Catheter Ablation of Atrial Fibrillation: Technique and Future Perspectives
Abstract
:1. Introduction
2. Brief Historical Overview
3. Transcatheter Ablation for Atrial Fibrillation: Techniques and Energy Sources
3.1. Radiofrequency Ablation vs. Cryoballoon Ablation
3.2. Emerging Alternative Energy Source for Catheter Ablation
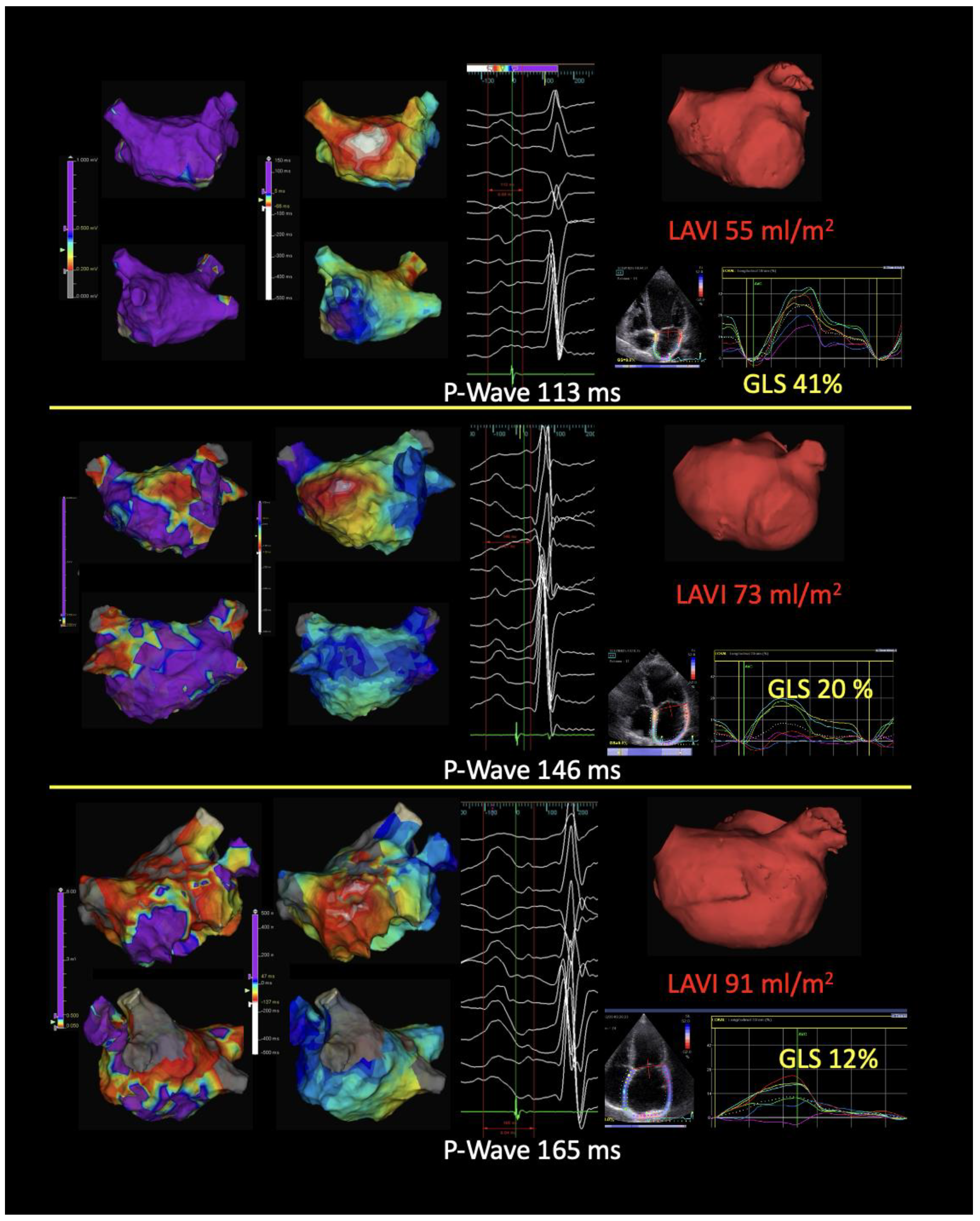
4. Atrial Cardiomyopathy and the Role of Imaging in Risk Stratification
4.1. Echocardiography
4.2. Cardiac Magnetic Resonance
4.3. Computed Tomography
5. Beyond Pulmonary Vein Isolation: Ablation of Persistent Atrial Fibrillation
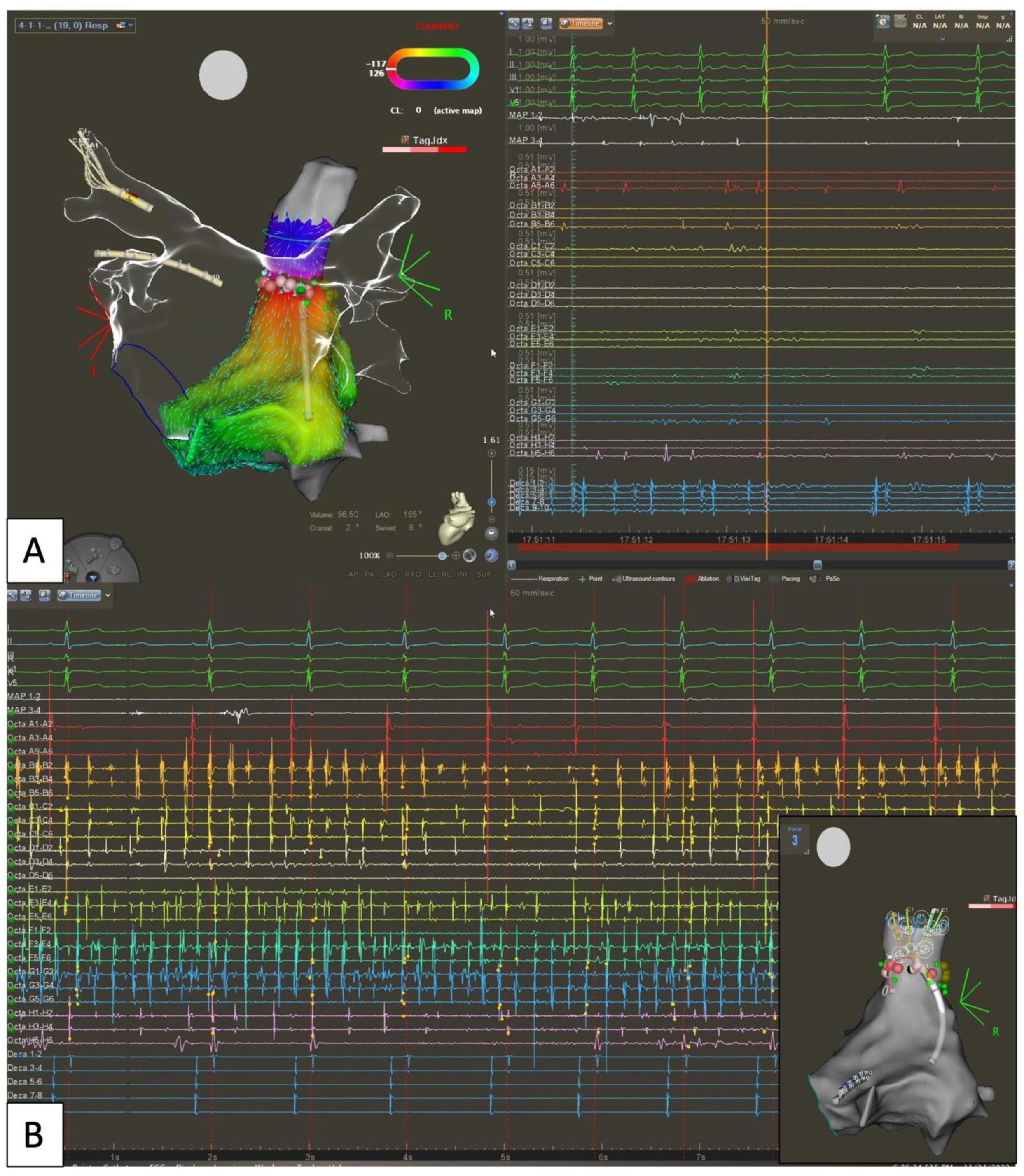
5.1. Extra PV Trigger Elimination
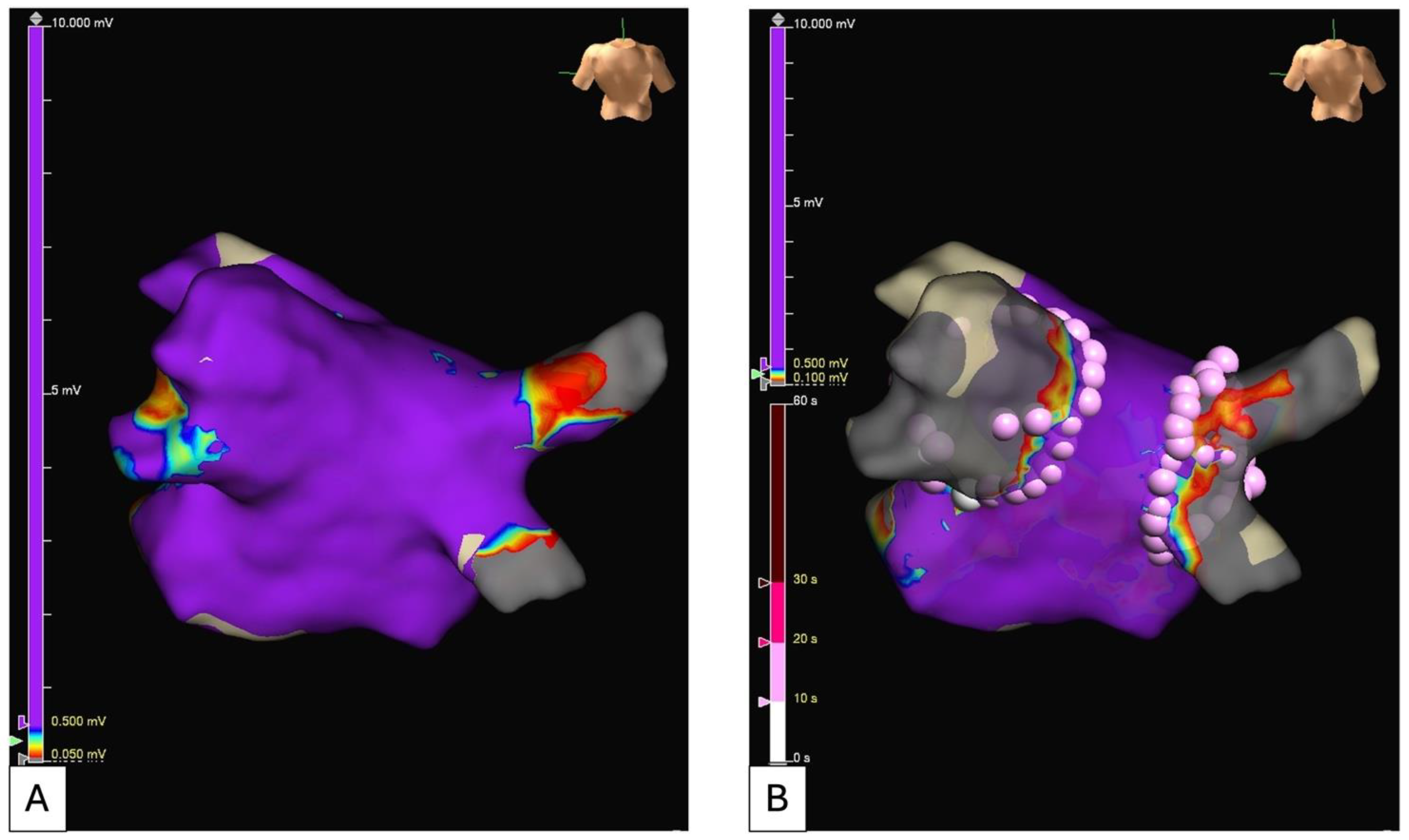
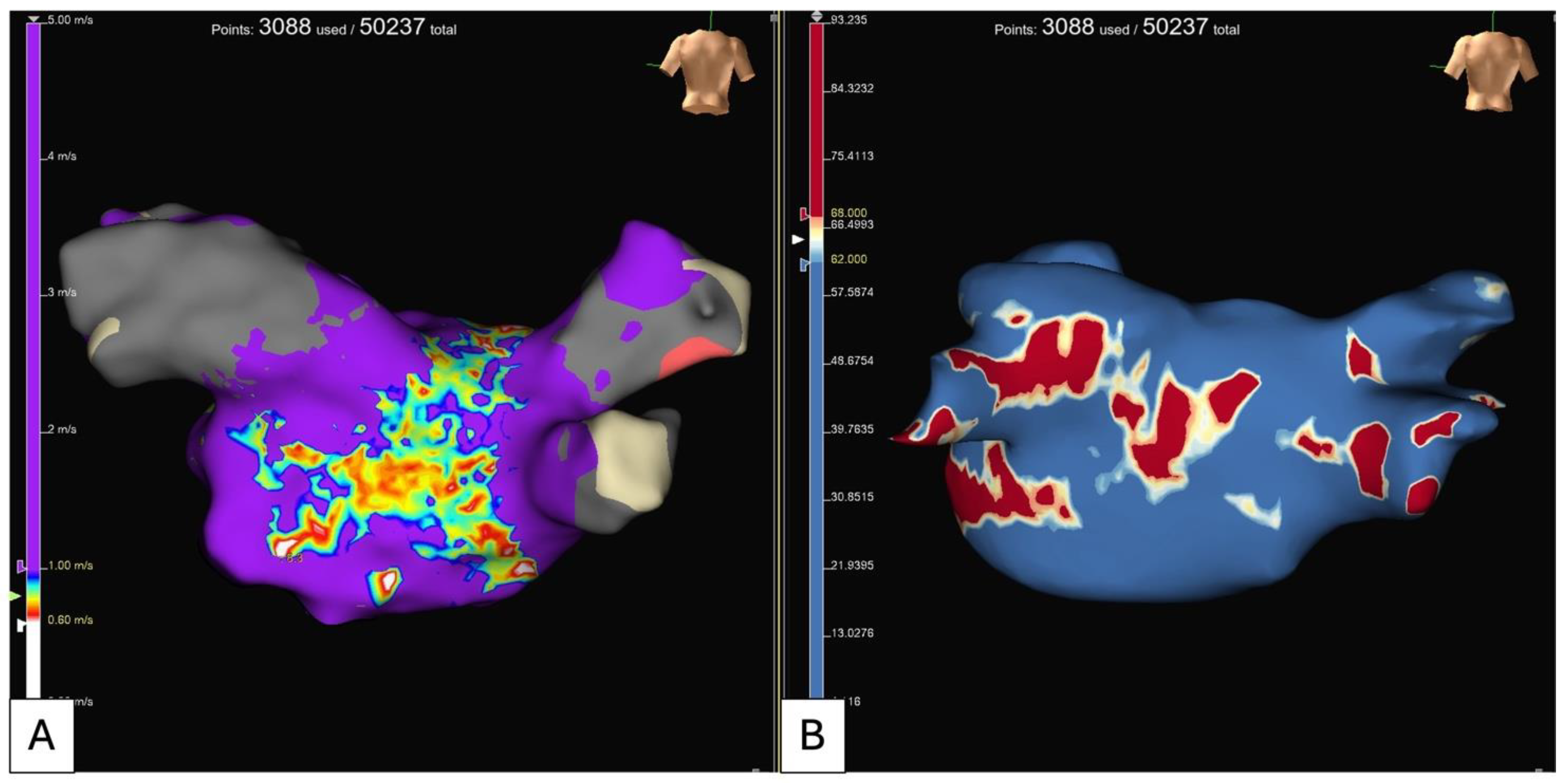
5.2. Electroanatomical Substrate Modification (Low Voltage and Complex Fractionated Electrograms Ablation)
5.3. Empirical Anatomical “Compartmentalization” (Cox-Maze-like Strategy) and Role of Epicardial Connections
5.4. Pulsed Field for Extra PV Trigger Ablation
5.5. Convergent Hybrid Ablation: Is This Technique Obsolete in the PFA Era?
6. Future Perspectives and Conclusions
Funding
Conflicts of Interest
References
- Holmes, D.N.; Piccini, J.P.; Allen, L.A.; Fonarow, G.C.; Gersh, B.J.; Kowey, P.R.; O’Brien, E.C.; Reiffel, J.A.; Naccarelli, G.V.; Ezekowitz, M.D.; et al. Defining Clinically Important Difference in the Atrial Fibrillation Effect on Quality-of-Life Score. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005358. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, D.G.; Emdin, C.A. Atrial Fibrillation and Risks of Cardiovascular Disease, Renal Disease, and Death: Systematic Review and Meta-Analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef] [PubMed]
- Bassand, J.-P.; Accetta, G.; Camm, A.J.; Cools, F.; Fitzmaurice, D.A.; Fox, K.A.A.; Goldhaber, S.Z.; Goto, S.; Haas, S.; Hacke, W.; et al. Two-Year Outcomes of Patients with Newly Diagnosed Atrial Fibrillation: Results from GARFIELD-AF. Eur. Heart J. 2016, 37, 2882–2889. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. Comparison of Antiarrhythmic Drug Therapy and Radiofrequency Catheter Ablation in Patients with Paroxysmal Atrial Fibrillation. JAMA 2010, 303, 333. [Google Scholar] [CrossRef]
- Calkins, H.; Reynolds, M.R.; Spector, P.; Sondhi, M.; Xu, Y.; Martin, A.; Williams, C.J.; Sledge, I. Treatment of Atrial Fibrillation with Antiarrhythmic Drugs or Radiofrequency Ablation. Circ. Arrhythmia Electrophysiol. 2009, 2, 349–361. [Google Scholar] [CrossRef]
- Jaïs, P.; Cauchemez, B.; Macle, L.; Daoud, E.; Khairy, P.; Subbiah, R.; Hocini, M.; Extramiana, F.; Sacher, F.; Bordachar, P.; et al. Catheter Ablation Versus Antiarrhythmic Drugs for Atrial Fibrillation. Circulation 2008, 118, 2498–2505. [Google Scholar] [CrossRef]
- Packer, D.L.; Kowal, R.C.; Wheelan, K.R.; Irwin, J.M.; Champagne, J.; Guerra, P.G.; Dubuc, M.; Reddy, V.; Nelson, L.; Holcomb, R.G.; et al. Cryoballoon Ablation of Pulmonary Veins for Paroxysmal Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 61, 1713–1723. [Google Scholar] [CrossRef]
- Cox, J.L.; Jaquiss, R.D.B.; Schuessler, R.B.; Boineau, J.P. Modification of the Maze Procedure for Atrial Flutter and Atrial Fibrillation. J. Thorac. Cardiovasc. Surg. 1995, 110, 485–495. [Google Scholar] [CrossRef]
- HAÍSSAGUERRE, M.; JAÍS, P.; SHAH, D.C.; GENCEL, L.; PRADEAU, V.; GARRIGUES, S.; CHOUAIRI, S.; HOCINI, M.; LE MÉTAYER, P.; ROUDAUT, R.; et al. Right and Left Atrial Radiofrequency Catheter Therapy of Paroxysmal Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 1996, 7, 1132–1144. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-A.; Hsieh, M.-H.; Tai, C.-T.; Tsai, C.-F.; Prakash, V.S.; Yu, W.-C.; Hsu, T.-L.; Ding, Y.-A.; Chang, M.-S. Initiation of Atrial Fibrillation by Ectopic Beats Originating from the Pulmonary Veins. Circulation 1999, 100, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.B.; Marrouche, N.F.; Natale, A. Ablation of Atrial Fibrillation. Curr. Cardiol. Rep. 2002, 4, 379–387. [Google Scholar] [CrossRef]
- Kanagaratnam, L.; Tomassoni, G.; Schweikert, R.; Pavia, S.; Bash, D.; Beheiry, S.; Lesh, M.; Niebauer, M.; Saliba, W.; Chung, M.; et al. Empirical Pulmonary Vein Isolation in Patients with Chronic Atrial Fibrillation Using a Three-Dimensional Nonfluoroscopic Mapping System: Long-Term Follow-Up. Pacing Clin. Electrophysiol. 2001, 24, 1774–1779. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Dresing, T.; Cole, C.; Bash, D.; Saad, E.; Balaban, K.; Pavia, S.V.; Schweikert, R.; Saliba, W.; Abdul-Karim, A.; et al. Circular Mapping and Ablation of Thepulmonary Vein for Treatment of Atrial Fibrillation. J. Am. Coll. Cardiol. 2002, 40, 464–474. [Google Scholar] [CrossRef]
