Immersive Virtual Reality in Stroke Rehabilitation: A Systematic Review and Meta-Analysis of Its Efficacy in Upper Limb Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Population: stroke patients of all ages, severity levels, and care settings with upper limb impairments, with no restrictions based on gender.
- Interventions: The interventions involved using imVR to target upper limb stroke rehabilitation. There were no exclusions based on the duration of interventions, the number of sessions per week, the care settings, or the use of controllers or hand tracking.
- Control: conventional rehabilitation
- Outcomes: the primary outcome was improvement in upper limb motor function in stroke patients, evaluated using the Fugl–Meyer Assessment Upper Extremity Scale (FMA-UE), the Box and Block Test (BBT), and the Action Research Arm Test (ARAT).
- Study sesign: all studies included in the meta-analysis were randomized controlled trials (RCTs), whereas interventional studies were included in the systematic review section.
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
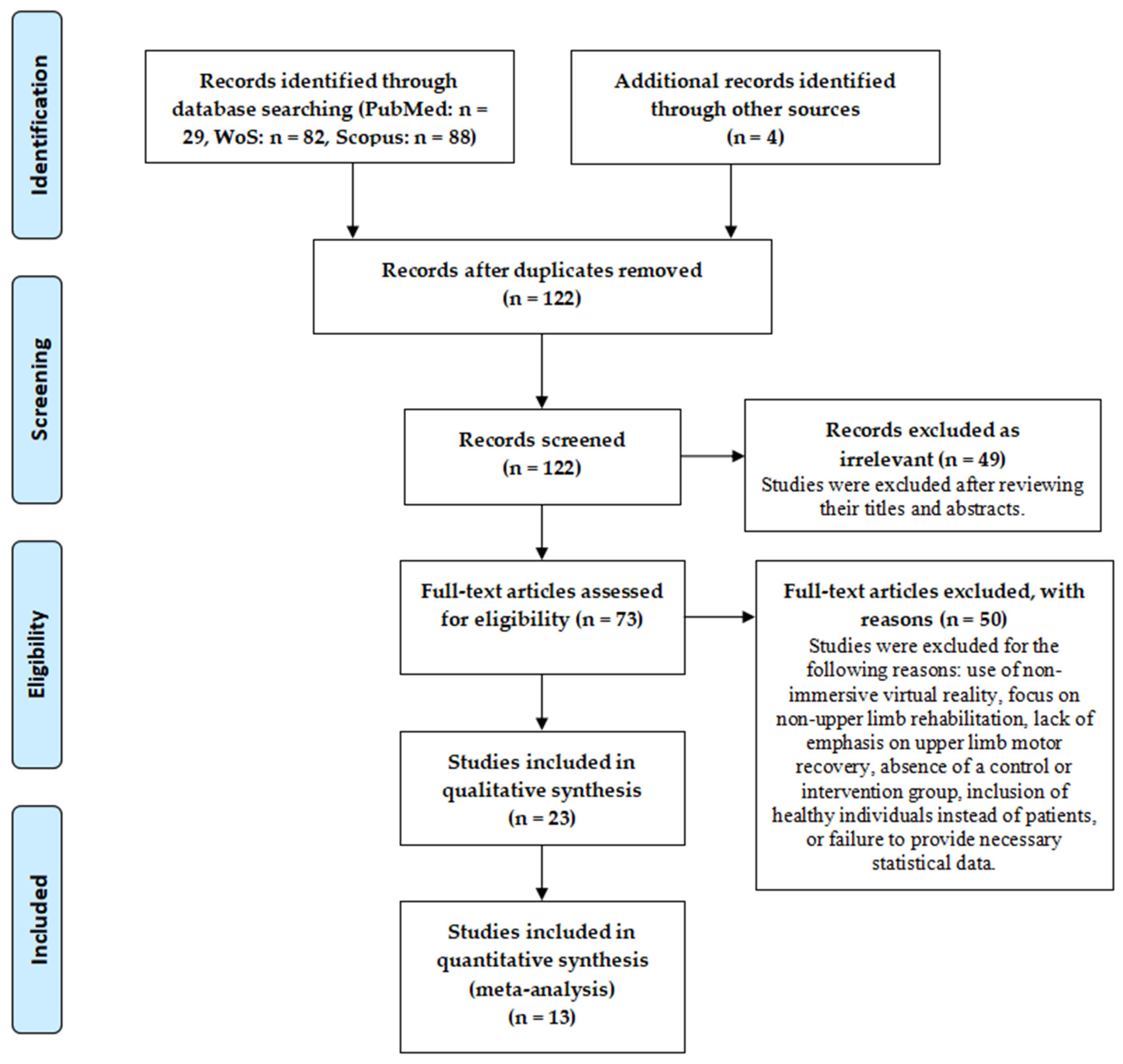
3.1. Quality Assessment
3.2. Participants
3.3. Interventions
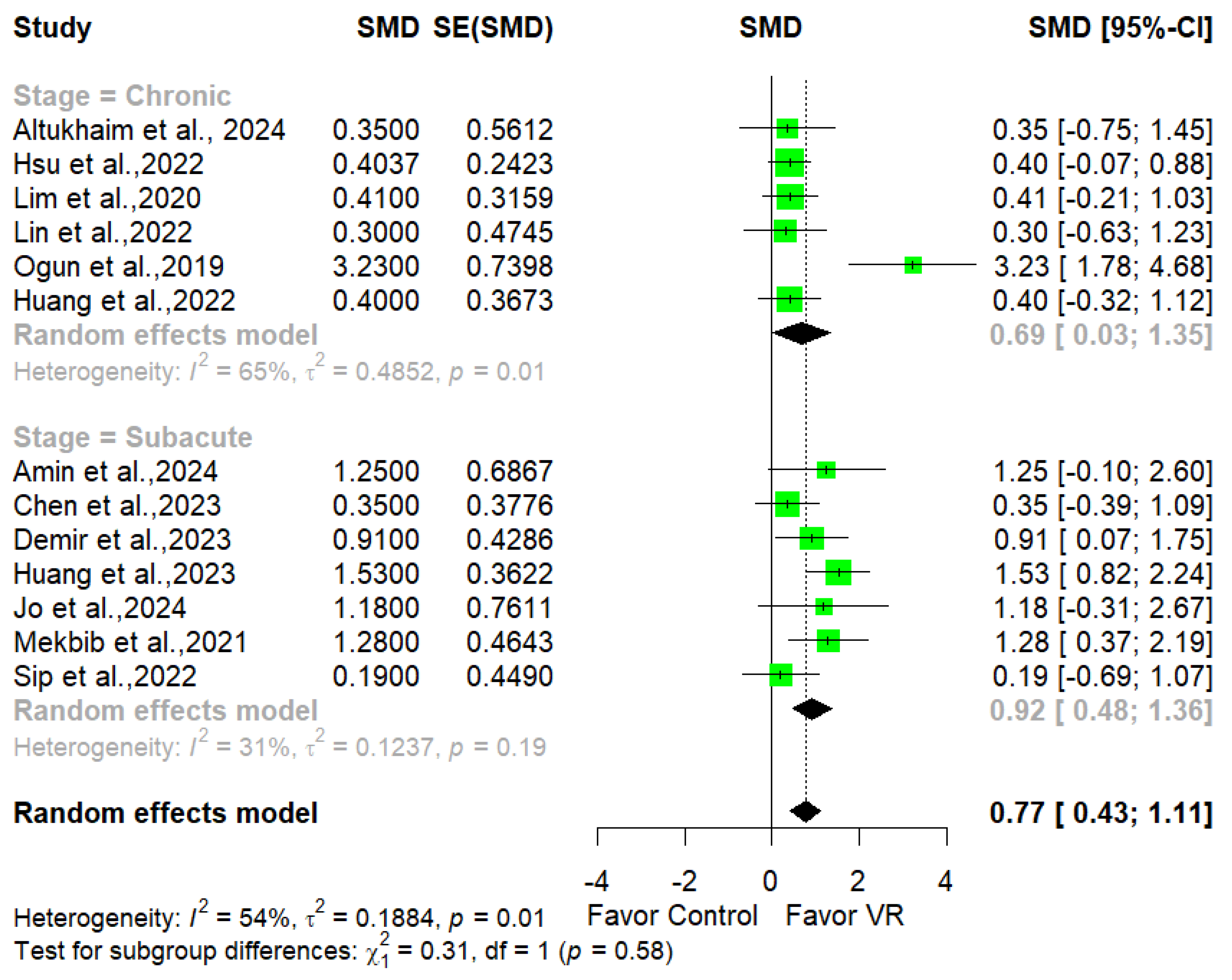
3.4. Clinical Effectiveness of imVR
3.5. Dose–Response Relationship
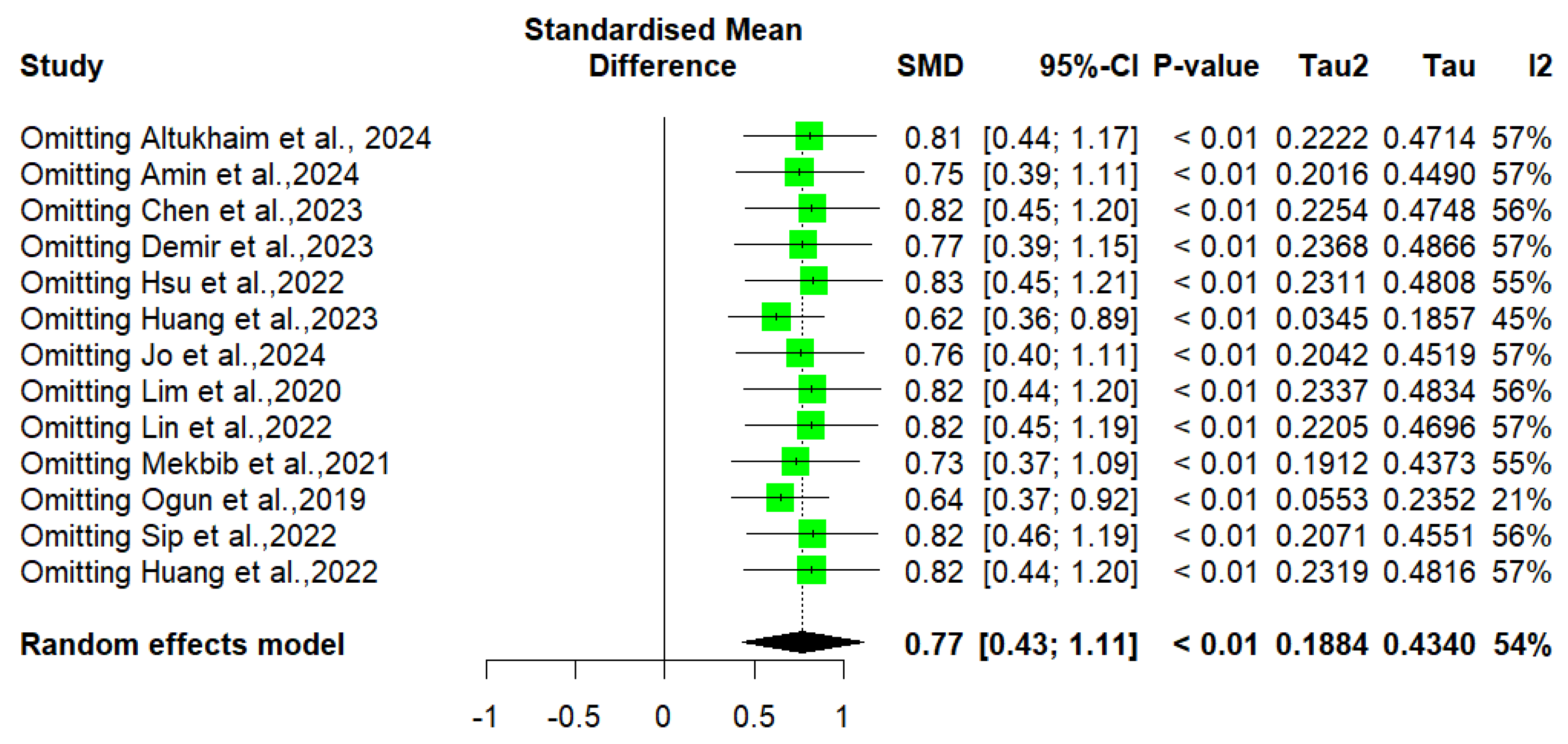
3.6. Risk of Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anwer, S.; Waris, A.; Gilani, S.O.; Iqbal, J.; Shaikh, N.; Pujari, A.N.; Niazi, I.K. Rehabilitation of Upper Limb Motor Impairment in Stroke: A Narrative Review on the Prevalence, Risk Factors, and Economic Statistics of Stroke and State of the Art Therapies. Healthcare 2022, 10, 190. [Google Scholar] [CrossRef]
- Prust, M.L.; Forman, R.; Ovbiagele, B. Addressing Disparities in the Global Epidemiology of Stroke. Nat. Rev. Neurol. 2024, 20, 207–221. [Google Scholar] [CrossRef]
- Thayabaranathan, T.; Kim, J.; Cadilhac, D.A.; Thrift, A.G.; Donnan, G.A.; Howard, G.; Howard, V.J.; Rothwell, P.M.; Feigin, V.; Norrving, B.; et al. Global Stroke Statistics 2022. Int. J. Stroke 2022, 17, 946–956. [Google Scholar] [CrossRef]
- Stucki, G.; Bickenbach, J.; Gutenbrunner, C.; Melvin, J.L. Rehabilitation: The Health Strategy of the 21st Century. J. Rehabil. Med. 2018, 50, 309–316. [Google Scholar] [CrossRef]
- Kayola, G.; Mataa, M.M.; Asukile, M.; Chishimba, L.; Chomba, M.; Mortel, D.; Nutakki, A.; Zimba, S.; Saylor, D. Stroke Rehabilitation in Low- and Middle-Income Countries: Challenges and Opportunities. Am. J. Phys. Med. Rehabil. 2023, 102, S24. [Google Scholar] [CrossRef]
- Yan, L.L.; Li, C.; Chen, J.; Miranda, J.J.; Luo, R.; Bettger, J.; Zhu, Y.; Feigin, V.; O’Donnell, M.; Zhao, D.; et al. Prevention, Management, and Rehabilitation of Stroke in Low- and Middle-Income Countries. eNeurologicalSci 2016, 2, 21–30. [Google Scholar] [CrossRef]
- Sommerfeld, D.K.; Eek, E.U.-B.; Svensson, A.-K.; Holmqvist, L.W.; von Arbin, M.H. Spasticity After Stroke. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef]
- Bleyenheuft, Y.; Gordon, A.M. Precision Grip in Congenital and Acquired Hemiparesis: Similarities in Impairments and Implications for Neurorehabilitation. Front. Hum. Neurosci. 2014, 8, 459. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Wang, D.; Rezaei, M.J. Stroke Rehabilitation: From Diagnosis to Therapy. Front. Neurol. 2024, 15, 1402729. [Google Scholar] [CrossRef]
- Nizamis, K.; Athanasiou, A.; Almpani, S.; Dimitrousis, C.; Astaras, A. Converging Robotic Technologies in Targeted Neural Rehabilitation: A Review of Emerging Solutions and Challenges. Sensors 2021, 21, 2084. [Google Scholar] [CrossRef]
- Patsaki, I.; Dimitriadi, N.; Despoti, A.; Tzoumi, D.; Leventakis, N.; Roussou, G.; Papathanasiou, A.; Nanas, S.; Karatzanos, E. The Effectiveness of Immersive Virtual Reality in Physical Recovery of Stroke Patients: A Systematic Review. Front. Syst. Neurosci. 2022, 16, 880447. [Google Scholar] [CrossRef]
