Low-Frequency Electrical Stimulation of the Auricular Branch of the Vagus Nerve in Patients with ST-Elevation Myocardial Infarction: A Randomized Clinical Trial
Abstract
:1. Introduction
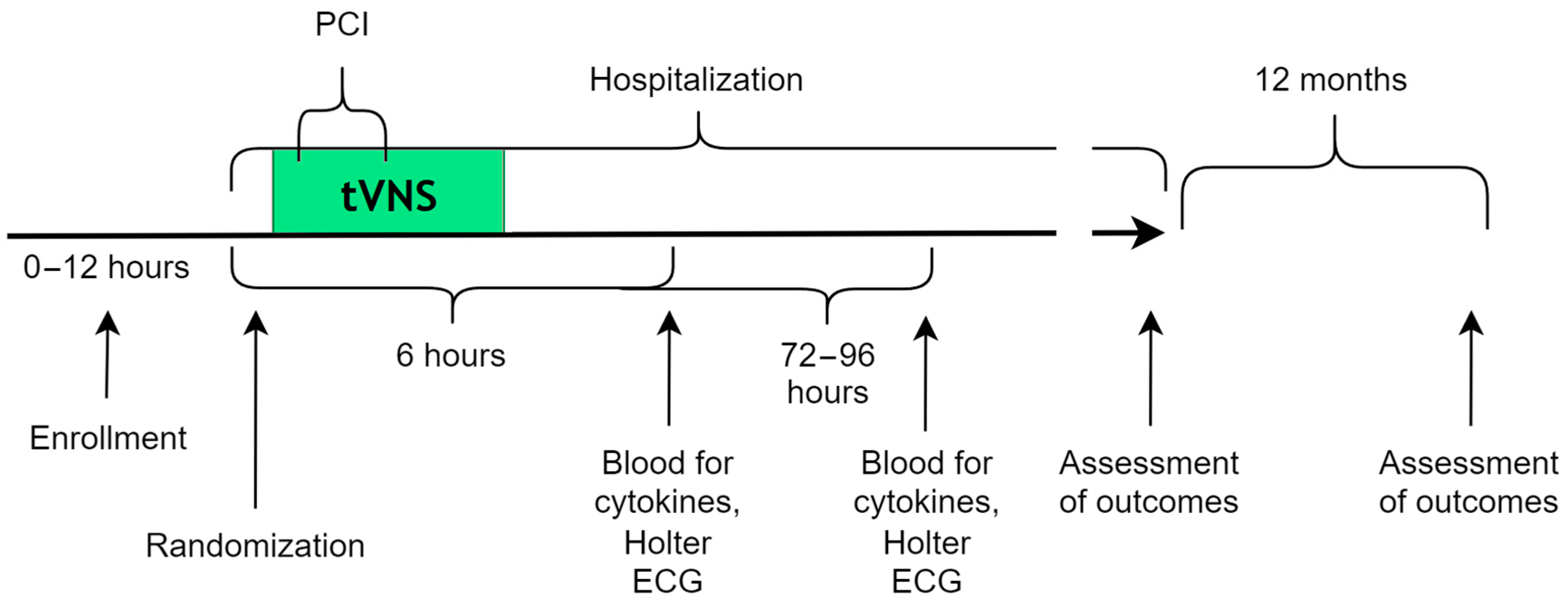
2. Materials and Method
2.1. Study Design
2.2. Inclusion Criteria
- Patients aged 40 to 90 years (adults and seniors);
- Patients afflicted with primary STEMI;
- Patients who had been treated in the first 12 h from the onset of pain;
- Patients who had undergone a primary percutaneous coronary intervention (PCI);
- Patients who had signed a voluntary informed consent form to participate in this study.
2.3. Non-Inclusion Criteria
- A history of MI;
- Acute heart failure (grades III–IV according to NYHA);
- Bradyarrhythmia;
- Atrial fibrillation/flutter at the time of physical examination;
- Thrombolytic therapy at the prehospital stage;
- PCI/coronary artery bypass grafting (CABG) in the anamnesis;
- Participation in another clinical trial as a patient.
2.4. Exclusion Criteria
- PCI cancellation or a failed PCI;
- Emergency change of PCI to CABG.