- Parameswaran, R.; Al-Kaisey, A.M.; Kalman, J.M. Catheter Ablation for Atrial Fibrillation: Current Indications and Evolving Technologies. Nat. Rev. Cardiol. 2021, 18, 210–225. [Google Scholar] [CrossRef]
- Arentz, T.; Weber, R.; Bürkle, G.; Herrera, C.; Blum, T.; Stockinger, J.; Minners, J.; Neumann, F.J.; Kalusche, D. Small or Large Isolation Areas Around the Pulmonary Veins for the Treatment of Atrial Fibrillation? Circulation 2007, 115, 3057–3063. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.-H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. EP Eur. 2018, 20, e1–e160. [Google Scholar] [CrossRef]
- Vogt, J.; Heintze, J.; Gutleben, K.J.; Muntean, B.; Horstkotte, D.; Nölker, G. Long-Term Outcomes After Cryoballoon Pulmonary Vein Isolation. J. Am. Coll. Cardiol. 2013, 61, 1707–1712. [Google Scholar] [CrossRef]
- Hussein, A.; Das, M.; Riva, S.; Morgan, M.; Ronayne, C.; Sahni, A.; Shaw, M.; Todd, D.; Hall, M.; Modi, S.; et al. Use of Ablation Index-Guided Ablation Results in High Rates of Durable Pulmonary Vein Isolation and Freedom from Arrhythmia in Persistent Atrial Fibrillation Patients. Circ. Arrhythmia Electrophysiol. 2018, 11, e006576. [Google Scholar] [CrossRef]
- Mattia, L. De Prospective Evaluation of Lesion Index-Guided Pulmonary Vein Isolation Technique in Patients with Paroxysmal Atrial Fibrillation: 1-Year Follow-Up. J. Atr. Fibrillation 2018, 10, 1858. [Google Scholar] [CrossRef] [PubMed]
- Taghji, P.; El Haddad, M.; Phlips, T.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Nakagawa, H.; Duytschaever, M. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins with Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Duytschaever, M.; De Pooter, J.; Demolder, A.; El Haddad, M.; Phlips, T.; Strisciuglio, T.; Debonnaire, P.; Wolf, M.; Vandekerckhove, Y.; Knecht, S.; et al. Long-Term Impact of Catheter Ablation on Arrhythmia Burden in Low-Risk Patients with Paroxysmal Atrial Fibrillation: The CLOSE to CURE Study. Heart Rhythm 2020, 17, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Grimaldi, M.; De Potter, T.; Vijgen, J.M.; Bulava, A.; Duytschaever, M.F.; Martinek, M.; Natale, A.; Knecht, S.; Neuzil, P.; et al. Pulmonary Vein Isolation with Very High Power, Short Duration, Temperature-Controlled Lesions. JACC Clin. Electrophysiol. 2019, 5, 778–786. [Google Scholar] [CrossRef]
- Aryana, A.; Mugnai, G.; Singh, S.M.; Pujara, D.K.; de Asmundis, C.; Singh, S.K.; Bowers, M.R.; Brugada, P.; d’Avila, A.; O’Neill, P.G.; et al. Procedural and Biophysical Indicators of Durable Pulmonary Vein Isolation during Cryoballoon Ablation of Atrial Fibrillation. Heart Rhythm 2016, 13, 424–432. [Google Scholar] [CrossRef]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Reichlin, T.; Kueffer, T.; Knecht, S.; Madaffari, A.; Badertscher, P.; Maurhofer, J.; Krisai, P.; Jufer, C.; Asatryan, B.; Heg, D.; et al. PolarX vs Arctic Front for Cryoballoon Ablation of Paroxysmal AF. JACC Clin. Electrophysiol. 2024, 10, 1367–1376. [Google Scholar] [CrossRef]
- Luik, A.; Radzewitz, A.; Kieser, M.; Walter, M.; Bramlage, P.; Hörmann, P.; Schmidt, K.; Horn, N.; Brinkmeier-Theofanopoulou, M.; Kunzmann, K.; et al. Cryoballoon Versus Open Irrigated Radiofrequency Ablation in Patients with Paroxysmal Atrial Fibrillation. Circulation 2015, 132, 1311–1319. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Boveda, S.; Providência, R.; Defaye, P.; Pavin, D.; Cebron, J.-P.; Anselme, F.; Halimi, F.; Khoueiry, Z.; Combes, N.; Combes, S.; et al. Outcomes after Cryoballoon or Radiofrequency Ablation for Persistent Atrial Fibrillation: A Multicentric Propensity-Score Matched Study. J. Interv. Card. Electrophysiol. 2016, 47, 133–142. [Google Scholar] [CrossRef]
- Gunawardene, M.A.; Hoffmann, B.A.; Schaeffer, B.; Chung, D.-U.; Moser, J.; Akbulak, R.O.; Jularic, M.; Eickholt, C.; Nuehrich, J.; Meyer, C.; et al. Influence of Energy Source on Early Atrial Fibrillation Recurrences: A Comparison of Cryoballoon vs. Radiofrequency Current Energy Ablation with the Endpoint of Unexcitability in Pulmonary Vein Isolation. Europace 2016, 20, 43–49. [Google Scholar] [CrossRef]