- Li, A.; Li, J.; Wu, W.; Zhao, J.; Qiang, Y. Effect of Virtual Reality Training on Cognitive Function and Motor Performance in Older Adults With Cognitive Impairment Receiving Health Care: A Randomized Controlled Trial. Int. J. Hum.–Comput. Interact. 2024, 40, 7755–7772. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, W.; Chen, X.; Wu, B.; Wu, L.; Huang, X.; Jiang, S.; Huang, L. Evaluating the Effect and Mechanism of Upper Limb Motor Function Recovery Induced by Immersive Virtual-Reality-Based Rehabilitation for Subacute Stroke Subjects: Study Protocol for a Randomized Controlled Trial. Trials 2019, 20, 104. [Google Scholar] [CrossRef]
- Hao, J.; He, Z.; Yu, X.; Remis, A. Comparison of Immersive and Non-Immersive Virtual Reality for Upper Extremity Functional Recovery in Patients with Stroke: A Systematic Review and Network Meta-Analysis. Neurol. Sci. 2023, 44, 2679–2697. [Google Scholar] [CrossRef]
- Herrera, V.; Reyes-Guzmán, A.; Vallejo, D.; Castro-Schez, J.J.; Monekosso, D.N.; González-Morcillo, C.; Albusac, J. Performance Analysis for Upper Limb Rehabilitation in Non-Immersive and Immersive Scenarios. In Proceedings of the ICEIS (2), Prague, Czech Republic, 24–26 April 2023; pp. 231–242. [Google Scholar]
- Jin, M.; Pei, J.; Bai, Z.; Zhang, J.; He, T.; Xu, X.; Zhu, F.; Yu, D.; Zhang, Z. Effects of Virtual Reality in Improving Upper Extremity Function after Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Rehabil. 2022, 36, 573–596. [Google Scholar] [CrossRef]
- Subramanian, S.K.; Lourenço, C.B.; Chilingaryan, G.; Sveistrup, H.; Levin, M.F. Arm Motor Recovery Using a Virtual Reality Intervention in Chronic Stroke: Randomized Control Trial. Neurorehabilit. Neural Repair 2013, 27, 13–23. [Google Scholar] [CrossRef]
- Kim, W.-S.; Cho, S.; Ku, J.; Kim, Y.; Lee, K.; Hwang, H.-J.; Paik, N.-J. Clinical Application of Virtual Reality for Upper Limb Motor Rehabilitation in Stroke: Review of Technologies and Clinical Evidence. J. Clin. Med. 2020, 9, 3369. [Google Scholar] [CrossRef]
- Combalia, A.; Sanchez-Vives, M.V.; Donegan, T. Immersive Virtual Reality in Orthopaedics—A Narrative Review. Int. Orthop. 2024, 48, 21–30. [Google Scholar] [CrossRef]
- Mani Bharathi, V.; Manimegalai, P.; George, S.T.; Pamela, D.; Mohammed, M.A.; Abdulkareem, K.H.; Jaber, M.M.; Damaševičius, R. A Systematic Review of Techniques and Clinical Evidence to Adopt Virtual Reality in Post-Stroke Upper Limb Rehabilitation. Virtual Real. 2024, 28, 172. [Google Scholar] [CrossRef]
- Chatterjee, K.; Buchanan, A.; Cottrell, K.; Hughes, S.; Day, T.W.; John, N.W. Immersive Virtual Reality for the Cognitive Rehabilitation of Stroke Survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 719–728. [Google Scholar] [CrossRef]
- Fregna, G.; Schincaglia, N.; Baroni, A.; Straudi, S.; Casile, A. A Novel Immersive Virtual Reality Environment for the Motor Rehabilitation of Stroke Patients: A Feasibility Study. Front. Robot. AI 2022, 9, 906424. [Google Scholar] [CrossRef]
- Bargeri, S.; Scalea, S.; Agosta, F.; Banfi, G.; Corbetta, D.; Filippi, M.; Sarasso, E.; Turolla, A.; Castellini, G.; Gianola, S. Effectiveness and Safety of Virtual Reality Rehabilitation after Stroke: An Overview of Systematic Reviews. EClinicalMedicine 2023, 64, 102220. [Google Scholar] [CrossRef]
- Castillo, J.F.V.; Vega, M.F.M.; Cardona, J.E.M.; Lopez, D.; Quiñones, L.; Gallo, O.A.H.; Lopez, J.F. Design of Virtual Reality Exergames for Upper Limb Stroke Rehabilitation Following Iterative Design Methods: Usability Study. JMIR Serious Games 2024, 12, e48900. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, H.; Yun, S.J.; Oh, B.; Seo, H.G. Upper Extremity Rehabilitation Using Fully Immersive Virtual Reality Games With a Head Mount Display: A Feasibility Study. PM&R 2020, 12, 257–262. [Google Scholar] [CrossRef]
