Single-Center Analysis of Soluble TREM2 as a Biomarker in Coronary Microvascular Dysfunction: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Evaluation of Microvascular Function
2.3. Plasma Collection and sTREM2 Concentration Quantification
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
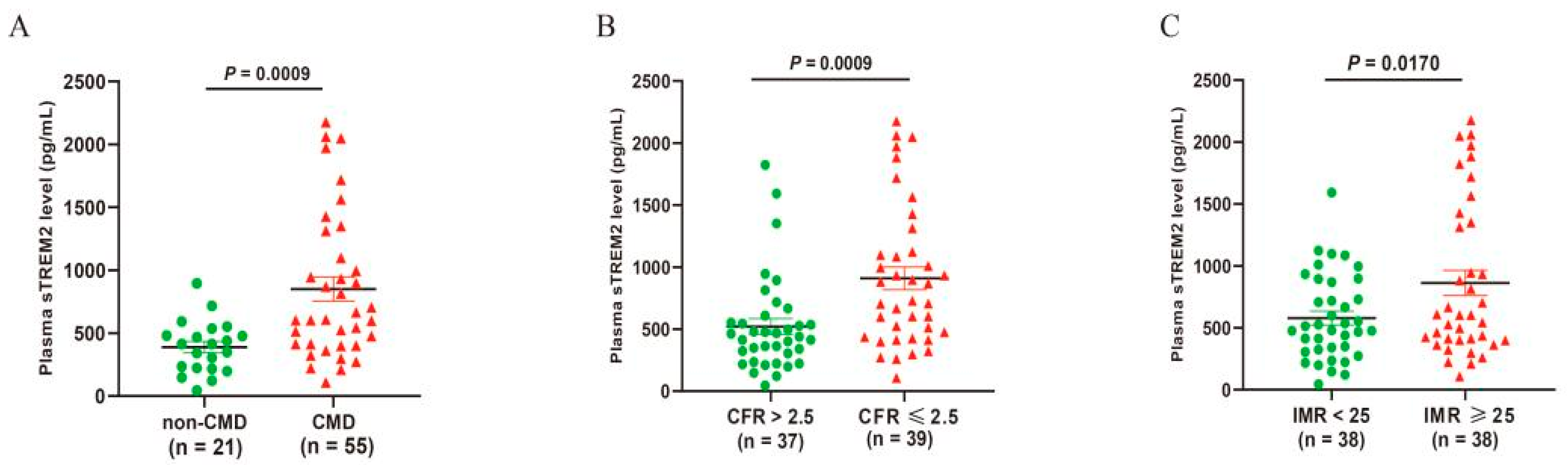
3.2. Plasma sTREM2 Level
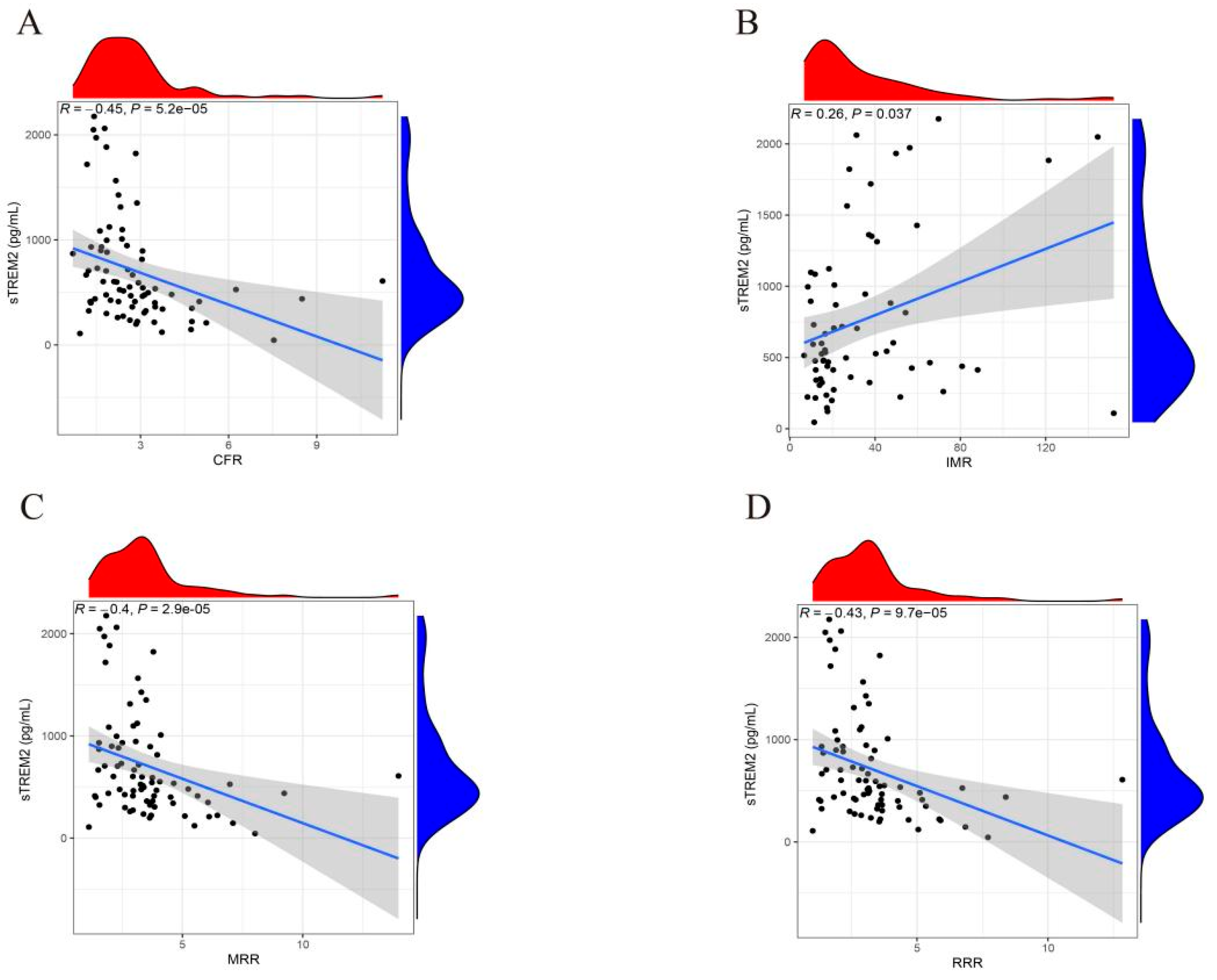
3.3. Correlation Between sTREM2 and Indices for Coronary Microcirculation Function
3.4. Logistic Regression and Subgroup Analysis of Factors Influencing CMD
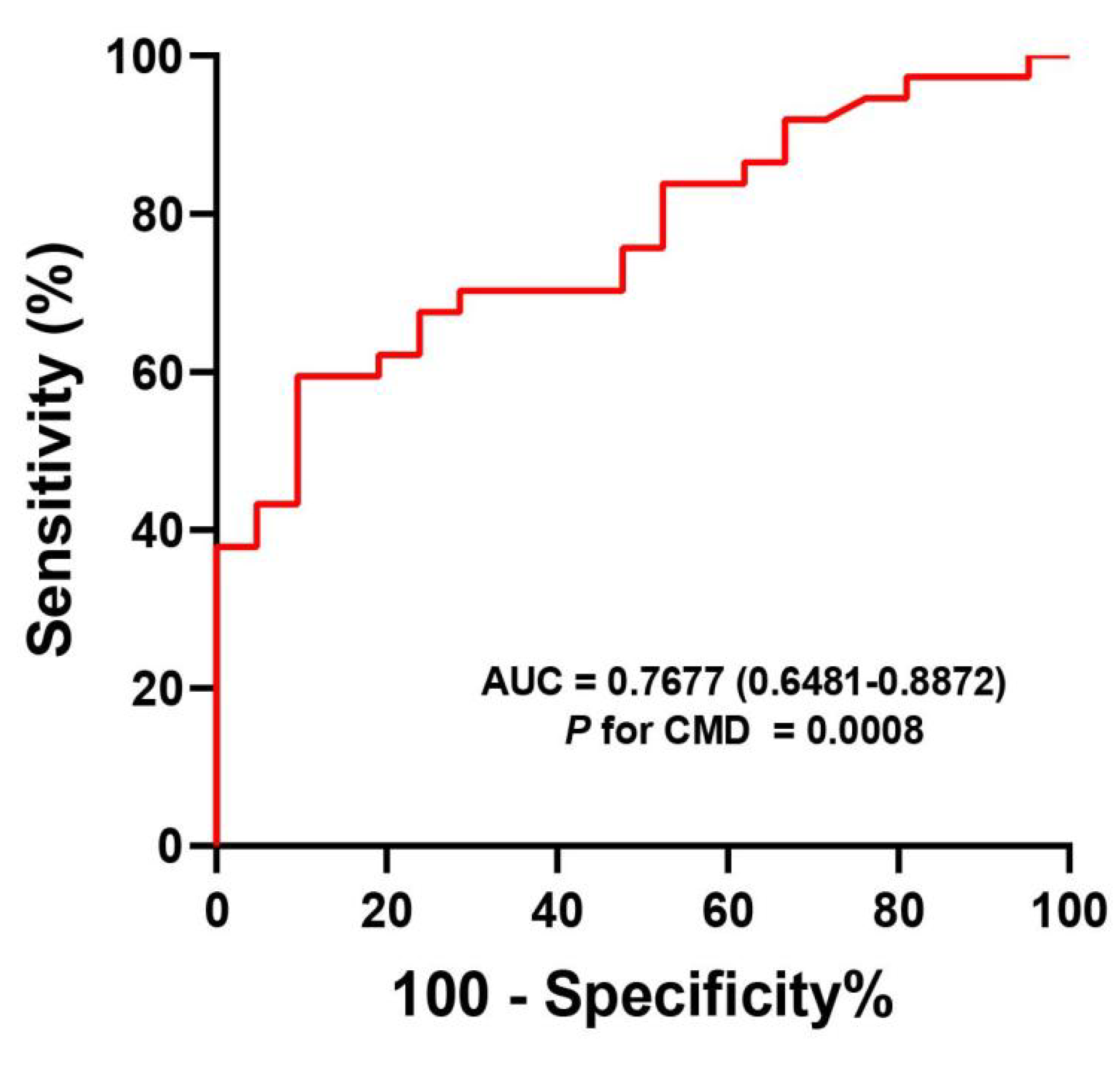
3.5. The Diagnostic Value of sTREM2 for CMD
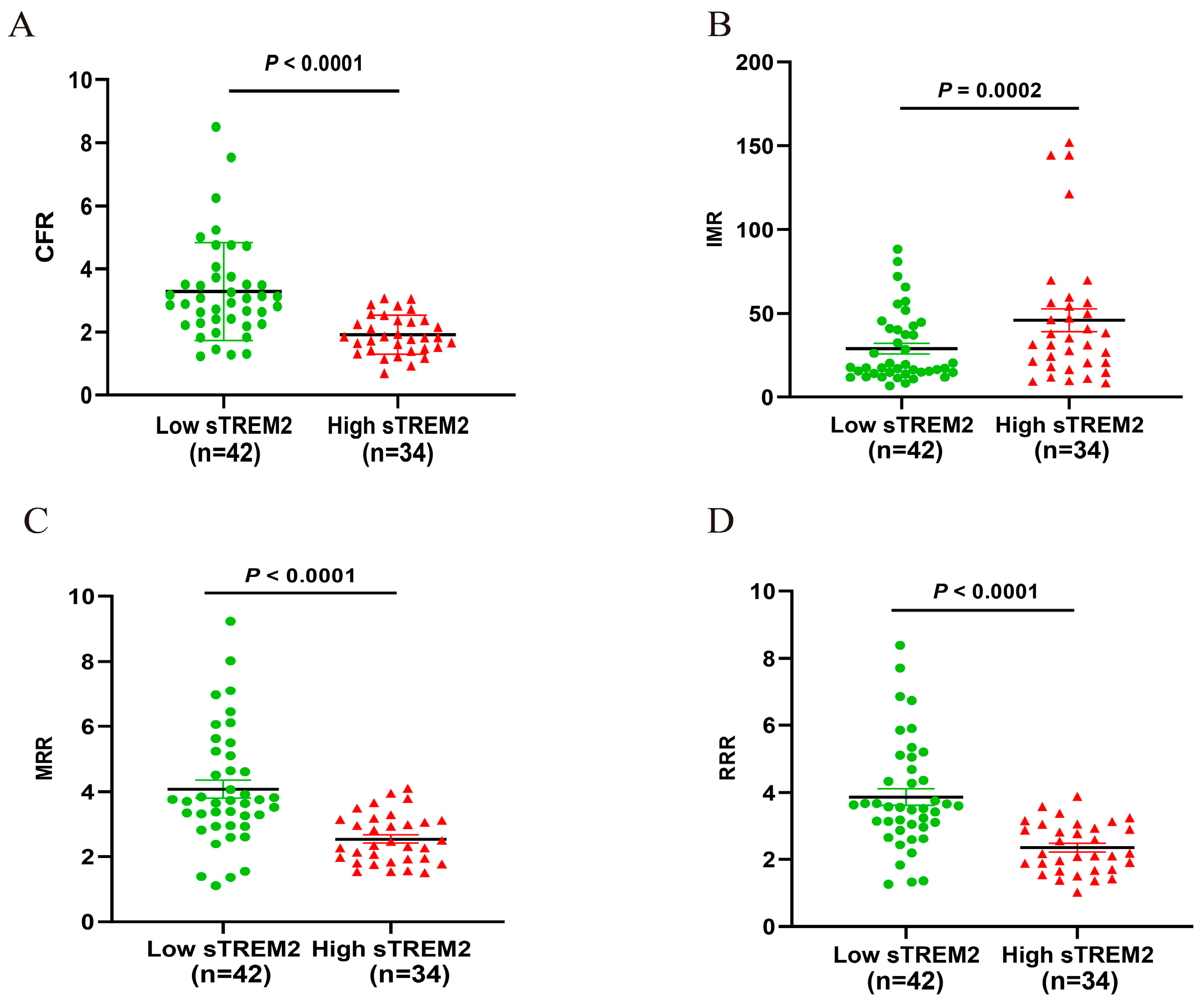
3.6. Comparison of CMD Parameters Based on Grouping of sTRME2 Cut-Off Value
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuels, B.A.; Shah, S.M.; Widmer, R.J.; Kobayashi, Y.; Miner, S.E.; Taqueti, V.R.; Jeremias, A.; Albadri, A.; Blair, J.A.; Kearney, K.E.; et al. Comprehensive Management of ANOCA, Part 1-Definition, Patient Population, and Diagnosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 82, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- Kardos, A.; Gaibazzi, N. Perivascular inflammation and the coronary microcirculation and vasoreactivity—A potential clue to ANOCA. Eur. J. Prev. Cardiol. 2024, 32, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Sakai, K.; Mizukami, T.; Ohashi, H.; Bouisset, F.; Caglioni, S.; van Hoe, L.; Gallinoro, E.; Bertolone, D.T.; Pardaens, S.; et al. Vascular Remodeling in Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2024, 17, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Boerhout, C.; de Waard, G.A.; Lee, J.M.; Mejia-Renteria, H.; Lee, S.H.; Jung, J.H.; Hoshino, M.; Echavarria-Pinto, M.; Meuwissen, M.; Matsuo, H.; et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease; from the multicentre international ILIAS registry. EuroIntervention 2022, 18, 719–728. [Google Scholar] [CrossRef]