- Watanabe, R.; Sairaku, A.; Yoshida, Y.; Nanasato, M.; Kamiya, H.; Suzuki, H.; Ogura, Y.; Aoyama, Y.; Maeda, M.; Ando, M.; et al. Head-to-head Comparison of Acute and Chronic Pulmonary Vein Stenosis for Cryoballoon versus Radiofrequency Ablation. Pacing Clin. Electrophysiol. 2018, 41, 376–382. [Google Scholar] [CrossRef]
- Buist, T.J.; Adiyaman, A.; Smit, J.J.J.; Ramdat Misier, A.R.; Elvan, A. Arrhythmia-Free Survival and Pulmonary Vein Reconnection Patterns after Second-Generation Cryoballoon and Contact-Force Radiofrequency Pulmonary Vein Isolation. Clin. Res. Cardiol. 2018, 107, 498–506. [Google Scholar] [CrossRef]
- Natale, A.; Mohanty, S.; Goldstein, L.; Gomez, T.; Hunter, T.D. Real-World Safety of Catheter Ablation for Atrial Fibrillation with Contact Force or Cryoballoon Ablation. J. Interv. Card. Electrophysiol. 2021, 60, 445–452. [Google Scholar] [CrossRef]
- Bisignani, A.; Cecchini, F.; Mugnai, G.; Overeinder, I.; Sieira, J.; Osório, T.G.; Miraglia, V.; Monaco, C.; Sofianos, D.; Boveda, S.; et al. Single Procedural Outcomes in the Setting of Percutaneous Ablation for Persistent Atrial Fibrillation: A Propensity-Matched Score Comparison between Different Strategies. J. Interv. Card. Electrophysiol. 2022, 64, 9–16. [Google Scholar] [CrossRef]
- Shi, L.-B.; Rossvoll, O.; Tande, P.; Schuster, P.; Solheim, E.; Chen, J. Cryoballoon vs. Radiofrequency Catheter Ablation: Insights from NOrwegian Randomized Study of PERSistent Atrial Fibrillation (NO-PERSAF Study). EP Eur. 2022, 24, 226–233. [Google Scholar] [CrossRef]
- Julian Chun, K.R.; Miklavčič, D.; Vlachos, K.; Bordignon, S.; Scherr, D.; Jais, P.; Schmidt, B. State-of-the-Art Pulsed Field Ablation for Cardiac Arrhythmias: Ongoing Evolution and Future Perspective. Europace 2024, 26, euae134. [Google Scholar] [CrossRef]
- Verma, A.; Asivatham, S.J.; Deneke, T.; Castellvi, Q.; Neal, R.E. Primer on Pulsed Electrical Field Ablation: Understanding the Benefits and Limitations. Circ. Arrhythmia Electrophysiol. 2021, 14, E010086. [Google Scholar] [CrossRef]
- Sugrue, A.; Maor, E.; Del-Carpio Munoz, F.; Killu, A.M.; Asirvatham, S.J. Cardiac Ablation with Pulsed Electric Fields: Principles and Biophysics. Europace 2022, 24, 1213–1222. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Gerstenfeld, E.P.; Natale, A.; Whang, W.; Cuoco, F.A.; Patel, C.; Mountantonakis, S.E.; Gibson, D.N.; Harding, J.D.; Ellis, C.R.; et al. Pulsed Field or Conventional Thermal Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.; Neuzil, P.; Reichlin, T.; Kautzner, J.; van der Voort, P.; Jais, P.; Chierchia, G.B.; Bulava, A.; Blaauw, Y.; Skala, T.; et al. Safety of Pulsed Field Ablation in More than 17,000 Patients with Atrial Fibrillation in the MANIFEST-17K Study. Nat. Med. 2024, 30, 2020–2029. [Google Scholar] [CrossRef]
- Schmidt, B.; Bordignon, S.; Neven, K.; Reichlin, T.; Blaauw, Y.; Hansen, J.; Adelino, R.; Ouss, A.; Füting, A.; Roten, L.; et al. EUropean Real-World Outcomes with Pulsed Field AblatiOn in Patients with Symptomatic AtRIAl Fibrillation: Lessons from the Multi-Centre EU-PORIA Registry. Europace 2023, 25, euad185. [Google Scholar] [CrossRef]
- Mohanty, S.; Torlapati, P.G.; Casella, M.; Della Rocca, D.G.; Schiavone, M.; Doty, B.; La Fazia, V.M.; Pahi, S.; Pierucci, N.; Valeri, Y.; et al. Redefining the Blanking Period after Pulsed-Field Ablation in Patients with Atrial Fibrillation. Heart Rhythm 2024. [Google Scholar] [CrossRef]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.-A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial Cardiomyopathy Revisited—Evolution of a Concept: A Clinical Consensus Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). Europace 2024, 26, euae204. [Google Scholar] [CrossRef]
- Henry, W.L.; Morganroth, J.; Pearlman, A.S.; Clark, C.E.; Redwood, D.R.; Itscoitz, S.B.; Epstein, S.E. Relation between Echocardiographically Determined Left Atrial Size and Atrial Fibrillation. Circulation 1976, 53, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Osranek, M.; Bursi, F.; Bailey, K.R.; Grossardt, B.R.; Brown, R.D.; Kopecky, S.L.; Tsang, T.S.; Seward, J.B. Left Atrial Volume Predicts Cardiovascular Events in Patients Originally Diagnosed with Lone Atrial Fibrillation: Three-Decade Follow-Up. Eur. Heart J. 2005, 26, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2019, 74, 104–132. [Google Scholar] [CrossRef]
- Hauser, R.; Nielsen, A.B.; Skaarup, K.G.; Lassen, M.C.H.; Duus, L.S.; Johansen, N.D.; Sengeløv, M.; Marott, J.L.; Jensen, G.; Schnohr, P.; et al. Left Atrial Strain Predicts Incident Atrial Fibrillation in the General Population: The Copenhagen City Heart Study. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 52–60. [Google Scholar] [CrossRef]