- Diriba Kenea, C.; Gemechu Abessa, T.; Lamba, D.; Bonnechère, B. Technological Features of Immersive Virtual Reality Systems for Upper Limb Stroke Rehabilitation: A Systematic Review. Sensors 2024, 24, 3546. [Google Scholar] [CrossRef]
- Kiper, P.; Godart, N.; Cavalier, M.; Berard, C.; Cieślik, B.; Federico, S.; Kiper, A.; Pellicciari, L.; Meroni, R. Effects of Immersive Virtual Reality on Upper-Extremity Stroke Rehabilitation: A Systematic Review with Meta-Analysis. J. Clin. Med. 2024, 13, 146. [Google Scholar] [CrossRef]
- Dawson, A.C. Upper Limb Movement Control After Stroke and in Healthy Ageing: Does Intensive Upper Limb Neurorehabilitation Improve Motor Control and Reduce Motor Impairment in the Chronic Phase of Stroke? Ph.D. Thesis, UCL (University College London), London, UK, 2023. [Google Scholar]
- Wei, X.; Sun, S.; Zhang, M.; Zhao, Z. A Systematic Review and Meta-Analysis of Clinical Efficacy of Early and Late Rehabilitation Interventions for Ischemic Stroke. BMC Neurol. 2024, 24, 91. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 2019, ED000142. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Oxford, UK, 2008. [Google Scholar]
- Carter, E.C.; Schönbrodt, F.D.; Gervais, W.M.; Hilgard, J. Correcting for Bias in Psychology: A Comparison of Meta-Analytic Methods. Adv. Methods Pract. Psychol. Sci. 2019, 2, 115–144. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. Bmj 2011, 343, d4002. [Google Scholar] [CrossRef]
- Pustejovsky, J.E.; Rodgers, M.A. Testing for Funnel Plot Asymmetry of Standardized Mean Differences. Res. Synth. Methods 2019, 10, 57–71. [Google Scholar] [CrossRef]
- Altukhaim, S.; Sakabe, N.; Nagaratnam, K.; Mannava, N.; Kondo, T.; Hayashi, Y. Immersive Virtual Reality Enhanced Reinforcement Induced Physical Therapy (EVEREST). Displays 2025, 87, 102962. [Google Scholar] [CrossRef]
- Amin, F.; Waris, A.; Syed, S.; Amjad, I.; Umar, M.; Iqbal, J.; Omer Gilani, S. Effectiveness of Immersive Virtual Reality-Based Hand Rehabilitation Games for Improving Hand Motor Functions in Subacute Stroke Patients. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 2060–2069. [Google Scholar] [CrossRef]
- Burton, Q.; Lejeune, T.; Dehem, S.; Lebrun, N.; Ajana, K.; Edwards, M.G.; Everard, G. Performing a Shortened Version of the Action Research Arm Test in Immersive Virtual Reality to Assess Post-Stroke Upper Limb Activity. J. Neuroeng. Rehabil. 2022, 19, 133. [Google Scholar] [CrossRef]
- Chen, J.; Or, C.K.; Li, Z.; Yeung, E.H.K.; Zhou, Y.; Hao, T. Effectiveness, Safety and Patients’ Perceptions of an Immersive Virtual Reality–Based Exercise System for Poststroke Upper Limb Motor Rehabilitation: A Proof-of-Concept and Feasibility Randomized Controlled Trial. Digit. Health 2023, 9, 20552076231203599. [Google Scholar] [CrossRef]
- Demir, O.B.; Gokbel, T.; Baydemir, C.; Dursun, E. Effects of Immersive Virtual Reality-Based Movement Therapy on Upper ExtremityFunctions and Cognitive Functions in Stroke Patients. Int. J. Health Stud. 2023, 9, 37–42. [Google Scholar]
- Elor, A.; Teodorescu, M.; Kurniawan, S. Project Star Catcher: A Novel Immersive Virtual Reality Experience for Upper Limb Rehabilitation. ACM Trans. Access. Comput. 2018, 11, 1–25. [Google Scholar] [CrossRef]
- Everard, G.; Otmane-Tolba, Y.; Rosselli, Z.; Pellissier, T.; Ajana, K.; Dehem, S.; Auvinet, E.; Edwards, M.G.; Lebleu, J.; Lejeune, T. Concurrent Validity of an Immersive Virtual Reality Version of the Box and Block Test to Assess Manual Dexterity among Patients with Stroke. J. Neuroeng. Rehabil. 2022, 19, 7. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Kuo, L.-C.; Lin, Y.-C.; Su, F.-C.; Yang, T.-H.; Lin, C.-W. Effects of a Virtual Reality–Based Mirror Therapy Program on Improving Sensorimotor Function of Hands in Chronic Stroke Patients: A Randomized Controlled Trial. Neurorehabil. Neural Repair 2022, 36, 335–345. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chiang, W.-C.; Yeh, Y.-C.; Fan, S.-C.; Yang, W.-H.; Kuo, H.-C.; Li, P.-C. Effects of Virtual Reality-Based Motor Control Training on Inflammation, Oxidative Stress, Neuroplasticity and Upper Limb Motor Function in Patients with Chronic Stroke: A Randomized Controlled Trial. BMC Neurol. 2022, 22, 21. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, X.; Jin, Y.; Wu, B.; Vigotsky, A.D.; Fan, L.; Gu, P.; Tu, W.; Huang, L.; Jiang, S. Immersive Virtual Reality-Based Rehabilitation for Subacute Stroke: A Randomized Controlled Trial. J. Neurol. 2024, 271, 1256–1266. [Google Scholar] [CrossRef]