- Ferrari, R.; Fox, K. Heart rate reduction in coronary artery disease and heart failure. Nat. Rev. Cardiol. 2016, 13, 493–501. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021, 16, 1049–1069. [Google Scholar]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- Eapen, D.J.; Manocha, P.; Patel, R.S.; Hammadah, M.; Veledar, E.; Wassel, C.; Nanjundappa, R.A.; Sikora, S.; Malayter, D.; Wilson, P.W.; et al. Aggregate risk score based on markers of inflammation, cell stress, and coagulation is an independent predictor of adverse cardiovascular outcomes. J. Am. Coll. Cardiol. 2013, 62, 329–337. [Google Scholar] [CrossRef]
- Jansen, T.; de Vos, A.; Paradies, V.; Damman, P.; Teerenstra, S.; Konst, R.E.; Dimitriu-Leen, A.; Maas, A.H.; Smits, P.C.; Elias-Smale, S.E.; et al. Absolute Flow and Resistance Have Superior Repeatability as Compared to CFR and IMR: EDIT-CMD Substudy. JACC Cardiovasc. Interv. 2023, 16, 872–874. [Google Scholar] [CrossRef]
- Ng, M.K.; Yeung, A.C.; Fearon, W.F. Invasive assessment of the coronary microcirculation: Superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation 2006, 113, 2054–2061. [Google Scholar] [CrossRef]
- Boerhout, C.; Lee, J.M.; A de Waard, G.; Mejia-Renteria, H.; Lee, S.H.; Jung, J.-H.; Hoshino, M.; Echavarria-Pinto, M.; Meuwissen, M.; Matsuo, H.; et al. Microvascular resistance reserve: Diagnostic and prognostic performance in the ILIAS registry. Eur. Heart J. 2023, 44, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Gallinoro, E.; Bertolone, D.T.; Mizukami, T.; Paolisso, P.; Bermpeis, K.; Munhoz, D.; Sakai, K.; Seki, R.; Ohashi, H.; Esposito, G.; et al. Continuous vs Bolus Thermodilution to Assess Microvascular Resistance Reserve. JACC Cardiovasc. Interv. 2023, 16, 2767–2777. [Google Scholar] [CrossRef] [PubMed]