2.5. Randomization
2.6. Intervention (tVNS)
2.7. Sample Size Determination
2.8. Statistical Analysis
2.9. Endpoints: Primary Outcome Measure
2.10. Endpoints: Secondary Outcome Measure
2.11. Monitoring
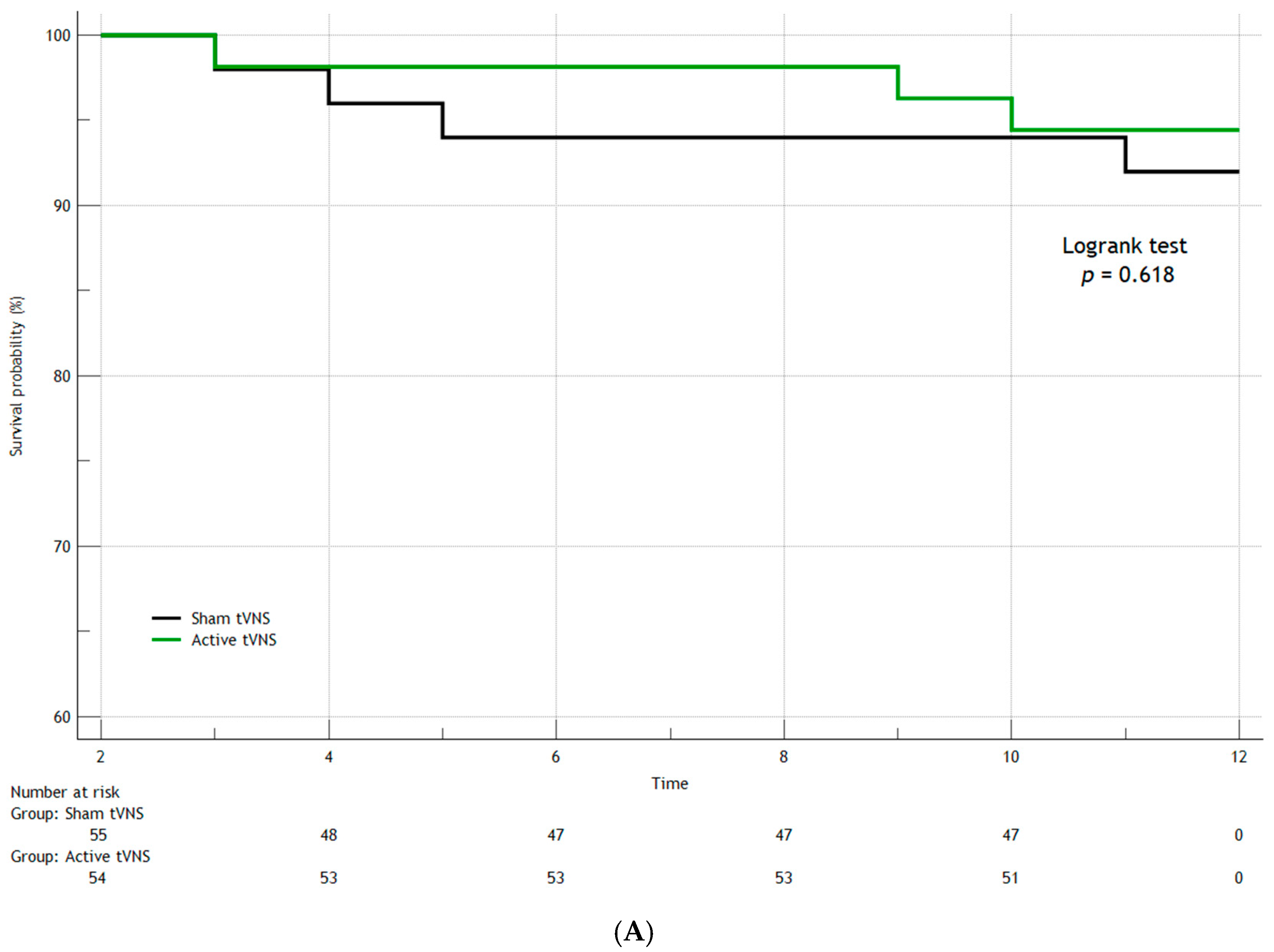
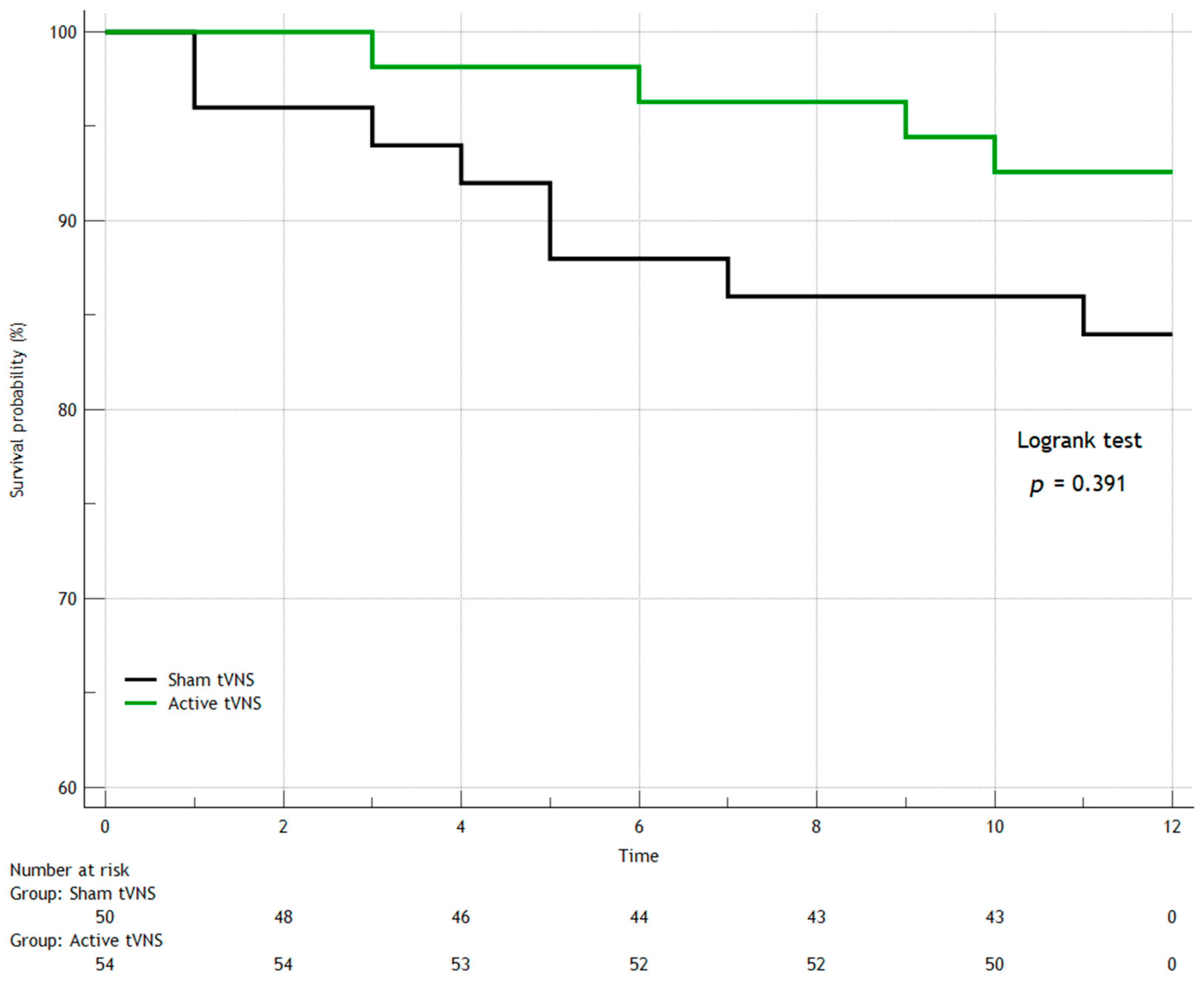
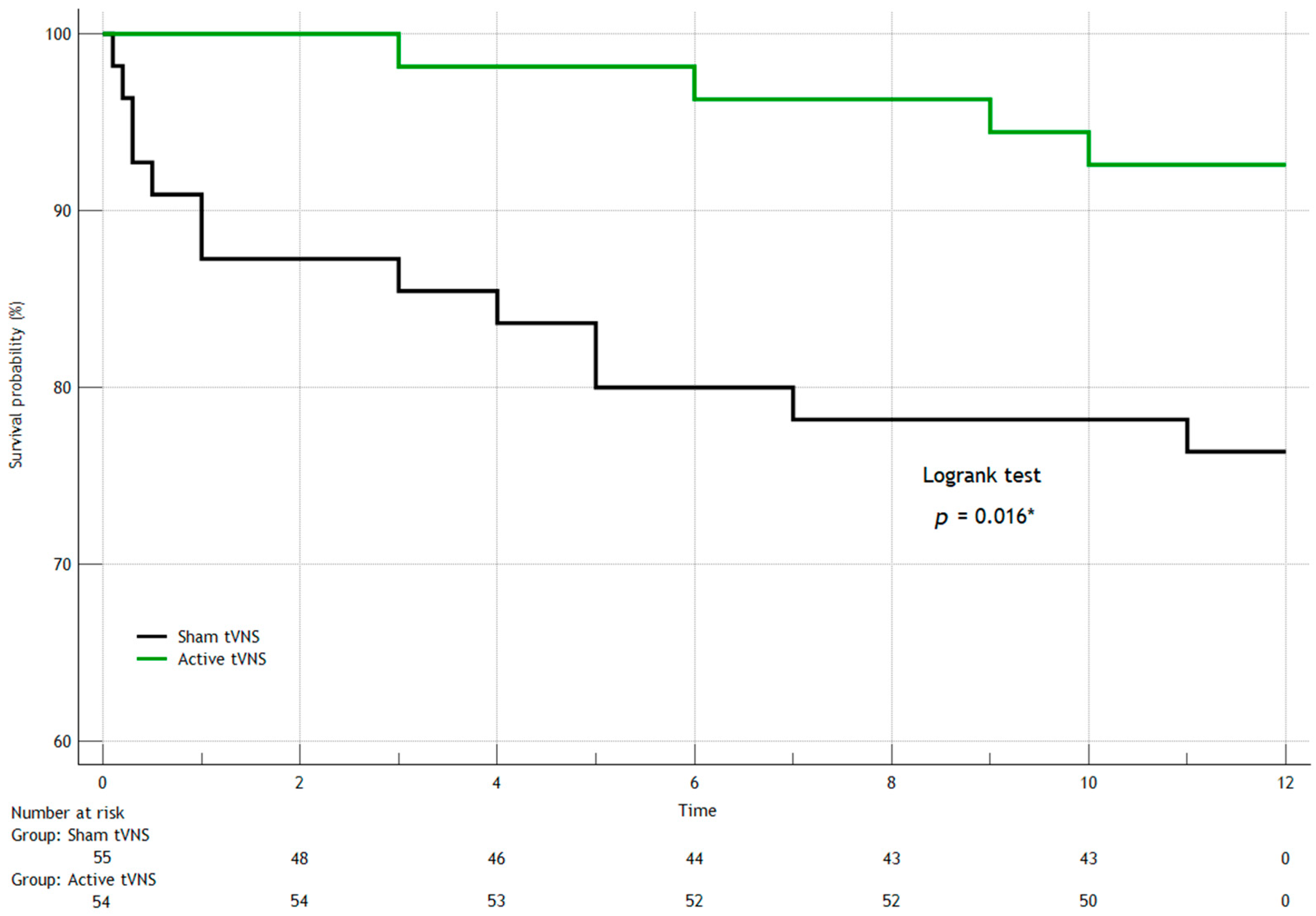
3. Results
3.1. Hospital Data
3.2. Adverse Events During 12-Month Monitoring
4. Discussion
Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braunwald, E. Cardiovascular science: Opportunities for translating research into improved care. J. Clin. Investig. 2013, 123, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Austelle, C.W.; O’Leary, G.H.; Thompson, S.; Gruber, E.; Kahn, A.; Manett, A.J.; Short, B.; Badran, B.W. A Comprehensive Review of Vagus Nerve Stimulation for Depression. Neuromodul. J. Int. Neuromodul. Soc. 2022, 25, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Efendieva, A.S.; Bulaeva, N.I.; Golukhova, E.Z. Evolution in treatment of ST-segment elevation myocardial infarction. Creat. Cardiol. 2023, 17, 203–216. [Google Scholar]
- Fetisova, V.I.; Namitokov, A.M.; Gilevich, I.V.; Kosmacheva, E.D. Soluble tumorigenicity suppression protein (sST2) as a possible biomarker in patients with acute coronary syndrome. South Russ. J. Ther. Pract. 2023, 4, 7–17. [Google Scholar] [CrossRef]