- Jo, S.; JANG, H.; KIM, H.; SONG, C. 360° Immersive Virtual Reality-Based Mirror Therapy for Upper Extremity Function and Satisfaction among Stroke Patients: A Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2024, 60, 207–215. [Google Scholar] [CrossRef]
- Juan, M.-C.; Elexpuru, J.; Dias, P.; Santos, B.S.; Amorim, P. Immersive Virtual Reality for Upper Limb Rehabilitation: Comparing Hand and Controller Interaction. Virtual Real. 2023, 27, 1157–1171. [Google Scholar] [CrossRef]
- Lim, D.H.; Hwang, D.M.; Cho, K.H.; Moon, C.W.; Ahn, S.Y. A Fully Immersive Virtual Reality Method for Upper Limb Rehabilitation in Spinal Cord Injury. Ann. Rehabil. Med. 2020, 44, 311–319. [Google Scholar] [CrossRef]
- Lin, C.-W.; Kuo, L.-C.; Lin, Y.-C.; Su, F.-C.; Lin, Y.-A.; Hsu, H.-Y. Development and Testing of a Virtual Reality Mirror Therapy System for the Sensorimotor Performance of Upper Extremity: A Pilot Randomized Controlled Trial. IEEE Access 2021, 9, 14725–14734. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Slater, M.; Sanchez-Vives, M.V. Impact of Virtual Embodiment and Exercises on Functional Ability and Range of Motion in Orthopedic Rehabilitation. Sci. Rep. 2022, 12, 5046. [Google Scholar] [CrossRef]
- Mekbib, D.B.; Debeli, D.K.; Zhang, L.; Fang, S.; Shao, Y.; Yang, W.; Han, J.; Jiang, H.; Zhu, J.; Zhao, Z.; et al. A Novel Fully Immersive Virtual Reality Environment for Upper Extremity Rehabilitation in Patients with Stroke. Ann. N. Y. Acad. Sci. 2021, 1493, 75–89. [Google Scholar] [CrossRef]
- Ögün, M.N.; Kurul, R.; Yaşar, M.F.; Turkoglu, S.A.; Avci, Ş.; Yildiz, N. Effect of Leap Motion-Based 3D Immersive Virtual Reality Usage on Upper Extremity Function in Ischemic Stroke Patients. Arq. Neuropsiquiatr. 2019, 77, 681–688. [Google Scholar] [CrossRef]
- Park, W.; Kim, J.; Kim, M. Efficacy of Virtual Reality Therapy in Ideomotor Apraxia Rehabilitation: A Case Report. Medicine 2021, 100, e26657. [Google Scholar] [CrossRef]
- Phelan, I.; Furness, P.J.; Dunn, H.D.; Carrion-Plaza, A.; Matsangidou, M.; Dimitri, P.; Lindley, S. Immersive Virtual Reality in Children with Upper Limb Injuries: Findings from a Feasibility Study. J. Pediatr. Rehabil. Med. 2021, 14, 401–414. [Google Scholar] [CrossRef]
- Sip, P.; Kozłowska, M.; Czysz, D.; Daroszewski, P.; Lisiński, P. Perspectives of Motor Functional Upper Extremity Recovery with the Use of Immersive Virtual Reality in Stroke Patients. Sensors 2023, 23, 712. [Google Scholar] [CrossRef]
- Song, Y.-H.; Lee, H.-M. Effect of Immersive Virtual Reality-Based Bilateral Arm Training in Patients with Chronic Stroke. Brain Sci. 2021, 11, 1032. [Google Scholar] [CrossRef]
- Tokgöz, P.; Wähnert, D.; Elsner, A.; Schack, T.; Cienfuegos Tellez, M.A.; Conrad, J.; Vordemvenne, T.; Dockweiler, C. Virtual Reality for Upper Extremity Rehabilitation—A Prospective Pilot Study. Healthcare 2023, 11, 1498. [Google Scholar] [CrossRef]
- Phelan, I.; Carrion-Plaza, A.; Furness, P.J.; Dimitri, P. Home-Based Immersive Virtual Reality Physical Rehabilitation in Paediatric Patients for Upper Limb Motor Impairment: A Feasibility Study. Virtual Real. 2023, 27, 3505–3520. [Google Scholar] [CrossRef]
- Smith, A.; Wyles, K.J.; Hernandez, S.M.; Clarke, S.; Schofield, P.; Hughes, S.W. Harnessing the Therapeutic Effects of Nature for Chronic Pain: A Role for Immersive Virtual Reality? A Narrative Review. Eur. J. Pain 2025, 29, e4727. [Google Scholar] [CrossRef]