- Serenelli, M.; Cantone, A.; Dal Passo, B.; Di Ienno, L.; Fiorio, A.; Pavasini, R.; Passarini, G.; Bertini, M.; Campo, G. Atrial Longitudinal Strain Predicts New-Onset Atrial Fibrillation. JACC Cardiovasc. Imaging 2023, 16, 392–395. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Europace 2024, 26, euae043. [Google Scholar] [CrossRef]
- Bertaglia, E.; Anselmino, M.; Zorzi, A.; Russo, V.; Toso, E.; Peruzza, F.; Rapacciuolo, A.; Migliore, F.; Gaita, F.; Cucchini, U.; et al. NOACs and Atrial Fibrillation: Incidence and Predictors of Left Atrial Thrombus in the Real World. Int. J. Cardiol. 2017, 249, 179–183. [Google Scholar] [CrossRef]
- Blackshear, J.L.; Odell, J.A. Appendage Obliteration to Reduce Stroke in Cardiac Surgical Patients with Atrial Fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.C.; Black, I.W.; Cranney, G.B.; Hopkins, A.P.; Walsh, W.F. Prognostic Implications of Left Artial Spontaneous Echo Contrast in Nonvalvular Atrial Fibrillation. J. Am. Coll. Cardiol. 1994, 24, 755–762. [Google Scholar] [CrossRef]
- Juey-Jen, H.; Jin-Jer, C.; Shen-Chang, L.; Yung-Zu, T.; Peiliang, K.; Wen-Pin, L.; Fang-Yue, L.; Shu-Hsun, C.; Chi-Ren, H.; Shu-Wen, H. Diagnostic Accuracy of Transesophageal Echocardiography for Detecting Left Atrial Thrombi in Patients with Rheumatic Heart Disease Having Undergone Mitral Valve Operations. Am. J. Cardiol. 1993, 72, 677–681. [Google Scholar] [CrossRef]
- Manning, W.J. Accuracy of Transesophageal Echocardiography for Identifying Left Atrial Thrombi: A Prospective, Intraoperative Study. Ann. Intern. Med. 1995, 123, 817. [Google Scholar] [CrossRef]
- Bernhardt, P.; Schmidt, H.; Hammerstingl, C.; Lüderitz, B.; Omran, H. Patients with Atrial Fibrillation and Dense Spontaneous Echo Contrast at High Risk. J. Am. Coll. Cardiol. 2005, 45, 1807–1812. [Google Scholar] [CrossRef]
- Handke, M.; Harloff, A.; Hetzel, A.; Olschewski, M.; Bode, C.; Geibel, A. Left Atrial Appendage Flow Velocity as a Quantitative Surrogate Parameter for Thromboembolic Risk: Determinants and Relationship to Spontaneous Echocontrast and Thrombus Formation–A Transesophageal Echocardiographic Study in 500 Patients with Cerebral Ischemia. J. Am. Soc. Echocardiogr. 2005, 18, 1366–1372. [Google Scholar] [CrossRef]
- Khurram, I.M.; Dewire, J.; Mager, M.; Maqbool, F.; Zimmerman, S.L.; Zipunnikov, V.; Beinart, R.; E. Marine, J.; Spragg, D.D.; Berger, R.D.; et al. Relationship between Left Atrial Appendage Morphology and Stroke in Patients with Atrial Fibrillation. Heart Rhythm 2013, 10, 1843–1849. [Google Scholar] [CrossRef]
- Safavi-Naeini, P.; Rasekh, A. Thromboembolism in Atrial Fibrillation. Card. Electrophysiol. Clin. 2020, 12, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Saenz, L.C.; Rosso, R.; Silvestry, F.E.; Callans, D.; Marchlinski, F.E.; Garcia, F. Use of Intracardiac Echocardiography in Interventional Cardiology: Working with the Anatomy Rather Than Fighting It. Circulation 2018, 137, 2278–2294. [Google Scholar] [CrossRef] [PubMed]
- Goya, M.; Frame, D.; Gache, L.; Ichishima, Y.; Tayar, D.O.; Goldstein, L.; Lee, S.H.Y. The Use of Intracardiac Echocardiography Catheters in Endocardial Ablation of Cardiac Arrhythmia: Meta-analysis of Efficiency, Effectiveness, and Safety Outcomes. J. Cardiovasc. Electrophysiol. 2020, 31, 664–673. [Google Scholar] [CrossRef]
- Rosu, R.; Cismaru, G.; Muresan, L.; Puiu, M.; Gusetu, G.; Istratoaie, S.; Pop, D.; Zdrenghea, D. Intracardiac Echocardiography for Transseptal Puncture. A Guide for Cardiac Electrophysiologists. Med. Ultrason. 2019, 21, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.K.; Di Biase, L.; Shah, K.; Alcazar, J.; Ziv-Ari, M.; Brem, E.; Farshchi-Heydari, S. Preclinical Experience Using 4-Dimensional Intracardiac Echocardiography with CARTO Integration. Heart Rhythm O2 2023, 4, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Tops, L.F.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Multi-Modality Imaging to Assess Left Atrial Size, Anatomy and Function. Heart 2007, 93, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation. JAMA 2014, 311, 498. [Google Scholar] [CrossRef]
- Akkaya, M.; Higuchi, K.; Koopmann, M.; Damal, K.; Burgon, N.S.; Kholmovski, E.; Mcgann, C.; Marrouche, N. Higher Degree of Left Atrial Structural Remodeling in Patients with Atrial Fibrillation and Left Ventricular Systolic Dysfunction. J. Cardiovasc. Electrophysiol. 2013, 24, 485–491. [Google Scholar] [CrossRef]