- Yellon, D.M.; Baxter, G.F. Protecting the ischaemic and reperfused myocardium in acute myocardial infarction: Distant dream or near reality? Heart (Br. Card. Soc.) 2000, 83, 381–387. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp. Ther. Med. 2022, 23, 430. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- Simon, T.; Kirk, J.; Dolezalova, N.; Guyot, M.; Panzolini, C.; Bondue, A.; Lavergne, J.; Hugues, S.; Hypolite, N.; Saeb-Parsy, K.; et al. The cholinergic anti-inflammatory pathway inhibits inflammation without lymphocyte relay. Front. Neurosci. 2023, 17, 1125492. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef]
- Le Maître, E.; Revathikumar, P.; Estelius, J.; Lampa, J. Increased Recovery Time and Decreased LPS Administration to Study the Vagus Nerve Stimulation Mechanisms in Limited Inflammatory Responses. J. Vis. Exp. JoVE 2017, 121, 54890. [Google Scholar]
- Jiang, Y.; Li, L.; Liu, B.; Zhang, Y.; Chen, Q.; Li, C. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS ONE 2014, 9, e102342. [Google Scholar] [CrossRef]
- Lu, X.X.; Hong, Z.Q.; Tan, Z.; Sui, M.H.; Zhuang, Z.Q.; Liu, H.H.; Zheng, X.Y.; Yan, T.B.; Geng, D.F.; Jin, D.M. Nicotinic Acetylcholine Receptor Alpha7 Subunit Mediates Vagus Nerve Stimulation-Induced Neuroprotection in Acute Permanent Cerebral Ischemia by a7nAchR/JAK2 Pathway. Med. Sci. Monit. 2017, 23, 6072–6081. [Google Scholar] [CrossRef] [PubMed]
- Calvillo, L.; Vanoli, E.; Andreoli, E.; Besana, A.; Omodeo, E.; Gnecchi, M.; Zerbi, P.; Vago, G.; Busca, G.; Schwartz, P.J. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J. Cardiovasc. Pharmacol. 2011, 58, 500–507. [Google Scholar] [CrossRef]