- Guerra-Armas, J.; Flores-Cortes, M.; Pineda-Galan, C.; Luque-Suarez, A.; La Touche, R. Role of Immersive Virtual Reality in Motor Behaviour Decision-Making in Chronic Pain Patients. Brain Sci. 2023, 13, 617. [Google Scholar] [CrossRef]
- Sokołowska, B. Being in Virtual Reality and Its Influence on Brain Health—An Overview of Benefits, Limitations and Prospects. Brain Sci. 2024, 14, 72. [Google Scholar] [CrossRef]
- Fayed, M.; Almadi, F.; Almadi, M.; Almudawah, R.T.; Alotaibi, F.; Adam, A.; Aldubaib, F.; Alshaikh, A.; Alhamad, L.; ElSayed, H. Immersion and Presence in Virtual Reality Applications for Physical Therapy and Upper Limb Rehabilitation. In Proceedings of the Social Computing and Social Media, Copenhagen, Denmark, 23–28 July 2023; Coman, A., Vasilache, S., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 217–227. [Google Scholar]
- Guy, M.; Normand, J.-M.; Jeunet-Kelway, C.; Moreau, G. The Sense of Embodiment in Virtual Reality and Its Assessment Methods. Front. Virtual Real. 2023, 4, 1141683. [Google Scholar] [CrossRef]
- Lang, C.E.; Edwards, D.F.; Birkenmeier, R.L.; Dromerick, A.W. Estimating Minimal Clinically Important Differences of Upper-Extremity Measures Early after Stroke. Arch. Phys. Med. Rehabil. 2008, 89, 1693–1700. [Google Scholar] [CrossRef]
- Huynh, B.P.; DiCarlo, J.A.; Vora, I.; Ranford, J.; Gochyyev, P.; Lin, D.J.; Kimberley, T.J. Sensitivity to Change and Responsiveness of the Upper Extremity Fugl-Meyer Assessment in Individuals With Moderate to Severe Acute Stroke. Neurorehabilit. Neural Repair 2023, 37, 545–553. [Google Scholar] [CrossRef]
- Cai, H.; Lin, T.; Chen, L.; Weng, H.; Zhu, R.; Chen, Y.; Cai, G. Evaluating the Effect of Immersive Virtual Reality Technology on Gait Rehabilitation in Stroke Patients: A Study Protocol for a Randomized Controlled Trial. Trials 2021, 22, 91. [Google Scholar] [CrossRef]
- McEwen, D.; Taillon-Hobson, A.; Bilodeau, M.; Sveistrup, H.; Finestone, H. Virtual Reality Exercise Improves Mobility After Stroke: An Inpatient Randomized Controlled Trial. Stroke 2014, 45, 1853–1855. [Google Scholar] [CrossRef]
- Demeco, A.; Zola, L.; Frizziero, A.; Martini, C.; Palumbo, A.; Foresti, R.; Buccino, G.; Costantino, C. Immersive Virtual Reality in Post-Stroke Rehabilitation: A Systematic Review. Sensors 2023, 23, 1712. [Google Scholar] [CrossRef]
- Khan, A.; Imam, Y.Z.; Muneer, M.; Al Jerdi, S.; Gill, S.K. Virtual Reality in Stroke Recovery: A Meta-Review of Systematic Reviews. Bioelectron. Med. 2024, 10, 23. [Google Scholar] [CrossRef]
- Hao, J.; Crum, G.; Siu, K.-C. Effects of Virtual Reality on Stroke Rehabilitation: An Umbrella Review of Systematic Reviews. Health Sci. Rep. 2024, 7, e70082. [Google Scholar] [CrossRef]
- Velmurugan, G.; Viswanath, S. Effectiveness of Virtual Reality Training on Upper Limb Motor Function in Stroke Patient’s: A Randomized Control Trial. Indian J. Physiother. Occup. Ther. 2023, 17, 60–66. [Google Scholar] [CrossRef]
- El-Kafy, E.M.A.; Alshehri, M.A.; El-Fiky, A.A.-R.; Guermazi, M.A. The Effect of Virtual Reality-Based Therapy on Improving Upper Limb Functions in Individuals With Stroke: A Randomized Control Trial. Front. Aging Neurosci. 2021, 13, 731343. [Google Scholar] [CrossRef]
- Brunner, I.; Skouen, J.S.; Hofstad, H.; Aßmus, J.; Becker, F.; Sanders, A.-M.; Pallesen, H.; Qvist Kristensen, L.; Michielsen, M.; Thijs, L.; et al. Virtual Reality Training for Upper Extremity in Subacute Stroke (VIRTUES): A Multicenter RCT. Neurology 2017, 89, 2413–2421. [Google Scholar] [CrossRef]
- Perez-Marcos, D.; Chevalley, O.; Schmidlin, T.; Garipelli, G.; Serino, A.; Vuadens, P.; Tadi, T.; Blanke, O.; Millán, J.d.R. Increasing Upper Limb Training Intensity in Chronic Stroke Using Embodied Virtual Reality: A Pilot Study. J. Neuroeng. Rehabil. 2017, 14, 119. [Google Scholar] [CrossRef]