- Daccarett, M.; Badger, T.J.; Akoum, N.; Burgon, N.S.; Mahnkopf, C.; Vergara, G.; Kholmovski, E.; McGann, C.J.; Parker, D.; Brachmann, J.; et al. Association of Left Atrial Fibrosis Detected by Delayed-Enhancement Magnetic Resonance Imaging and the Risk of Stroke in Patients with Atrial Fibrillation. J. Am. Coll. Cardiol. 2011, 57, 831–838. [Google Scholar] [CrossRef]
- Akoum, N.; Fernandez, G.; Wilson, B.; Mcgann, C.; Kholmovski, E.; Marrouche, N. Association of Atrial Fibrosis Quantified Using LGE-MRI with Atrial Appendage Thrombus and Spontaneous Contrast on Transesophageal Echocardiography in Patients with Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2013, 24, 1104–1109. [Google Scholar] [CrossRef]
- Hopman, L.H.G.A.; Bhagirath, P.; Mulder, M.J.; Eggink, I.N.; van Rossum, A.C.; Allaart, C.P.; Götte, M.J.W. Quantification of Left Atrial Fibrosis by 3D Late Gadolinium-Enhanced Cardiac Magnetic Resonance Imaging in Patients with Atrial Fibrillation: Impact of Different Analysis Methods. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1182–1190. [Google Scholar] [CrossRef]
- Deneke, T.; Kutyifa, V.; Hindricks, G.; Sommer, P.; Zeppenfeld, K.; Carbucicchio, C.; Pürerfellner, H.; Heinzel, F.R.; Traykov, V.B.; De Riva, M.; et al. Pre- and Post-Procedural Cardiac Imaging (Computed Tomography and Magnetic Resonance Imaging) in Electrophysiology: A Clinical Consensus Statement of the European Heart Rhythm Association and European Association of Cardiovascular Imaging of the European Society of Cardiology. Europace 2024, 26, euae108. [Google Scholar] [CrossRef]
- Falasconi, G.; Penela, D.; Soto-Iglesias, D.; Francia, P.; Teres, C.; Saglietto, A.; Jauregui, B.; Viveros, D.; Bellido, A.; Alderete, J.; et al. Personalized Pulmonary Vein Antrum Isolation Guided by Left Atrial Wall Thickness for Persistent Atrial Fibrillation. Europace 2023, 25, euad118. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Ganesan, A.N.; Selvanayagam, J.B. Epicardial Fat and Atrial Fibrillation: Current Evidence, Potential Mechanisms, Clinical Implications, and Future Directions. Eur. Heart J. 2016, 38, 1294–1302. [Google Scholar] [CrossRef]
- Saglietto, A.; Falasconi, G.; Soto-Iglesias, D.; Francia, P.; Penela, D.; Alderete, J.; Viveros, D.; Bellido, A.F.; Franco-Ocaña, P.; Zaraket, F.; et al. Assessing Left Atrial Intramyocardial Fat Infiltration from Computerized Tomography Angiography in Patients with Atrial Fibrillation. Europace 2023, 25, euad351. [Google Scholar] [CrossRef]
- Huber, A.T.; Fankhauser, S.; Chollet, L.; Wittmer, S.; Lam, A.; Baldinger, S.; Madaffari, A.; Seiler, J.; Servatius, H.; Haeberlin, A.; et al. The Relationship between Enhancing Left Atrial Adipose Tissue at CT and Recurrent Atrial Fibrillation. Radiology 2022, 305, 56–65. [Google Scholar] [CrossRef]
- Santangeli, P.; Marchlinski, F.E. Techniques for the Provocation, Localization, and Ablation of Non–Pulmonary Vein Triggers for Atrial Fibrillation. Heart Rhythm 2017, 14, 1087–1096. [Google Scholar] [CrossRef]
- Keelani, A.; Alothman, O.; Borisov, G.; Frommhold, M.; Bartoli, L.; Abdelwahab, H.; D’Ambrosio, G.; Al Shehri, S.; Raffa, S.; Geller, J.C. Feasibility and Clinical Efficacy of Focal Pulsed Field Ablation in Patients with Non-Pulmonary Vein Triggered Atrial Arrhythmia from the Superior Caval Vein. J. Cardiovasc. Electrophysiol. 2024, 36, 359–366. [Google Scholar] [CrossRef] [PubMed]
- William, J.; Chieng, D.; Curtin, A.G.; Sugumar, H.; Ling, L.H.; Segan, L.; Crowley, R.; Iyer, A.; Prabhu, S.; Voskoboinik, A.; et al. Radiofrequency Catheter Ablation of Persistent Atrial Fibrillation by Pulmonary Vein Isolation with or without Left Atrial Posterior Wall Isolation: Long-Term Outcomes of the CAPLA Trial. Eur. Heart J. 2025, 46, 132–143. [Google Scholar] [CrossRef]
- Tilz, R.R.; Schmidt, V.; Pürerfellner, H.; Maury, P.; Chun, K.R.J.u.; Martinek, M.; Sohns, C.; Schmidt, B.; Mandel, F.; Gandjbakhch, E.; et al. A Worldwide Survey on Incidence, Management, and Prognosis of Oesophageal Fistula Formation Following Atrial Fibrillation Catheter Ablation: The POTTER-AF Study. Eur. Heart J. 2023, 44, 2458–2469. [Google Scholar] [CrossRef]
- Turagam, M.K.; Neuzil, P.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Impact of Left Atrial Posterior Wall Ablation During Pulsed-Field Ablation for Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2024, 10, 900–912. [Google Scholar] [CrossRef]