- Giannino, G.; Nocera, L.; Andolfatto, M.; Braia, V.; Giacobbe, F.; Bruno, F.; Saglietto, A.; Angelini, F.; De Filippo, O.; D’Ascenzo, F.; et al. Vagal nerve stimulation in myocardial ischemia/reperfusion injury: From bench to bedside. Bioelectron. Med. 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Hadaya, J.; Dajani, A.H.; Cha, S.; Hanna, P.; Challita, R.; Hoover, D.B.; Ajijola, O.A.; Shivkumar, K.; Ardell, J.L. Vagal Nerve Stimulation Reduces Ventricular Arrhythmias and Mitigates Adverse Neural Cardiac Remodeling Post-Myocardial Infarction. JACC Basic Transl. Sci. 2023, 8, 1100–1118. [Google Scholar] [CrossRef]
- Yu, L.; Huang, B.; Po, S.S.; Tan, T.; Wang, M.; Zhou, L.; Meng, G.; Yuan, S.; Zhou, X.; Li, X.; et al. Low-Level Tragus Stimulation for the Treatment of Ischemia and Reperfusion Injury in Patients With ST-Segment Elevation Myocardial Infarction: A Proof-of-Concept Study. JACC Cardiovasc. Interv. 2017, 10, 1511–1520. [Google Scholar] [CrossRef]
- Lehr, R. Sixteen S-squared over D-squared: A relation for crude sample size estimates. Stat. Med. 1992, 11, 1099–1102. [Google Scholar] [CrossRef]
- Capilupi, M.J.; Kerath, S.M.; Becker, L.B. Vagus Nerve Stimulation and the Cardiovascular System. Cold Spring Harb. Perspect. Med. 2020, 10, a034173. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, L.; Tan, X.; Liu, B.; Zhang, Y.; Li, C. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J. Neurochem. 2015, 134, 173–181. [Google Scholar] [CrossRef]