- Wilson, J.; Heinsch, M.; Betts, D.; Booth, D.; Kay-Lambkin, F. Barriers and Facilitators to the Use of E-Health by Older Adults: A Scoping Review. BMC Public Health 2021, 21, 1556. [Google Scholar] [CrossRef] [PubMed]
- Khundam, C.; Vorachart, V.; Preeyawongsakul, P.; Hosap, W.; Noël, F. A Comparative Study of Interaction Time and Usability of Using Controllers and Hand Tracking in Virtual Reality Training. Informatics 2021, 8, 60. [Google Scholar] [CrossRef]
- Peng, Q.; Yin, L.; Cao, Y. Effectiveness of Virtual Reality in the Rehabilitation of Motor Function of Patients with Subacute Stroke: A Meta-Analysis. Front. Neurol. 2021, 12, 639535. [Google Scholar] [CrossRef]
- Helou, S.; Khalil, N.; Daou, M.; El Helou, E. Virtual Reality for Healthcare: A Scoping Review of Commercially Available Applications for Head-Mounted Displays. Digit. Health 2023, 9, 20552076231178619. [Google Scholar] [CrossRef]
- Herrera, V.; Albusac, J.; Castro-Schez, J.J.; González-Morcillo, C.; Monekosso, D.N.; Pacheco, S.; Perales, R.; de los Reyes-Guzmán, A. Creating Adapted Environments: Enhancing Accessibility in Virtual Reality for Upper Limb Rehabilitation through Automated Element Adjustment. Virtual Real. 2025, 29, 28. [Google Scholar] [CrossRef]
- Ceradini, M.; Losanno, E.; Micera, S.; Bandini, A.; Orlandi, S. Immersive VR for Upper-Extremity Rehabilitation in Patients with Neurological Disorders: A Scoping Review. J. Neuroeng. Rehabil. 2024, 21, 75. [Google Scholar] [CrossRef]
- Goumopoulos, C.; Chartomatsidis, M.; Koumanakos, G. Participatory Design of Fall Prevention Exergames Using Multiple Enabling Technologies. In Proceedings of the 8th International Conference on Information and Communication Technologies for Ageing Well and e-Health (ICT4AWE 2022), Online, 23–25 April 2022; pp. 70–80. [Google Scholar]
- Hernández-Martínez, A.; Fernandez-Escabias, M.; Amaya-Pascasio, L.; Carrilho-Candeias, S.; Ramos-Teodoro, M.; Gil-Rodríguez, M.; Orellana-Jaen, A.; Martínez-Rosales, E.; Ruiz-González, D.; Esteban-Simón, A.; et al. Evaluation of the Effects of a Gamified, Fully Immersive and Stroke-Specific Virtual Reality Intervention for Improving Disability and Quality of Life in Patients with Stroke in the Subacute Phase: Study Protocol of the RESET Randomised Trial. BMJ Open Sport Exerc. Med. 2024, 10, e002123. [Google Scholar] [CrossRef]
- Birckhead, B.; Khalil, C.; Liu, X.; Conovitz, S.; Rizzo, A.; Danovitch, I.; Bullock, K.; Spiegel, B. Recommendations for Methodology of Virtual Reality Clinical Trials in Health Care by an International Working Group: Iterative Study. JMIR Ment. Health 2019, 6, e11973. [Google Scholar] [CrossRef]
- Bryant, L.; Sedlarevic, N.; Stubbs, P.; Bailey, B.; Nguyen, V.; Bluff, A.; Barnett, D.; Estela, M.; Hayes, C.; Jacobs, C.; et al. Collaborative Co-Design and Evaluation of an Immersive Virtual Reality Application Prototype for Communication Rehabilitation (DISCOVR Prototype). Disabil. Rehabil. Assist. Technol. 2024, 19, 90–99. [Google Scholar] [CrossRef]
- Antonopoulos, P.; Fokides, E.; Koutromanos, G. Understanding Learning and Learning Experience in Immersive Virtual Reality. Technol. Knowl. Learn. 2024, 1–30. [Google Scholar] [CrossRef]
- Abdlkarim, D.; Di Luca, M.; Aves, P.; Maaroufi, M.; Yeo, S.-H.; Miall, R.C.; Holland, P.; Galea, J.M. A Methodological Framework to Assess the Accuracy of Virtual Reality Hand-Tracking Systems: A Case Study with the Meta Quest 2. Behav. Res. Methods 2024, 56, 1052–1063. [Google Scholar] [CrossRef]
- Li, X.; Elnagar, D.; Song, G.; Ghannam, R. Advancing Medical Education Using Virtual and Augmented Reality in Low- and Middle-Income Countries: A Systematic and Critical Review. Virtual Worlds 2024, 3, 384–403. [Google Scholar] [CrossRef]
- Iserhienrhein, I. Understanding Challenges Faced by Rehabilitation Professionals Using Digital Rehabilitation in Kenya, Tanzania, Rwanda. Available online: http://www.theseus.fi/handle/10024/860069 (accessed on 29 January 2025).