- Al Rawahi, M.; Liang, J.J.; Kapa, S.; Lin, A.; Shirai, Y.; Kuo, L.; Zado, E.S.; Hyman, M.C.; Riley, M.P.; Nazarian, S.; et al. Incidence of Left Atrial Appendage Triggers in Patients with Atrial Fibrillation Undergoing Catheter Ablation. JACC Clin. Electrophysiol. 2020, 6, 21–30. [Google Scholar] [CrossRef]
- Madaffari, A.; Große, A.; Conci, E.; Geller, J.C. Left Atrial Appendage Electrical Isolation for Persistent Atrial Fibrillation. PACE-Pacing Clin. Electrophysiol. 2019, 42, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Rillig, A.; Tilz, R.R.; Lin, T.; Fink, T.; Heeger, C.-H.; Arya, A.; Metzner, A.; Mathew, S.; Wissner, E.; Makimoto, H.; et al. Unexpectedly High Incidence of Stroke and Left Atrial Appendage Thrombus Formation After Electrical Isolation of the Left Atrial Appendage for the Treatment of Atrial Tachyarrhythmias. Circ. Arrhythmia Electrophysiol. 2016, 9, e003461. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Brunelli, M.; Grosse, A.; Raffa, S.; Daehne, T.; Frommhold, M.; Cima, A.; Madaffari, A.; Gelle, J.C. Reference values for conduction times to identify achievement of bidirectional block after left atrial linear ablation (roof, inferolateral or superoseptal mitral isthmus). In Proceedings of the EHRA Congress, Milan, Italy, 21–24 June 2015. [Google Scholar]
- Rolf, S.; Kircher, S.; Arya, A.; Eitel, C.; Sommer, P.; Richter, S.; Gaspar, T.; Bollmann, A.; Altmann, D.; Piedra, C.; et al. Tailored Atrial Substrate Modification Based on Low-Voltage Areas in Catheter Ablation of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2014, 7, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, C.; Zhu, X.; Wang, X.; Ye, P.; Jiang, W.; Wu, S.; Xu, K.; Li, X.; Wang, Y.; et al. Multi-Centre, Prospective Randomized Comparison of Three Different Substrate Ablation Strategies for Persistent Atrial Fibrillation. Europace 2023, 25, euad090. [Google Scholar] [CrossRef]
- Sugumar, H.; Prabhu, S.; Voskoboinik, A.; Young, S.; Gutman, S.J.; Wong, G.R.; Parameswaran, R.; Nalliah, C.J.; Lee, G.; McLellan, A.J.; et al. Atrial Remodeling Following Catheter Ablation for Atrial Fibrillation-Mediated Cardiomyopathy: Long-Term Follow-Up of CAMERA-MRI Study. JACC Clin. Electrophysiol. 2019, 5, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Deisenhofer, I.; Albenque, J.-P.; Busch, S.; Gitenay, E.; Mountantonakis, S.; Roux, A.; Horvilleur, J.; Bakouboula, B.; Oza, S.R.; Abbey, S.; et al. Lb-469805-01 Tailored Cardiac Ablation Procedure for Persistent Atrial Fibrillation Guided by Artificial Intelligence: The Tailored-Af Randomized Clinical Trial. Heart Rhythm 2024, 21, 1199. [Google Scholar] [CrossRef]
- Knecht, S.; Hocini, M.; Wright, M.; Lellouche, N.; O’Neill, M.D.; Matsuo, S.; Nault, I.; Chauhan, V.S.; Makati, K.J.; Bevilacqua, M.; et al. Left Atrial Linear Lesions Are Required for Successful Treatment of Persistent Atrial Fibrillation. Eur. Heart J. 2008, 29, 2359–2366. [Google Scholar] [CrossRef]
- Cabrera, J.A.; Ho, S.Y.; Climent, V.; Fuertes, B.; Murillo, M.; Sánchez-Quintana, D. Morphological Evidence of Muscular Connections between Contiguous Pulmonary Venous Orifices: Relevance of the Interpulmonary Isthmus for Catheter Ablation in Atrial Fibrillation. Heart Rhythm 2009, 6, 1192–1198. [Google Scholar] [CrossRef]
- Ho, S.Y.; Sánchez-Quintana, D. The Importance of Atrial Structure and Fibers. Clin. Anat. 2009, 22, 52–63. [Google Scholar] [CrossRef]
- Madaffari, A.; Knecht, S.; Spies, F.; Schaer, B.; Kühne, M.; Sticherling, C.; Osswald, S. Epicardial Connection: The Achilles Heel of Gap Mapping After Wide Antral Pulmonary Veins Isolation. JACC Clin. Electrophysiol. 2019, 5, 1356–1357. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Wu, T.-J.; Doshi, R.N.; Peter, C.T.; Chen, P.-S. Vein of Marshall Cannulation for the Analysis of Electrical Activity in Patients with Focal Atrial Fibrillation. Circulation 2000, 101, 1503–1505. [Google Scholar] [CrossRef]
- Valderrábano, M.; Chen, H.R.; Sidhu, J.; Rao, L.; Ling, Y.; Khoury, D.S. Retrograde Ethanol Infusion in the Vein of Marshall. Circ. Arrhythmia Electrophysiol. 2009, 2, 50–56. [Google Scholar] [CrossRef]