- Dusi, V.; Angelini, F.; De Ferrari, G.M. Vagus Nerve Stimulation for Myocardial Ischemia: The Sooner the Better. JACC Basic Transl. Sci. 2023, 8, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Strzelczyk, A.; Finisguerra, A.; Gourine, A.V.; Gharabaghi, A.; Hasan, A.; Burger, A.M.; Jaramillo, A.M.; Mertens, A.; Majid, A.; et al. International Consensus Based Review and Recommendations for Minimum Reporting Standards in Research on Transcutaneous Vagus Nerve Stimulation (Version 2020). Front. Hum. Neurosci. 2021, 14, 568051. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Active tVNS (n = 54) | Sham tVNS (n = 55) | p |
|---|---|---|---|
| Clinical parameters of patients | |||
| Male, n (%) | 34 (63) | 37 (67) | 0.641 |
| Age, yrs | 67 (60; 72) | 65 (55; 71) | 0.117 |
| Weight, kg | 80 (70; 96) | 85 (75; 90) | 0.954 |
| Height, cm | 172 (166; 175) | 173 (164; 178) | 0.785 |
| Angina pectoris, n (%) | 15 (28) | 15 (27) | 0.956 |
| Hypertension, n (%) | 51 (94) | 50 (91) | 0.485 |
| Diabetes, n (%) | 9 (17) | 18 (32) | 0.054 |
| Stroke, n (%) | 1 (1.8) | 2 (3.6) | 0.578 |
| PAD, n (%) | 4 (7) | 1 (1.8) | 0.168 |
| COPD, n (%) | 0 (0) | 0 (0) | - |
| Smoking, n (%) | 11 (20) | 12 (22) | 0.857 |
| Hospital admission data | |||
| Pain-to-admission time, min | 90 (60; 120) | 80 (60; 120) | 0.639 |
| Door-to-balloon time, min | 20 (15; 25) | 20 (15; 25) | 0.399 |
| tVNS-to-balloon time, min | 10 (8; 12) | 10 (8; 15) | 0.722 |
| Total duration of tVNS, min | 70 (65; 75) | 65 (60; 75) | 0.114 |
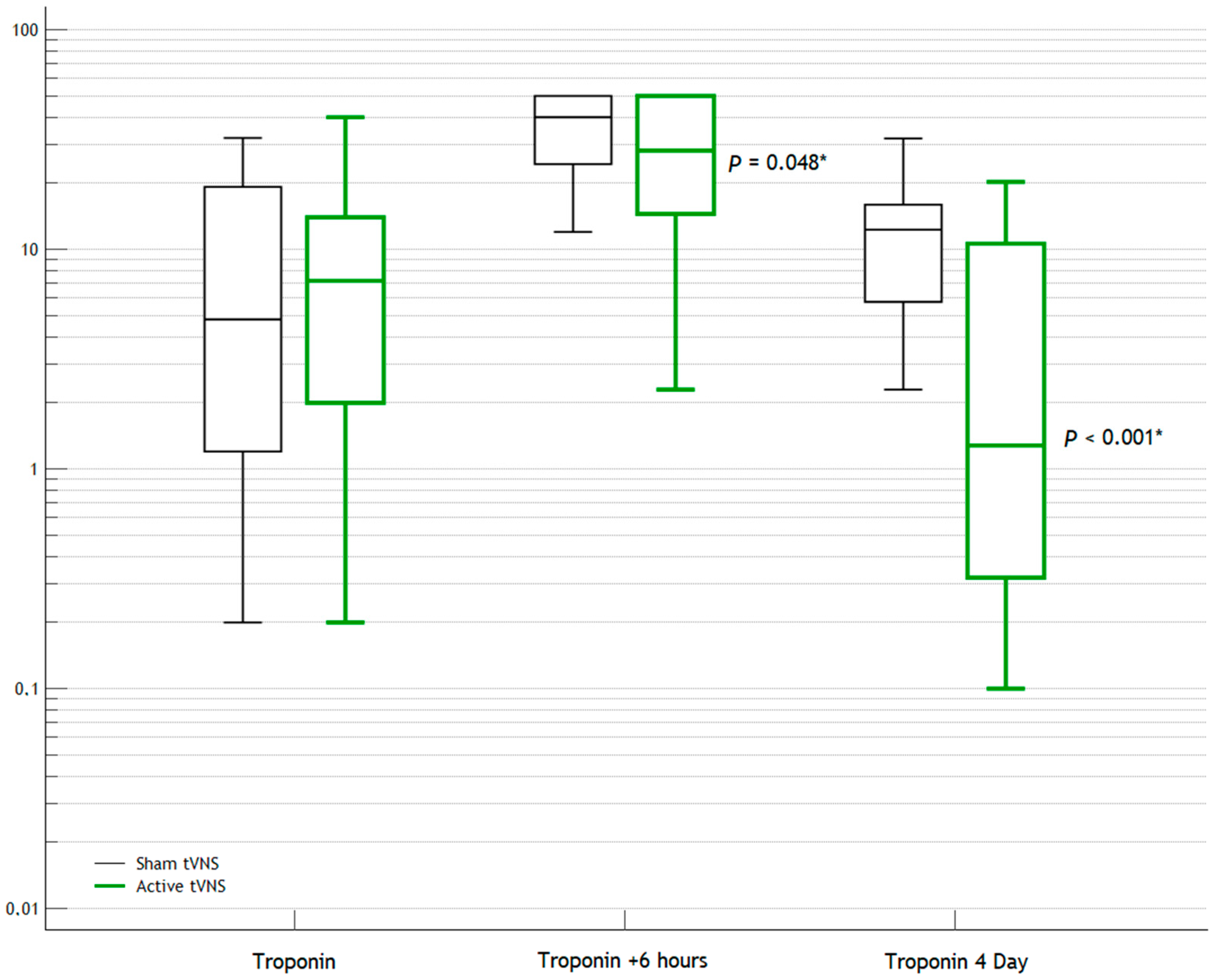