- Cano-de-la-Cuerda, R.; Blázquez-Fernández, A.; Marcos-Antón, S.; Sánchez-Herrera-Baeza, P.; Fernández-González, P.; Collado-Vázquez, S.; Jiménez-Antona, C.; Laguarta-Val, S. Economic Cost of Rehabilitation with Robotic and Virtual Reality Systems in People with Neurological Disorders: A Systematic Review. J. Clin. Med. 2024, 13, 1531. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.F.V.; López, J.F.; Muñoz, J.E.; Gallo, O.H. Clinical Perceptions and Feasibility Analysis of a Virtual Reality Game for Post-Stroke Rehabilitation. TecnoLógicas 2024, 27, e3180. [Google Scholar] [CrossRef]





| Databases | Strings | Numbers Results |
|---|---|---|
| Web of Science | TS = (“immersive virtual reality”) AND (“upper extremity” OR “upper limb”) AND stroke AND rehabilitat*) | 82 |
| Scopus | TITLE-ABS (“immersive virtual reality”) AND (“upper extremity” OR “upper limb”) AND stroke AND rehabilitat*) | 88 |
| PubMed | (“immersive virtual reality”[Title/Abstract]) AND (“upper extremity”[MeSH Terms]) AND (stroke[MeSH Terms]) AND (rehabilitat*[MeSH Terms]) | 29 |
| Study | Country | N (% Female) | Control Group | Experimental Group | Age | Stroke Stage |
|---|---|---|---|---|---|---|
| Altukhaim et al., 2024 [36] | UK | 12 (46) | 6 | 7 | 72.87 | Chronic |
| Amin et al., 2024 [37] | Pakistan | 52 (35) | 26 | 26 | 50.8 | Subacute |
| Burton et al., 2022 [38] | Belgium | 55 (42) | 30 | 25 | 60 | Acute, subacute, and chronic |
| Chen et al., 2023 [39] | China | 28 (36) | 14 | 14 | 57.75 | Subacute |
| Demir et al., 2023 [40] | Turkey | 35 (52) | 10 | 15 | 51 | Subacute |
| Elor et al., 2018 [41] | USA | 6 (17) | / | 6 | 26.5 | Chronic |
| Everard et al., 2022 [42] | Belgium | 45 (40) | 23 | 22 | 64 | Subacute and chronic |
| Fregna et al., 2022 [22] | Italy | 16 (25) | / | 16 | 62 | Subacute and chronic |
| Hsu et al., 2022 [43] | Taiwan | 35 (57) | 17 | 18 | 54.6 | Chronic |
| Huang et al., 2022 [44] | Taiwan | 30 (67) | 15 | 15 | 54.57 | Chronic |
| Huang et al., 2023 [45] | Taiwan | 40 (31) | 20 | 20 | 64.2 | Subacute |
| Jo et al., 2024 [46] | South Korea | 30 (50) | 15 | 15 | 49.43 | Subacute |
| Juan et al., 2023 [47] | Spain | 14 (36) | / | 14 | 40.61 | Chronic |
| Lee et al., 2020 [25] | South Korea | 12 (42) | / | 12 | 40.2 | Chronic |
| Lim et al., 2020 [48] | Korea | 20 (30) | 10 | 10 | 60.25 | Chronic |
| Lin et al., 2020 [49] | Taiwan | 18 (50) | 9 | 9 | 22 | Chronic |
| Matamala-Gomez et al., 2022 [50] | Spain | 20 (100) | / | 20 | 60.05 | Chronic |
| Mekbib et al., 2021 [51] | China | 23 (26) | 12 | 11 | 55 | Subacute |
| Ogun et al., 2019 [52] | Turkey | 65 (22) | 32 | 33 | 60.62 | Chronic |
| Park et al., 2021 [53] | Korea | 1 (0) | / | 1 | 56 | Subacute |
| Phelan et al., 2021 [54] | UK | 10 (60) | / | 10 | 11 | Chronic |
| Sip et al., 2022 [55] | Poland | 20 (NS) | 10 | 10 | 57 | Subacute |
| Song and Lee, 2021 [56] | Korea | 10 (40) | 5 | 5 | 64 | Chronic |
| Study | Study Design | VR Headset | VR Interactions | Type of Exercises/Games | Description | DoI | NoSPW | DoOS (min) | Setting | Outcome Measures | Main Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Altukhaim et al., 2024 [36] | RCT | Oculus Rift | Hand tracking | Reach the target objects | The game requires players to reach target objects in seven semi-circular positions. A total of 35 balls are presented, each target receiving five balls. | 1 | 5 | 30 | Hospital | FMA-UE | The study revealed that imVR has the potential to enhance motor function in stroke patients with upper limb impairment. |
| Amin et al., 2024 [37] | RCT | Oculus Quest 2 | Hand tracking | Hit a rolling ball, grasp a balloon, switch hands, and grip a pencil. | In the first game, the patient hits randomly generated, colored rolling balls. In the second game, the patient grasps a virtual balloon to reach nearby balls. In the third game, the patient swipes incoming balls in different directions. The final game involves gripping and holding a virtual pencil. | 6 | 4 | 24 | Hospital | FMA-UE, ARAT, BBT | The main result showed that VR was effective in improving hand motor functions. |
| Chen et al., 2023 [39] | RCT | HTC Vive Pro | Controllers | Dumbbell lifting, fishing, sheep whacking, apple picking, and balloon popping. | Patients hold the controller at shoulder level for 1 to 3 s for the dumbbell exercise. In the fishing game, participants use the controller as a rod to catch fish and pull them out of the water. In the sheep game, participants stand before two holes, whacking the sheep back into the holes. In the apple-picking game, participants use the controller as a bird to pick apples from a tree and drop them onto a stump. For balloon popping, participants reach their hand toward the balloon to pop it. | 2 | 6 | 30 | Hospital | FMA-UE | Immersive VR statistically significant improvements in shoulder flexion, shoulder abduction, upper limb motor function, and QoL were observed in both groups. |
| Hsu et al., 2022 [43] | RCT | Oculus Rift | Hand tracking | VR-MT system | VR-MT included movements such as forearm supination/pronation, wrist extension/flexion, finger extension/flexion, thumb opposition with the little finger, thumb extension/flexion, and tendon-gliding exercises. | 9 | 3 | 30 | Hospital | FMA_UE | VR-MT has potential effects on restoring upper limb motor function in chronic stroke patients, compared to COT. |
| Huang et al., 2022 [44] | RCT | HTC vive | Controllers | Twenty VR exercises | There was no list of the names and descriptions of the exercises. | 5 | 3 | 60 | Hospital | FMA-UE | The results showed that the imVR group demonstrated significantly more improvements in FMA-UE and AROM than the COT group. |
| Huang et al., 2023 [45] | RCT | Oculus Rift | Controllers | Immersive VR system | NS | 3 | 5 | 30 | Hospital | FMA-UE, BI | The FMA-UE score was more significant in the imVR compared with the Control at the post-intervention. |
| Jo et al., 2024 [46] | RCT | Pico GO VR 4K | Hand tracking | Novel 360° imVR- MT | NS | 4 | 3 | 30 | Hospital | FMA-UE, BBT | Results revealed that the 360 imVR-MT group showed significantly more improvements in FMA-UE and BBT than conventional rehabilitation. |
| Lin et al., 2020 [49] | RCT | Oculus Rift | Hand tracking | Immersive VR-MT system | Supination, thumb-to-the-tip of the finger movement, thumb circling, wrist flexion and extension, tendon gliding exercise, finger flexion and extension, and key pinch. | 2 | 2 | 45 | Hospital | FMA-UE | The findings suggest that imVR-MT resulted in better clinical effects for upper limb motor facilitation than traditional MT. |
| Matamala-Gomez et al., 2022 [50] | RCT | Oculus quest | Hand tracking | Virtual arm illusion | They used exercises, organized into six modules of increasing complexity, but did not describe the exercises. | 5 | 3 | 20 | Hospital | FMA-UE, ROM | The imVR training group presented higher functional motor ability recovery after cast removal (T1) and six weeks later (T2) than non-imVR training groups. |
| Mekbib et al., 2021 [51] | RCT | HTC vive | Hand tracking | Grasping, transporting, and releasing ball | The patients pick up each ball individually and place it into a basket at the virtual table’s center. | 2 | 4 | 60 | Hospital | BI, FMA-UE | The VR group revealed significant improvements compared to the control group. |