- de Vos, A.; Jansen, T.P.; van’t Veer, M.; Dimitriu-Leen, A.; Konst, R.E.; Elias-Smale, S.; Paradies, V.; Rodwell, L.; van den Oord, S.; Smits, P.; et al. Microvascular Resistance Reserve to Assess Microvascular Dysfunction in ANOCA Patients. JACC Cardiovasc. Interv. 2023, 16, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; Weiner, A.; Amit, I. The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell 2020, 181, 1207–1217. [Google Scholar] [CrossRef]
- Colonna, M. The biology of TREM receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, L.; Yang, J.; Meng, L.; Chen, J.; Zhou, L.; Wang, J.; Xiong, M.; Zhang, Z. Soluble TREM2 ameliorates tau phosphorylation and cognitive deficits through activating transgelin-2 in Alzheimer’s disease. Nat. Commun. 2023, 14, 6670. [Google Scholar] [CrossRef]
- Deming, Y.; Filipello, F.; Cignarella, F.; Cantoni, C.; Hsu, S.; Mikesell, R.; Li, Z.; Del-Aguila, J.L.; Dube, U.; Farias, F.G.; et al. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 2019, 11, eaau2291. [Google Scholar] [CrossRef]
- Liu, W.; Weng, S.; Liu, H.; Cao, C.; Wang, S.; Wu, S.; He, J.; Yang, Y.; Hu, D.; Luo, Y.; et al. Serum soluble TREM2 is an independent biomarker associated with coronary heart disease. Clin. Chim. Acta 2023, 548, 117499. [Google Scholar] [CrossRef]
- Smart, C.D.; Fehrenbach, D.J.; Wassenaar, J.W.; Agrawal, V.; Fortune, N.L.; Dixon, D.D.; A Cottam, M.; Hasty, A.H.; Hemnes, A.R.; Doran, A.C.; et al. Immune profiling of murine cardiac leukocytes identifies triggering receptor expressed on myeloid cells 2 as a novel mediator of hypertensive heart failure. Cardiovasc. Res. 2023, 119, 2312–2328. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Y.; Zhang, Q.; Fang, C.; Bao, A.; Dong, W.; Peng, Y.; Peng, H.; Ju, Z.; He, J.; et al. Soluble TREM2 is associated with death and cardiovascular events after acute ischemic stroke: An observational study from CATIS. J. Neuroinflammation 2022, 19, 88. [Google Scholar] [CrossRef]