| Troponin | 6.2 (2; 14) | 4.8 (1.2; 20) | 0.813 |
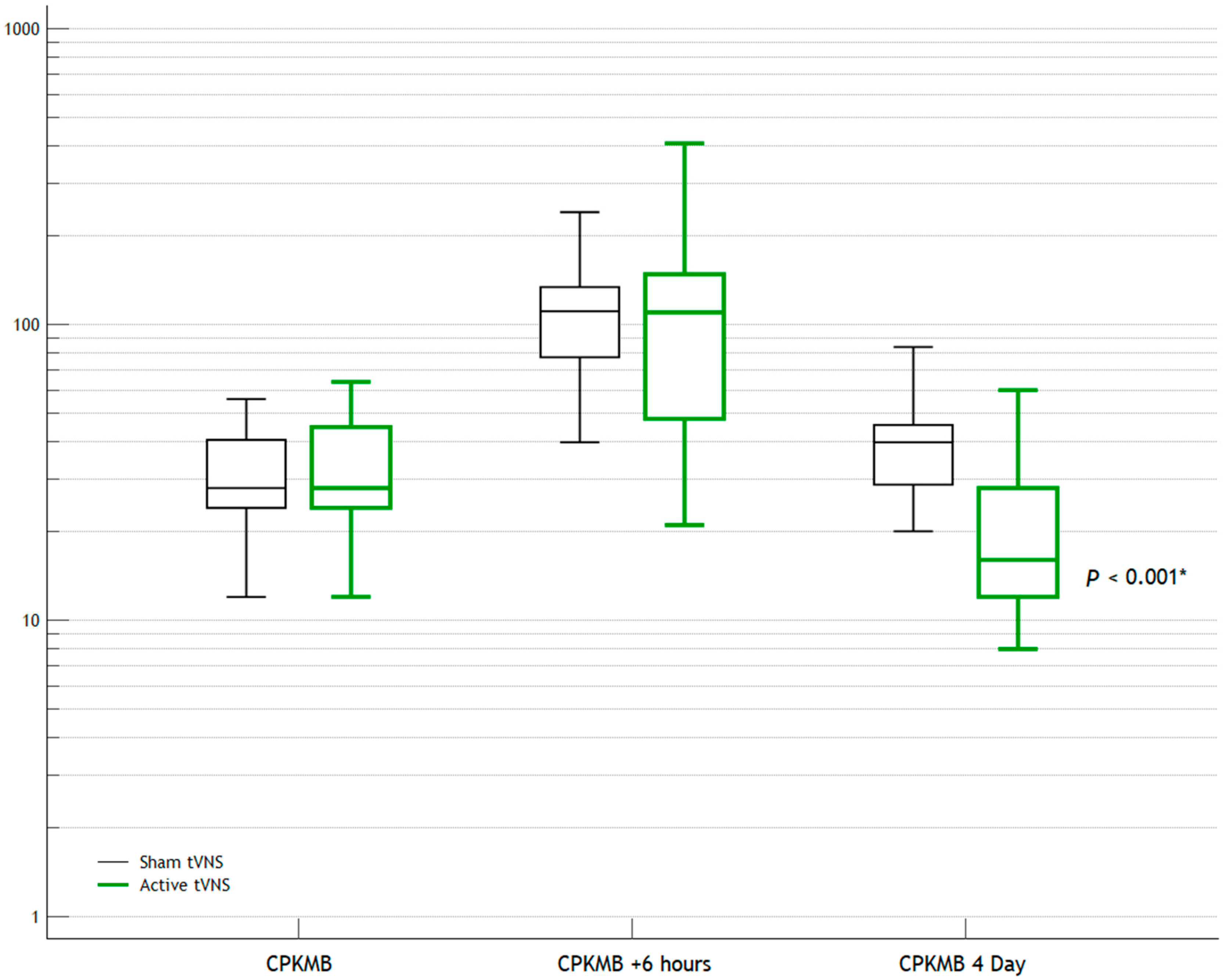
| CPK-MB | 28 (24; 45) | 28 (24; 41) | 0.958 |
| hs-CRP | 22 (14; 22) | 18 (12; 24) | 0.072 |
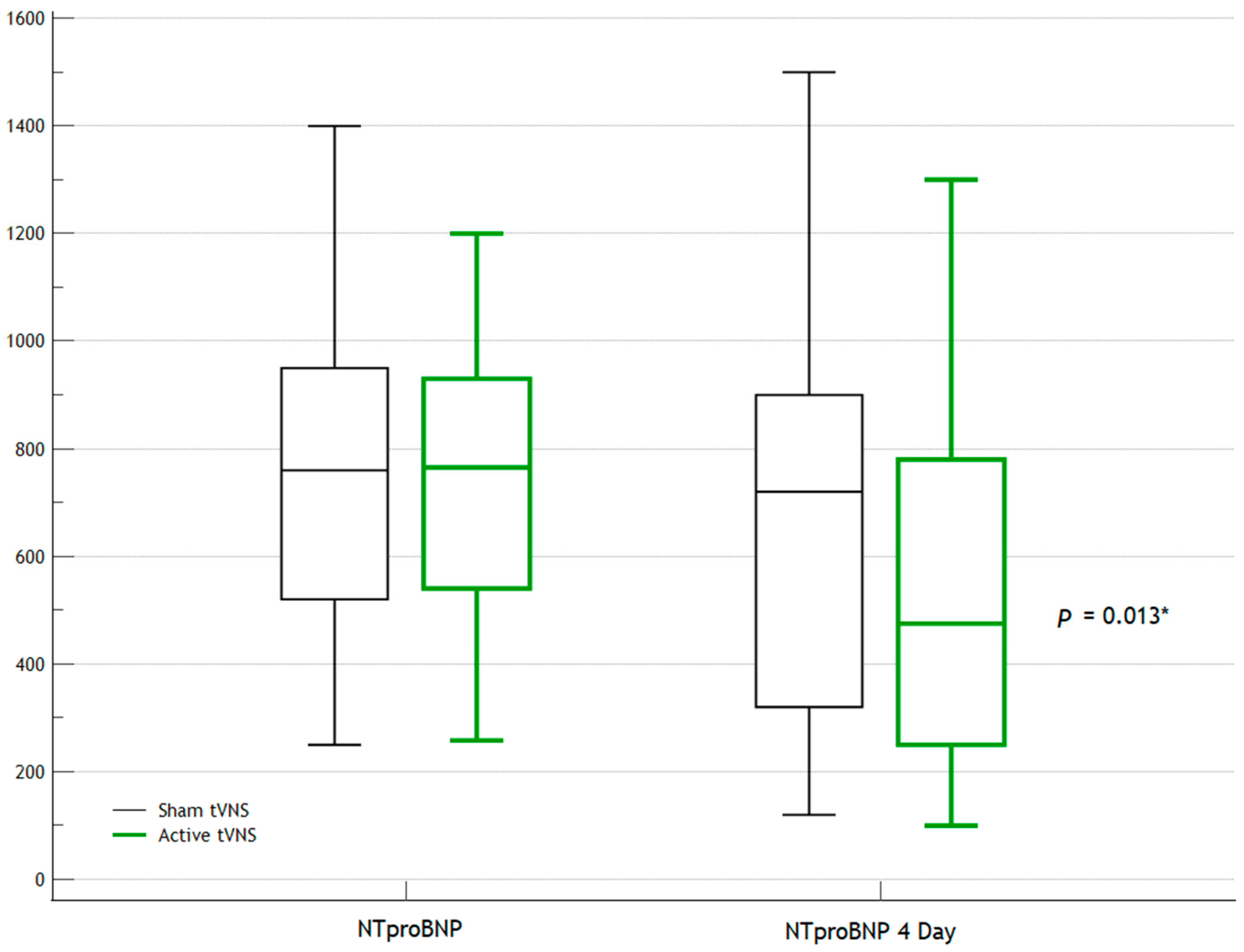
| NT-proBNP | 765 (540; 930) | 760 (520; 950) | 0.978 |
| ST2 | 60 (40; 124) | 64 (28; 118) | 0.750 |
| WBC | 7.2 (7.2; 10.2) | 9.2 (6.3; 12.2) | 0.624 |
| HR | 75 (65; 80) | 80 (70; 85) | 0.289 |
| Operating room data | |||
| LDA, n (%) | 28 (52) | 22 (40) | 0.218 |
| CA/OMA, n (%) | 13 (24) | 21 (38) | 0.114 |
| Right coronary artery, n (%) | 13 (24) | 15 (27) | 0.706 |
| Main left coronary artery, n (%) | 1 (1.8) | 1 (1.8) | 1.000 |
| Stenting of 2 arteries, n (%) | 1 (1.8) | 4 (7) | 0.181 |
| Parameters | Active tVNS (n = 54) | Sham tVNS (n = 55) | p |
|---|---|---|---|
| Troponin (+6 h) | 28.2 (14.5; 50) | 40 (24; 50) | 0.048 * |
| CPK-MB (+6 h) | 110 (48; 148) | 111 (78; 134) | 0.392 |
| Hospital admission data | |||
| LVEF, % | 42 (35; 47) | 45 (38; 48) | 0.317 |
| LA, mm | 37 (35; 38) | 38 (37; 39) | 0.402 |
| IVS, mm | 10 (9; 10) | 10 (9; 11) | 0.269 |
| Posterior wall, mm | 9 (8; 11) | 10 (9; 11) | 0.089 |
| LVEDD, mm | 45 (42; 46) | 45 (45; 52) | 0.587 |
| Holter | |||
| VE events | 19 (10; 23) | 120 (2; 630) | 0.110 |
| PVE events | 0 (0; 0) | 0 (0; 40) | 0.002 * |
| VT | 0 (0; 0) | 0 (0; 0) | - |
| VF, n (%) | 0 (0) | 1 (1.8) | 0.330 |
| Daytime HR | 78 (74; 85) | 86 (84; 92) | <0.001 * |
| Nighttime HR | 68 (63; 72) | 75 (70; 80) | <0.001 * |