| Ogun et al., 2019 [52] | RCT | HTC vive | Hand tracking | Types of VR programs | Cube handling, decorating a tree with leaves, picking vegetables from a bowl, kitchen experience games, and drumming. | 6 | 3 | 60 | Hospital | FMA-UE, ARAT | The pre-test and post-test results of the FMA-UE and ARAT showed a significant difference, favoring the VR group. |
| Sip et al., 2022 [55] | RCT | Oculus quest | Hand tracking | VR mirror therapy and classical mirror therapy | NS | 3 | 6 | 30 | Hospital | FMA-UE | FMA-UE obtained a statistically significant outcome. |
| Song and Lee, 2021 [56] | RCT | Oculus Rift | Controllers | Living room, kitchen, veranda, and convenience store | The content of this imVR rehabilitation game includes a daily life training component featuring environments like a living room, kitchen, and veranda. | 4 | 5 | 30 | Hospital | EMG and MFT | The findings indicate that imVR-based bilateral is an effective intervention for improving upper limb functions in patients with chronic stroke. |
| Demir et al., 2023 [40] | RCT | Oculus Rift | Hand tracking | Climb game | An immersive Oculus Rift VR climbing game was used. | 6 | 7 | 30 | Hospital | BBT | There were significant improvements in the imVR group compared to control group. |
| Lim et al., 2020 [48] | RCT | Oculus Rift | Hand tracking | Catching balls, playing xylophones, moving cherry tomatoes into a bowl, avoiding stones, throwing objects towards a target, and popping bubbles. | The patient sat in a chair with a backrest and performed six games (catching balls, playing the xylophone, moving cherry tomatoes into a bowl, avoiding stones, throwing objects) with a target and popped bubbles using both hands. | 4 | 4 | 30 | Hospital | BBT, ARAT | This study demonstrated that VR training combined with CR significantly improved functional improvement compared to CR alone. |
| Elor et al., 2018 [41] | Pilot study | HTC vive | Controllers | Catching falling stars | Patients catch descending stars that fall in a straight line (0°) in mode 1, at a 45° angle in mode 2, and 90° angle in mode 3. | 1 | 1 | 5 | NGO center | Questionnaire | The results suggest that an imVR intervention provides a motivating and cost-effective solution for real-time data capture during rehabilitation. |
| Burton et al., 2022 [38] | Observational | Oculus quest | Hand tracking | Grasp, grip, pinch and gross movement | The patients grasp and lift wooden cubes of various sizes and weights. Next, they pour water from one glass to another, grabbing and moving marbles of different diameters. Finally, they touch their neck, head, and mouth with their contralesional hand. | 2 | NS | NS | Hospital | ARAT, SUS | The ARAT-VR is a valid, usable, and reliable tool to improve paretic hands among individuals with stroke. |
| Fregna et al., 2022 [22] | Feasibility study | Oculus quest | Hand tracking | Ball in hole, cloud, glasses and rolling pin | Patients push a ball into a designated hole using their corresponding hand. Next, a cloud appears to the left or right, prompting them to pop all bubbles with the matching hand. In the third task, a glass appears on one of four pedestals arranged in a circle, and patients must push it. The final task involves using both hands to make a rolling pin a set distance along the table. | 1 | 1 | 50 | Hospital | FMA-UE | The results revealed that patients showed high comfort in imVR game development. |
| Lee et al., 2020 [25] | Feasibility Study | HTC vive | Controllers | Hammering, ball catch, cup pour, bubble touch, and playing a xylophone | The patient holds a virtual hammer to strike a nail using their affected hand, and the nail is automatically generated in virtual space. In the second activity, the patient catches a ball from the front of the virtual space and throws it back. The third activity involves pouring strawberries from a cup into a bowl. The fourth activity focuses on touching and popping a floating bubble. The final activity consists of playing a xylophone with the affected hand. | 3 | 3 | 30 | Hospital | ARAT | The results of the study showed significant functional improvement in all outcome measures. |
| Phelan et al., 2021 [54] | Feasibility study | Oculus quest | Controllers | Climbing | In this game, the child must ascend to the top by performing an overhead arm raise exercise. The game includes highlighted bricks and ropes. To climb up, the child grabs a brick and lowers their arm. Failure to grasp the brick results in the child falling off the climbing wall. | 1 | 1 | 15 | Hospital | ROM | Findings suggested that imVR was an engaging, enjoyable experience that distracted children from the pain and boredom of rehabilitation. |
| Juan et al., 2023 [47] | Comparative study | Oculus quest | Hand tracking | Lifting barbells, eating an apple and inflating a balloon. | In the first game, patients lift a barbell above a target height with their affected hand, holding it for a specified time. In the second game, they reach for an apple and bring it to their mouth, involving hand opening and closing. They also touch each finger with their thumb. The third game involves inflating a balloon to assess hand-closing ability. | NS | NS | NS | Hospital | LMS | The result of the study showed that 78.5% of the users preferred interaction using their hands. |
| Everard et al., 2022 [42] | Clinical trial | Oculus quest 1 | Controllers | Grasping cube object | Patients move the cubes from one compartment to another. | 1 | 1 | 45 | Hospital | BBT | The study results revealed that test–retest reliability was excellent, and usability was nearly excellent. |
| Park et al., 2021 [53] | Case report | HTC vive | Hand tracking | Grasp and release | Eating, grooming, and dressing | 4 | 5 | 20 | Hospital | TULIA | The study reveals that an incomparably best motor response of the left hand during the imVR condition, OT, AR, and VR was 8 (26.7%), 20 (66.7%), and 28 (93.3%), respectively. |
| Condition | Duration (One Session, in min) | Frequency (Session/Week) | Duration (Total, in min) | |||
|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | |
| Subacute | 0.0032 (0.027) | 0.90 | −0.1599 (0.2200) | 0.46 | −0.0001 (0.0011) | 0.89 |
| Chronic | 0.0604 (0.0251) | 0.0163 | −0.3030 (0.5728) | 0.59 | 0.0024 (0.0011) | 0.0254 |
| TOTAL | 0.0323 (0.0170) | 0.047 | −0.1148 (0.2028) | 0.46 | 0.0013 (0.0008) | 0.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenea, C.D.; Abessa, T.G.; Lamba, D.; Bonnechère, B. Immersive Virtual Reality in Stroke Rehabilitation: A Systematic Review and Meta-Analysis of Its Efficacy in Upper Limb Recovery. J. Clin. Med. 2025, 14, 1783. https://doi.org/10.3390/jcm14061783
Kenea CD, Abessa TG, Lamba D, Bonnechère B. Immersive Virtual Reality in Stroke Rehabilitation: A Systematic Review and Meta-Analysis of Its Efficacy in Upper Limb Recovery. Journal of Clinical Medicine. 2025; 14(6):1783. https://doi.org/10.3390/jcm14061783
Chicago/Turabian StyleKenea, Chala Diriba, Teklu Gemechu Abessa, Dheeraj Lamba, and Bruno Bonnechère. 2025. "Immersive Virtual Reality in Stroke Rehabilitation: A Systematic Review and Meta-Analysis of Its Efficacy in Upper Limb Recovery" Journal of Clinical Medicine 14, no. 6: 1783. https://doi.org/10.3390/jcm14061783
APA StyleKenea, C. D., Abessa, T. G., Lamba, D., & Bonnechère, B. (2025). Immersive Virtual Reality in Stroke Rehabilitation: A Systematic Review and Meta-Analysis of Its Efficacy in Upper Limb Recovery. Journal of Clinical Medicine, 14(6), 1783. https://doi.org/10.3390/jcm14061783








