, J.M.; Cha, A.; Ständer, S. Similarities and differences in peripheral itch and pain pathways in atopic dermatitis. J. Allergy Clin. Immunol. 2024, 153, 904–912. [Google Scholar] [CrossRef]
- Renkhold, L.; Wiegmann, H.; Pfleiderer, B.; Süer, A.; Zeidler, C.; Pereira, M.P.; Schmelz, M.; Ständer, S.; Agelopoulos, K. Scratching increases epidermal neuronal branching and alters psychophysical testing responses in atopic dermatitis and brachioradial pruritus. Front. Mol. Neurosci. 2023, 16, 1260345. [Google Scholar] [CrossRef]
- Navarro, X.; Verdú, E.; Wendelschafer-Crabb, G.; Kennedy, W.R. Immunohistochemical study of skin reinnervation by regenerative axons. J. Comp. Neurol. 1997, 380, 164–174. [Google Scholar] [CrossRef]
- Nolano, M.; Simone, D.A.; Wendelschafer-Crabb, G.; Johnson, T.; Hazen, E.; Kennedy, W.R. Topical capsaicin in humans: Parallel loss of epidermal nerve fibers and pain sensation. Pain 1999, 81, 135–145. [Google Scholar] [CrossRef]
- Dhand, A.; Aminoff, M.J. The neurology of itch. Brain 2014, 137, 313–322. [Google Scholar] [CrossRef]
- Gao, N.; Li, M.; Wang, W.; Liu, Z.; Guo, Y. The dual role of TRPV1 in peripheral neuropathic pain: Pain switches caused by its sensitization or desensitization. Front. Mol. Neurosci. 2024, 17, 1400118. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Nau, C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 2005, 280, 13424–13432. [Google Scholar] [CrossRef]
- Jung, J.; Shin, J.S.; Lee, S.Y.; Hwang, S.W.; Koo, J.; Cho, H.; Oh, U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 2004, 279, 7048–7054. [Google Scholar] [CrossRef]