- Cuciuc, V.; Tshori, S.; Grib, L.; Sella, G.; Tuvali, O.; Volodarsky, I.; Welt, M.; Fassler, M.; Shimoni, S.; George, J. Circulating Soluble TREM2 and Cardiovascular Outcome in Cohort Study of Coronary Atherosclerosis Patients. Int. J. Mol. Sci. 2022, 23, 13121. [Google Scholar] [CrossRef] [PubMed]
- Akhiyat, N.; Ozcan, I.; Gulati, R.; Prasad, A.; Tchkonia, T.; Kirkland, J.L.; Lewis, B.; Lerman, L.O.; Lerman, A. Patients With Coronary Microvascular Dysfunction Have Less Circulating α-Klotho. J. Am. Heart Assoc. 2024, 13, e031972. [Google Scholar] [CrossRef] [PubMed]
- Shetrit, A.; Freund, O.; Banai, A.; Shamir, R.A.; Avivi, I.; Zornitzki, L.; Ben-Shoshan, J.; Szekely, Y.; Arbel, Y.; Halkin, A.; et al. Coronary microvascular dysfunction in patients with Takotsubo syndrome. Heart Lung 2024, 68, 46–51. [Google Scholar] [CrossRef]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.O.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel index for invasively assessing the coronary microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Toya, T.; Ahmad, A.; Corban, M.T.; Özcan, I.; Sara, J.D.; Sebaali, F.; Escaned, J.; Lerman, L.O.; Lerman, A. Risk Stratification of Patients With NonObstructive Coronary Artery Disease Using Resistive Reserve Ratio. J. Am. Heart Assoc. 2021, 10, e020464. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Smilowitz, N.R.; Toleva, O.; Chieffo, A.; Perera, D.; Berry, C. Coronary Microvascular Disease in Contemporary Clinical Practice. Circ. Cardiovasc. Interv. 2023, 16, e012568. [Google Scholar] [CrossRef]
- Mangiacapra, F.; Viscusi, M.M.; Verolino, G.; Paolucci, L.; Nusca, A.; Melfi, R.; Ussia, G.P.; Grigioni, F. Invasive Assessment of Coronary Microvascular Function. J. Clin. Med. 2021, 11, 228. [Google Scholar] [CrossRef]