- Pambrun, T.; Derval, N.; Duchateau, J.; Denis, A.; Chauvel, R.; Tixier, R.; Welte, N.; André, C.; Nakashima, T.; Nakatani, Y.; et al. Epicardial Course of the Musculature Related to the Great Cardiac Vein: Anatomical Considerations and Clinical Implications for Mitral Isthmus Block after Vein of Marshall Ethanol Infusion. Heart Rhythm 2021, 18, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, T.; Martin, R.; Denis, A.; Takigawa, M.; Duparc, A.; Rollin, A.; Frontera, A.; Thompson, N.; Massoullié, G.; Cheniti, G.; et al. Characteristics of Single-Loop Macroreentrant Biatrial Tachycardia Diagnosed by Ultrahigh-Resolution Mapping System. Circ. Arrhythmia Electrophysiol. 2018, 11, e005558. [Google Scholar] [CrossRef]
- Mikhaylov, E.N.; Mitrofanova, L.B.; Vander, M.A.; Tatarskiy, R.B.; Kamenev, A.V.; Abramov, M.L.; Szili-Torok, T.; Lebedev, D.S. Biatrial Tachycardia Following Linear Anterior Wall Ablation for the Perimitral Reentry: Incidence and Electrophysiological Evaluations. J. Cardiovasc. Electrophysiol. 2015, 26, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Báez-Escudero, J.L.; Keida, T.; Dave, A.S.; Okishige, K.; Valderrábano, M. Ethanol Infusion in the Vein of Marshall Leads to Parasympathetic Denervation of the Human Left Atrium. J. Am. Coll. Cardiol. 2014, 63, 1892–1901. [Google Scholar] [CrossRef]
- Valderrábano, M.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Effect of Catheter Ablation with Vein of Marshall Ethanol Infusion vs Catheter Ablation Alone on Persistent Atrial Fibrillation. JAMA 2020, 324, 1620. [Google Scholar] [CrossRef]
- Lador, A.; Peterson, L.E.; Swarup, V.; Schurmann, P.A.; Makkar, A.; Doshi, R.N.; DeLurgio, D.; Athill, C.A.; Ellenbogen, K.A.; Natale, A.; et al. Determinants of Outcome Impact of Vein of Marshall Ethanol Infusion When Added to Catheter Ablation of Persistent Atrial Fibrillation: A Secondary Analysis of the VENUS Randomized Clinical Trial. Heart Rhythm 2021, 18, 1045–1054. [Google Scholar] [CrossRef]
- Derval, N.; Duchateau, J.; Denis, A.; Ramirez, F.D.; Mahida, S.; André, C.; Krisai, P.; Nakatani, Y.; Kitamura, T.; Takigawa, M.; et al. Marshall Bundle Elimination, Pulmonary Vein Isolation, and Line Completion for ANatomical Ablation of Persistent Atrial Fibrillation (Marshall-PLAN): Prospective, Single-Center Study. Heart Rhythm 2021, 18, 529–537. [Google Scholar] [CrossRef]
- Takagi, T.; Derval, N.; Duchateau, J.; Chauvel, R.; Tixier, R.; Marchand, H.; Bouyer, B.; André, C.; Kamakura, T.; Krisai, P.; et al. Gaps after Linear Ablation of Persistent Atrial Fibrillation (Marshall-PLAN): Clinical Implication. Heart Rhythm 2023, 20, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sang, C.; Liu, Q.; Lai, Y.; Xia, S.; Jiang, R.; Li, S.; Guo, Q.; Li, Q.; Gao, M.; Guo, X.; et al. Pulmonary Vein Isolation with Optimized Linear Ablation vs Pulmonary Vein Isolation Alone for Persistent AF: The PROMPT-AF Randomized Clinical Trial. JAMA 2024, 333, 381. [Google Scholar] [CrossRef]
- Khiabani, A.J.; MacGregor, R.M.; Bakir, N.H.; Manghelli, J.L.; Sinn, L.A.; Maniar, H.S.; Moon, M.R.; Schuessler, R.B.; Melby, S.J.; Damiano, R.J. The Long-Term Outcomes and Durability of the Cox-Maze IV Procedure for Atrial Fibrillation. J. Thorac. Cardiovasc. Surg. 2022, 163, 629–641.e7. [Google Scholar] [CrossRef]
- Kueffer, T.; Tanner, H.; Madaffari, A.; Seiler, J.; Haeberlin, A.; Maurhofer, J.; Noti, F.; Herrera, C.; Thalmann, G.; Kozhuharov, N.A.; et al. Posterior Wall Ablation by Pulsed-Field Ablation: Procedural Safety, Efficacy, and Findings on Redo Procedures. Europace 2024, 26, euae006. [Google Scholar] [CrossRef]
- Kueffer, T.; Seiler, J.; Madaffari, A.; Mühl, A.; Asatryan, B.; Stettler, R.; Haeberlin, A.; Noti, F.; Servatius, H.; Tanner, H.; et al. Pulsed-Field Ablation for the Treatment of Left Atrial Reentry Tachycardia. J. Interv. Card. Electrophysiol. 2023, 66, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Anter, E.; Rackauskas, G.; Peichl, P.; Koruth, J.S.; Petru, J.; Funasako, M.; Minami, K.; Natale, A.; Jais, P.; et al. Lattice-Tip Focal Ablation Catheter That Toggles Between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation: A First-in-Human Trial. Circ. Arrhythmia Electrophysiol. 2020, 13, e008718. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Peichl, P.; Anter, E.; Rackauskas, G.; Petru, J.; Funasako, M.; Minami, K.; Koruth, J.S.; Natale, A.; Jais, P.; et al. A Focal Ablation Catheter Toggling Between Radiofrequency and Pulsed Field Energy to Treat Atrial Fibrillation. JACC Clin. Electrophysiol. 2023, 9, 1786–1801. [Google Scholar] [CrossRef]