- Lee, S.; Lim, N.Y.; Kang, M.S.; Jeong, Y.; Ahn, J.O.; Choi, J.H.; Chung, J.Y. IL-31RA and TRPV1 Expression in Atopic Dermatitis Induced with Trinitrochlorobenzene in Nc/Nga Mice. Int. J. Mol. Sci. 2023, 24, 13521. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Ono, K.; Ye, Y.; Viet, C.T.; Dang, D.; Schmidt, B.L. TRPV1 expression level in isolectin B₄-positive neurons contributes to mouse strain difference in cutaneous thermal nociceptive sensitivity. J. Neurophysiol. 2015, 113, 3345–3355. [Google Scholar] [CrossRef]

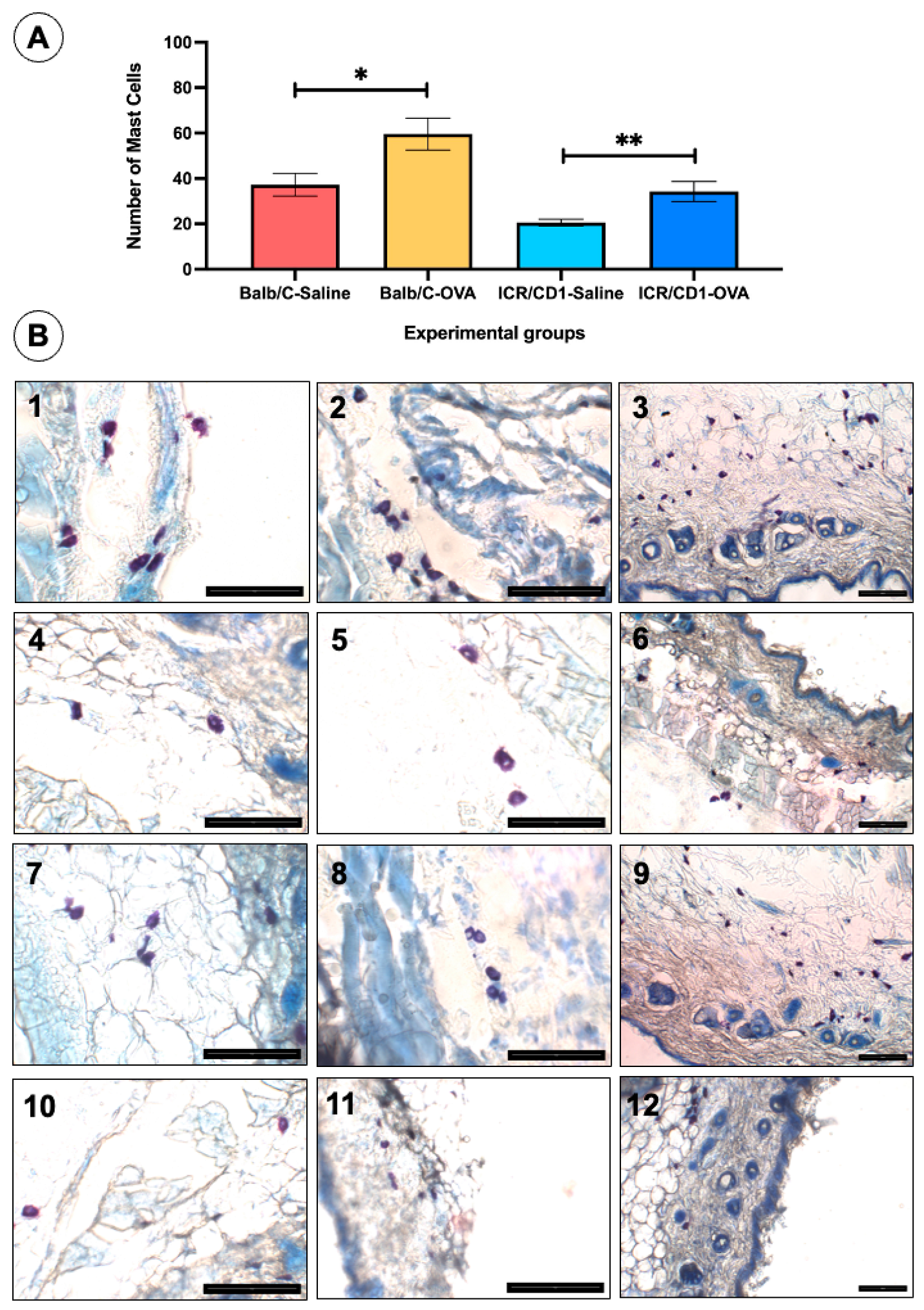
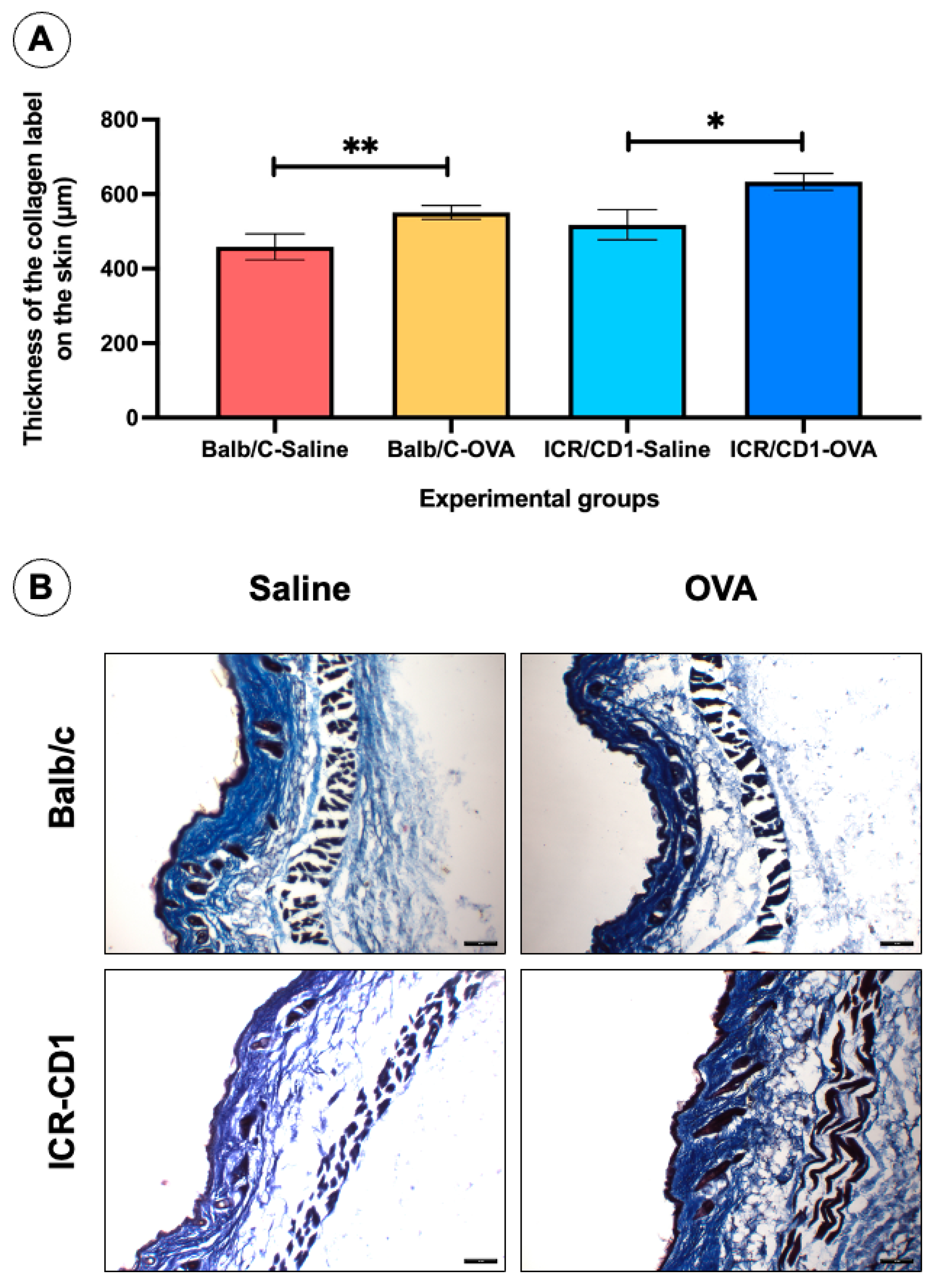

| Groups | Research 1 | Research 2 | |
|---|---|---|---|
| Balb/C-Saline | 37.64 ± 6.05 | 36.82 ± 8.42 | p = 0.7599 |
| Balb/C-OVA | 55.40 ± 9.13 | 63.80 ± 11.03 | p = 0.5421 |
| ICR/CD1-Saline | 21.33 ± 2.30 | 19.75 ± 1.96 | p = 0.6325 |
| ICR/CD1-OVA | 33.11 ± 6.56 | 35.33 ± 6.29 | p = 0.6823 |
| Groups | Research 1 | Research 2 | |
|---|---|---|---|
| Balb/C-Saline | 546.6 ± 30.57 | 554.9 ± 23.9 | 0.7283 |
| Balb/C-OVA | 458.7 ± 54.42 | 458.9 ± 46.16 | 0.6058 |
| ICR/CD1-Saline | 623.9 ± 34.40 | 642.7 ± 30.70 | 0.9430 |
| ICR/CD1-OVA | 517.7 ± 60.11 | 518.1 ± 58.45 | 0.7984 |
| Balb/c Mice | ICR-CD1 Mice | |
|---|---|---|
| Alloknesis | None | None |
| Dermatitis score | ++ | +/none |
| Mast cells | +++ | ++ |
| Thickness of epidermis | = | = |
| Thickness of dermis | = | = |
| Thickness of Masson’s trichomic labeling | = | = |
| Parameter | 35 Days of Follow-Up | 49 Days of Follow-Up |
|---|---|---|
| Alloknesis | No | No |