- Ciaramella, L.; Di Serafino, L.; Mitrano, L.; De Rosa, M.L.; Carbone, C.; Rea, F.S.; Monaco, S.; Scalamogna, M.; Cirillo, P.; Esposito, G. Invasive Assessment of Coronary Microcirculation: A State-of-the-Art Review. Diagnostics 2023, 14, 86. [Google Scholar] [CrossRef]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Lipscomb, K.; Hamilton, G.W. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am. J. Cardiol. 1974, 33, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.J.; Lerman, A.; Bech, J.W.; De Bruyne, B.; Eeckhout, E.; Fearon, W.F.; Higano, S.T.; Lim, M.J.; Meuwissen, M.; Piek, J.J.; et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 2006, 114, 1321–1341. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Morita, K.; Naya, M.; Katoh, C.; Inubushi, M.; Kuge, Y.; Tsutsui, H.; Tamaki, N. Myocardial flow reserve is influenced by both coronary artery stenosis severity and coronary risk factors in patients with suspected coronary artery disease. Eur. J. Nucl. Med. Mol. Imaging. 2006, 33, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A. New tools for coronary risk assessment: What are their advantages and limitations? Circulation 2002, 105, 886–892. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cochain, C.; Vafadarnejad, E.; Arampatzi, P.; Pelisek, J.; Winkels, H.; Ley, K.; Wolf, D.; Saliba, A.-E.; Zernecke, A. Single-Cell RNA-Seq Reveals the Transcriptional Landscape and Heterogeneity of Aortic Macrophages in Murine Atherosclerosis. Circ. Res. 2018, 122, 1661–1674. [Google Scholar] [CrossRef]
- Kothari, V.; Savard, C.; Tang, J.; Lee, S.P.; Subramanian, S.; Wang, S.; Hartigh, L.J.D.; Bornfeldt, K.E.; Ioannou, G.N. sTREM2 is a plasma biomarker for human NASH and promotes hepatocyte lipid accumulation. Hepatol. Commun. 2023, 7, e0265. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Xu, W.; Li, Z.; Chen, X.; Luo, Q.; Wang, D.; Peng, L. Baicalein upregulates macrophage TREM2 expression via TrKB-CREB1 pathway to attenuate acute inflammatory injury in acute-on-chronic liver failure. Int. Immunopharmacol. 2024, 139, 112685. [Google Scholar] [CrossRef]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef]

| Variables | Non-CMD (n = 21) | CMD (n = 55) | p-Value |
|---|---|---|---|
| Age, y | 62.9 ± 10.8 | 64.8 ± 11.9 | 0.533 |
| Female Gender, n (%) | 11 (52.4) | 30 (54.5) | 0.866 |
| BMI, kg/m2 | 25.87 ± 3.63 | 25.61 ± 3.18 | 0.770 |
| Current smoking, n (%) | 3 (14.3) | 14 (25.5) | 0.296 |
| Diabetes, n (%) | 5 (23.8) | 19 (34.5) | 0.368 |
| Hypertension, n (%) | 13 (61.9) | 35 (63.6) | 0.889 |
| Hyperlipidemia, n (%) | 19 (90.5) | 41 (75.9) | 0.157 |
| Chronic kidney disease, n (%) | 1 (4.8) | 1 (1.8) | 0.473 |
| Chronic heart failure, n (%) | 1 (4.8) | 1 (1.8) | 0.534 |
| Atrial fibrillation, n (%) | 0 (0) | 2 (3.6) | 0.822 |
| Troponin T, ng/mL | 0.01 ± 0.004 | 0.01 ± 0.012 | 0.261 |
| NT-proBNP, pg/mL | 201.15 (32.0, 170.7) | 133.23 (31.7, 131.0) | 0.369 |
| ALT, IU/L | 29.75 (15.0, 37.0) | 24.35 (16.0, 29.0) | 0.225 |
| Fasting blood glucose, mmol/L | 6.15 ± 1.68 | 6.94 ± 2.24 | 0.149 |
| HbA1c, % | 6.08 ± 0.81 | 6.40 ± 1.04 | 0.228 |
| Total cholesterol, mmol/L | 4.17 ± 0.92 | 3.95 ± 0.93 | 0.354 |
| Triglyceride, mmol/L | 1.37 ± 0.71 | 1.47 ± 0.72 | 0.559 |
| LDL-C, mmol/L | 2.52 ± 0.72 | 2.36 ± 0.72 | 0.401 |
| HDL-C, mmol/L | 1.32 ± 0.27 | 1.26 ± 0.26 | 0.392 |
| Creatinine, μmol/L | 66.56 (57.3, 76.8) | 74.11 (50.9, 78.3) | 0.659 |
| eGFR, mL/min/1.73 m2 | 91.9 (85.7, 104.0) | 93.4 (85.4, 101.3) | 0.722 |
| FFR | 0.89 ± 0.03 | 0.90 ± 0.04 | 0.084 |
| Variable | CMD | |
|---|---|---|
| OR (95%CI) | p-Value | |
| Age | 1.014 (0.971–1.059) | 0.527 |
| Gender | 1.091(0.398–2.988) | 0.866 |
| BMI | 0.977 (0.839–1.138) | 0.767 |