| Parameters | Active tVNS (n = 54) | Sham tVNS (n = 55) | p |
|---|---|---|---|
| Day 3 | |||
| hs-CRP | 10 (10; 14) | 15 (8; 20) | 0.093 |
| Day 4 | |||
| NT-proBNP | 475 (250; 780) | 720 (320; 900) | 0.013 * |
| Troponin | 1.3 (0.32; 10.2) | 12.3 (5.6; 16) | <0.001 * |
| CPK-MB | 16 (12; 28) | 40 (29; 45) | <0.001 * |
| LVEF | 46 (40; 50) | 48 (42; 50) | 0.300 |
| VE events | 0 (0; 0) | 12 (0; 0) | <0.001 * |
| PVE events | 0 (0; 0) | 0 (0; 2) | 0.029 * |
| VT | 0 (0; 0) | 0 (0; 0) | - |
| VF, n (%) | 0 (0) | 1 (1.8) | 0.330 |
| Daytime HR | 72 (70; 76) | 82 (58; 94) | 0.179 |
| Nighttime HR | 60 (58; 65) | 67 (50; 86) | 0.596 |
| Hospital Events | Active tVNS (n = 54) | Sham tVNS (n = 55) | p |
|---|---|---|---|
| In-hospital mortality, n (%) | 0 (0) | 5 (9) | 0.024 * |
| Pulmonary edema, n (%) | 2 (3.7) | 6 (11) | 0.153 |
| Cardiogenic shock, n (%) | 3 (5.6) | 10 (18) | 0.044 * |
| AF, n (%) | 2 (3.7) | 6 (11) | 0.153 |
| VT, n (%) | 3 (5.6) | 4 (7) | 0.721 |
| VF, n (%) | 0 (0) | 2 (3.6) | 0.163 |
| AIVR, n (%) | 0 (0) | 0 (0) | - |
| AV block 2, n (%) | 1 (1.8) | 4 (7) | 0.181 |
| AV block 3, n (%) | 0 (0) | 6 (11) | 0.013 * |
| Stroke/TIA, n (%) | 0 (0) | 1 (1.8) | 0.330 |
| Pacemaker implantation, n (%) | 0 (0) | 3 (5.5) | 0.085 |
| Electric cardioversion, n (%) | 0 (0) | 2 (3.6) | 0.163 |