| Skin lesion | Yes (dryness) | Yes (dryness) |
| Mast cells in dorsal skin | OVA > saline | OVA > saline |
| Collagen staining on dorsal skin (Masson’s trichome stain) | OVA > saline | OVA > saline |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siquier-Dameto, G.; Iguaran-Pérez, A.; Gimeno-Beltrán, J.; Bellia, G.; Giori, A.M.; Boadas-Vaello, P.; Verdú, E. Subcutaneous Injection and Brush Application of Ovalbumin–Aluminum Salt Solution Induces Dermatitis-like Changes in Mice. J. Clin. Med. 2025, 14, 1701. https://doi.org/10.3390/jcm14051701
Siquier-Dameto G, Iguaran-Pérez A, Gimeno-Beltrán J, Bellia G, Giori AM, Boadas-Vaello P, Verdú E. Subcutaneous Injection and Brush Application of Ovalbumin–Aluminum Salt Solution Induces Dermatitis-like Changes in Mice. Journal of Clinical Medicine. 2025; 14(5):1701. https://doi.org/10.3390/jcm14051701
Chicago/Turabian StyleSiquier-Dameto, Gabriel, Ainhoa Iguaran-Pérez, Javier Gimeno-Beltrán, Gilberto Bellia, Andrea Maria Giori, Pere Boadas-Vaello, and Enrique Verdú. 2025. "Subcutaneous Injection and Brush Application of Ovalbumin–Aluminum Salt Solution Induces Dermatitis-like Changes in Mice" Journal of Clinical Medicine 14, no. 5: 1701. https://doi.org/10.3390/jcm14051701
APA StyleSiquier-Dameto, G., Iguaran-Pérez, A., Gimeno-Beltrán, J., Bellia, G., Giori, A. M., Boadas-Vaello, P., & Verdú, E. (2025). Subcutaneous Injection and Brush Application of Ovalbumin–Aluminum Salt Solution Induces Dermatitis-like Changes in Mice. Journal of Clinical Medicine, 14(5), 1701. https://doi.org/10.3390/jcm14051701