| Smoking | 1.319 (0.328–5.298) | 0.696 |
| LDL-C | 0.744 (0.375–1.476) | 0.397 |
| HbA1c | 1.464 (0.786–2.729) | 0.230 |
| eGFR | 1.006 (0.975–1.037) | 0.718 |
| sTREM2 | 1.004 (1.001–1.006) | 0.003 |
| Variable | CMD | |
|---|---|---|
| OR (95% CI) | p-Value | |
| sTREM2 | 1.003 (1.001–1.007) | 0.008 |
| Age | 0.985 (0.871–1.114) | 0.527 |
| Gender | 4.324 (0.501–37.312) | 0.183 |
| BMI | 1.023 (0.770–1.047) | 0.770 |
| Smoking | 6.163 (0.590–64.374) | 0.129 |
| LDL-C | 0.436 (0.148–1.284) | 0.132 |
| HbA1c | 2.553 (0.418–15.612) | 0.310 |
| eGFR | 0.990 (0.913–1.073) | 0.803 |
| Diabetes | 0.301 (0.013–7.204) | 0.459 |
| Hypertension | 1.163 (0.163–8.296) | 0.670 |
| Hyperlipidemia | 0.223 (0.014–3.646) | 0.293 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Sheng, Z.; He, H.; Li, Y.; Chen, Q.; Gao, Y.; Zheng, J. Single-Center Analysis of Soluble TREM2 as a Biomarker in Coronary Microvascular Dysfunction: A Cross-Sectional Study. J. Clin. Med. 2025, 14, 1816. https://doi.org/10.3390/jcm14061816
Xie Y, Sheng Z, He H, Li Y, Chen Q, Gao Y, Zheng J. Single-Center Analysis of Soluble TREM2 as a Biomarker in Coronary Microvascular Dysfunction: A Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(6):1816. https://doi.org/10.3390/jcm14061816
Chicago/Turabian StyleXie, Yingying, Zhaoxue Sheng, Haoming He, Yike Li, Qiang Chen, Yanxiang Gao, and Jingang Zheng. 2025. "Single-Center Analysis of Soluble TREM2 as a Biomarker in Coronary Microvascular Dysfunction: A Cross-Sectional Study" Journal of Clinical Medicine 14, no. 6: 1816. https://doi.org/10.3390/jcm14061816
APA StyleXie, Y., Sheng, Z., He, H., Li, Y., Chen, Q., Gao, Y., & Zheng, J. (2025). Single-Center Analysis of Soluble TREM2 as a Biomarker in Coronary Microvascular Dysfunction: A Cross-Sectional Study. Journal of Clinical Medicine, 14(6), 1816. https://doi.org/10.3390/jcm14061816