| In-hospital mortality, n (%) | 0 (0) | 5 (9) | 0.024 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruchinova, S.; Gendugova, M.; Namitokov, A.; Sokolskaya, M.; Gilevich, I.; Tatarintseva, Z.; Karibova, M.; Danilov, V.; Simakin, N.; Shvartz, E.; et al. Low-Frequency Electrical Stimulation of the Auricular Branch of the Vagus Nerve in Patients with ST-Elevation Myocardial Infarction: A Randomized Clinical Trial. J. Clin. Med. 2025, 14, 1866. https://doi.org/10.3390/jcm14061866
Kruchinova S, Gendugova M, Namitokov A, Sokolskaya M, Gilevich I, Tatarintseva Z, Karibova M, Danilov V, Simakin N, Shvartz E, et al. Low-Frequency Electrical Stimulation of the Auricular Branch of the Vagus Nerve in Patients with ST-Elevation Myocardial Infarction: A Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(6):1866. https://doi.org/10.3390/jcm14061866
Chicago/Turabian StyleKruchinova, Sofia, Milana Gendugova, Alim Namitokov, Maria Sokolskaya, Irina Gilevich, Zoya Tatarintseva, Maria Karibova, Vasiliy Danilov, Nikita Simakin, Elena Shvartz, and et al. 2025. "Low-Frequency Electrical Stimulation of the Auricular Branch of the Vagus Nerve in Patients with ST-Elevation Myocardial Infarction: A Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 6: 1866. https://doi.org/10.3390/jcm14061866
APA StyleKruchinova, S., Gendugova, M., Namitokov, A., Sokolskaya, M., Gilevich, I., Tatarintseva, Z., Karibova, M., Danilov, V., Simakin, N., Shvartz, E., Kosmacheva, E., & Shvartz, V. (2025). Low-Frequency Electrical Stimulation of the Auricular Branch of the Vagus Nerve in Patients with ST-Elevation Myocardial Infarction: A Randomized Clinical Trial. Journal of Clinical Medicine, 14(6), 1866. https://doi.org/10.3390/jcm14061866