- van der Heijden, C.A.J.; Vroomen, M.; Luermans, J.G.; Vos, R.; Crijns, H.J.G.M.; Gelsomino, S.; La Meir, M.; Pison, L.; Maesen, B. Hybrid versus Catheter Ablation in Patients with Persistent and Longstanding Persistent Atrial Fibrillation: A Systematic Review and Meta-Analysis†. Eur. J. Cardio-Thorac. Surg. 2019, 56, 433–443. [Google Scholar] [CrossRef]
- DeLurgio, D.B.; Crossen, K.J.; Gill, J.; Blauth, C.; Oza, S.R.; Magnano, A.R.; Mostovych, M.A.; Halkos, M.E.; Tschopp, D.R.; Kerendi, F.; et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, e009288. [Google Scholar] [CrossRef]
- Bianchini, L.; Schiavone, M.; Vettor, G.; Gasperetti, A.; Penza, E.; Ballotta, A.; Pirola, S.; Brambillasca, C.; Zito, E.; De Lio, F.; et al. Hybrid-Convergent Procedure or Pulsed Field Ablation in Long-Standing Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2024, 10, 1700–1710. [Google Scholar] [CrossRef]


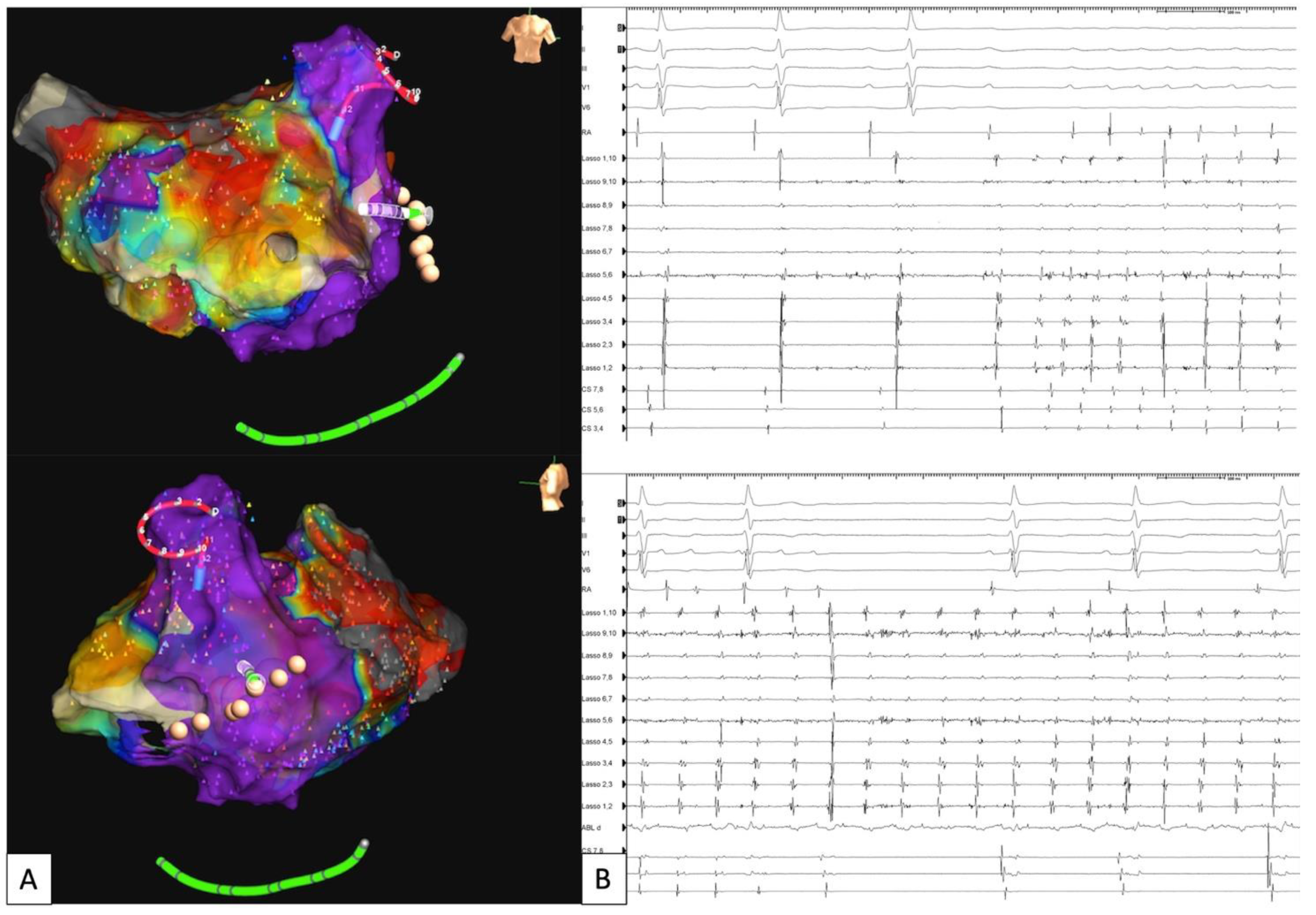
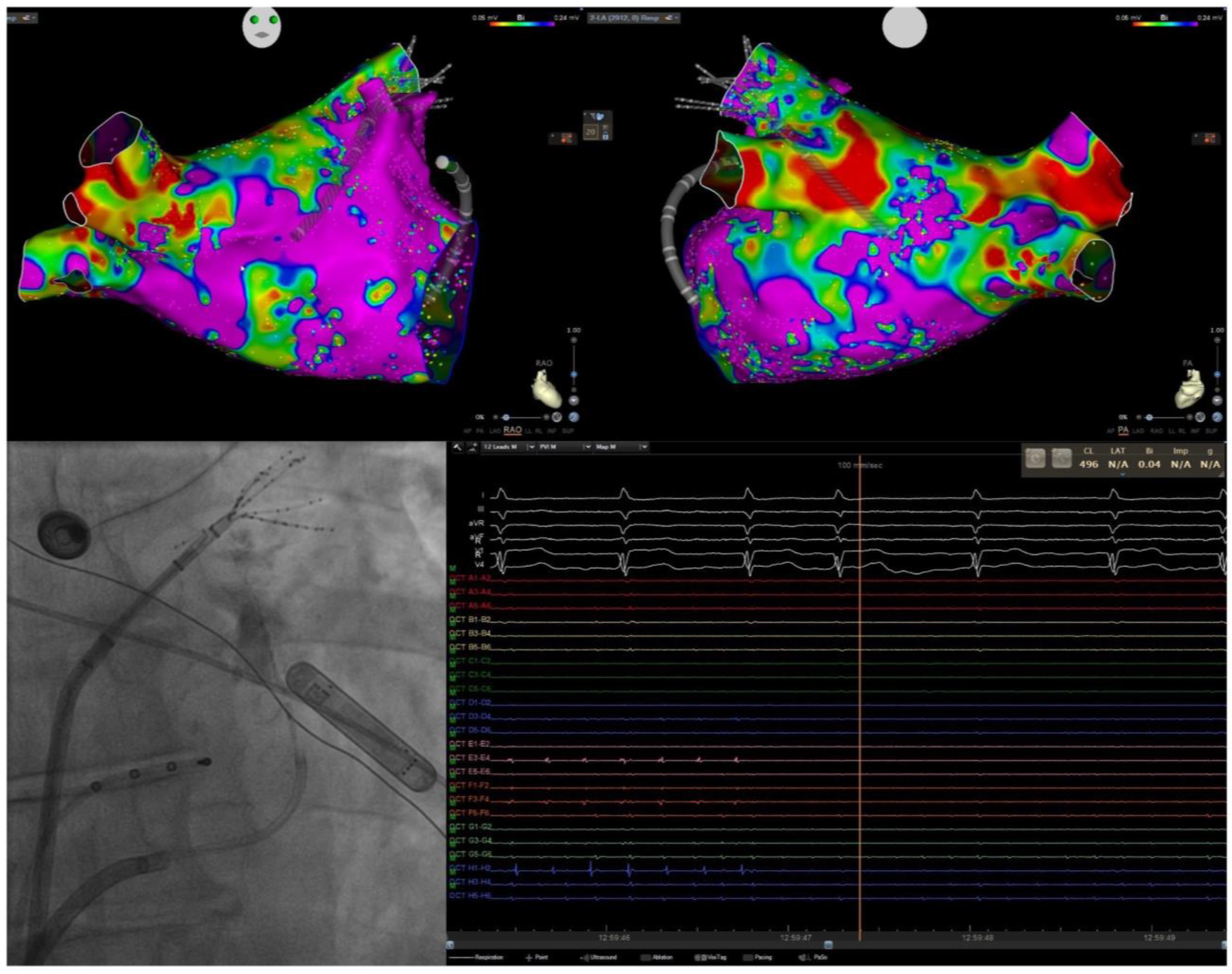
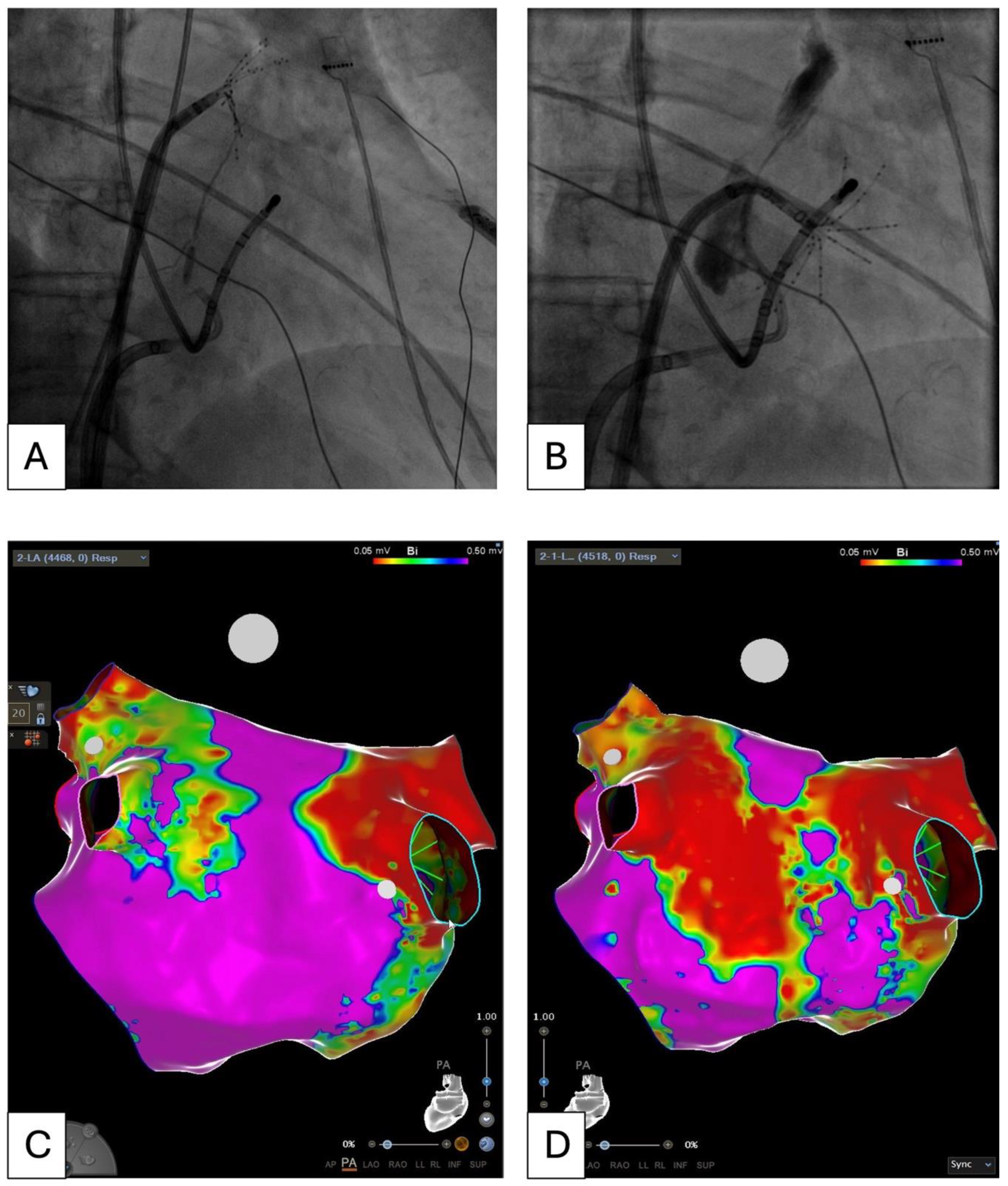
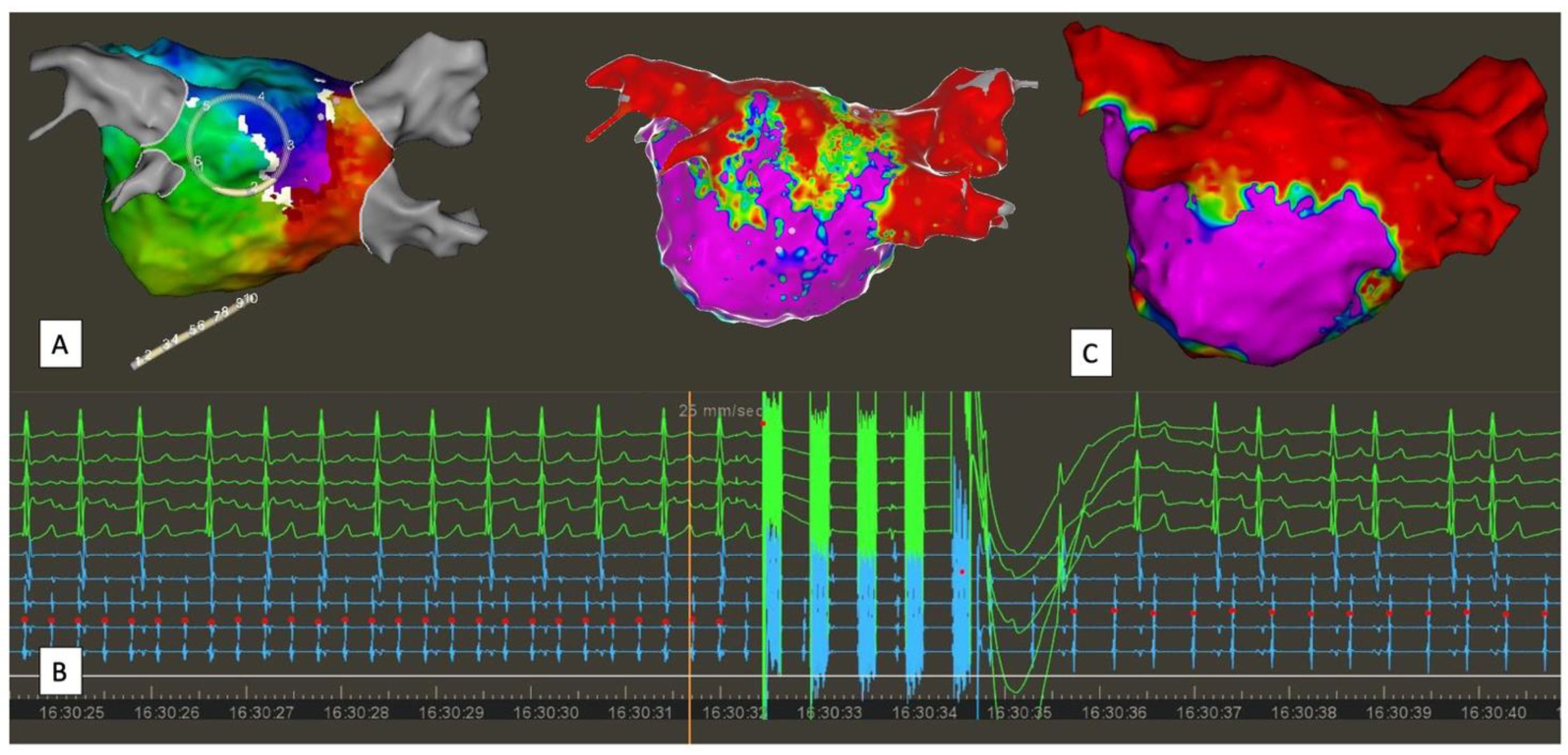
| Study | Study Type | N Patients | Results | Safety |
|---|---|---|---|---|
| Luik et al. FreezeAF (2015) [28] | Multicenter RCT | 159 to RFA, 141 to CBA | Efficacy: CBA was non- inferior to RFA. | 5.0% RFA versus 12.2% CBA, p = 0.022 |
| Kuck et al. (FIRE AND ICE) 2016 [29] | Multicenter RCT | 762 (378 RFA-384 CBA) PAF only | Efficacy: CBA is non-inferior to RFA. | no difference |
| Boveda et al., 2016 [30] | Multicenter, prospective observational | 59 CBA 59 RFA Ps AF | Efficacy: CBA was non-inferior to RFA. | Patients undergoing RFA presented a numerically, but non-significantly, lower complication rate (6.8% vs. 10.2%, p = 0.51). |
| Gunawardene et al., 2016 [31] | RCT | 30 CBA (2 gen) 30 RFA PAF only | Efficacy: No difference in early recurrence rates of atrial fibrillation (ERAF). | No difference. |
| Watanabe et al., 2018 [32] | RCT | 24 CBA 25 RFA PAF only | - | CBA may reduce the acute narrowing of the left-sided PVs as compared to RFA ablation. |
| Buist et al., 2018 [33] | RCT | 136 CBA 133 RFA PAF and PsAF | Single procedure freedom from atrial arrhythmias was significantly higher in CBA as compared to RFA (75.2 vs. 57.4%, p = 0.013). | No difference. |
| Andrade et al., 2019 [26] (CIRCA DOSE) | Multicenter RCT | 346 (RFA115:CBA 4 min 116:CBA 2 min 115) PAF only | No difference in 1-year efficacy (time to first recurrence and burden re- duction assessed by ILR). Less fluoroscopy time for RFA. | no difference. |
| Natale et al., 2021 [34] | Retrospective observational | 407 RFA 1066 CBA | - | No difference |
| Bisignani et al., 2022 [35] | Retrospective observational | 30 patients undergoing PVI + LA posterior wall isolation (LAPWI) with CBA, 30 patients who underwent PVI + linear ablation (roof and mitral lines) using RFA, 60 patients with PVI alone using CBA, and 60 patients who had PVI alone using RFA PsAF | LAPW ablation in addition to PVI with CBA seems to improve 1-year outcomes in comparison to PVI + linear ablation using RFA and to PVI alone using RFA or CBA. | No difference. |
| Shi et al., 2022 [36] | RCT | 49 RFA 52 CBA PsAF | No difference in AF recurrence, less atrial flutter recurrence documented in the CBA group compared with the RFA group (3.9% vs. 18.0%, p = 0.020). | No difference. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peruzza, F.; Candelora, A.; Angheben, C.; Maines, M.; Laurente, M.; Catanzariti, D.; Del Greco, M.; Madaffari, A. Catheter Ablation of Atrial Fibrillation: Technique and Future Perspectives. J. Clin. Med. 2025, 14, 1788. https://doi.org/10.3390/jcm14061788
Peruzza F, Candelora A, Angheben C, Maines M, Laurente M, Catanzariti D, Del Greco M, Madaffari A. Catheter Ablation of Atrial Fibrillation: Technique and Future Perspectives. Journal of Clinical Medicine. 2025; 14(6):1788. https://doi.org/10.3390/jcm14061788
Chicago/Turabian StylePeruzza, Francesco, Andrea Candelora, Carlo Angheben, Massimiliano Maines, Mauro Laurente, Domenico Catanzariti, Maurizio Del Greco, and Antonio Madaffari. 2025. "Catheter Ablation of Atrial Fibrillation: Technique and Future Perspectives" Journal of Clinical Medicine 14, no. 6: 1788. https://doi.org/10.3390/jcm14061788
APA StylePeruzza, F., Candelora, A., Angheben, C., Maines, M., Laurente, M., Catanzariti, D., Del Greco, M., & Madaffari, A. (2025). Catheter Ablation of Atrial Fibrillation: Technique and Future Perspectives. Journal of Clinical Medicine, 14(6), 1788. https://doi.org/10.3390/jcm14061788







