Effects of a Supervised-As-Needed Home Exercise Program on Scoliosis and Motor Function in Rett Syndrome: A Multiple-Baseline Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. RTT Severity Level
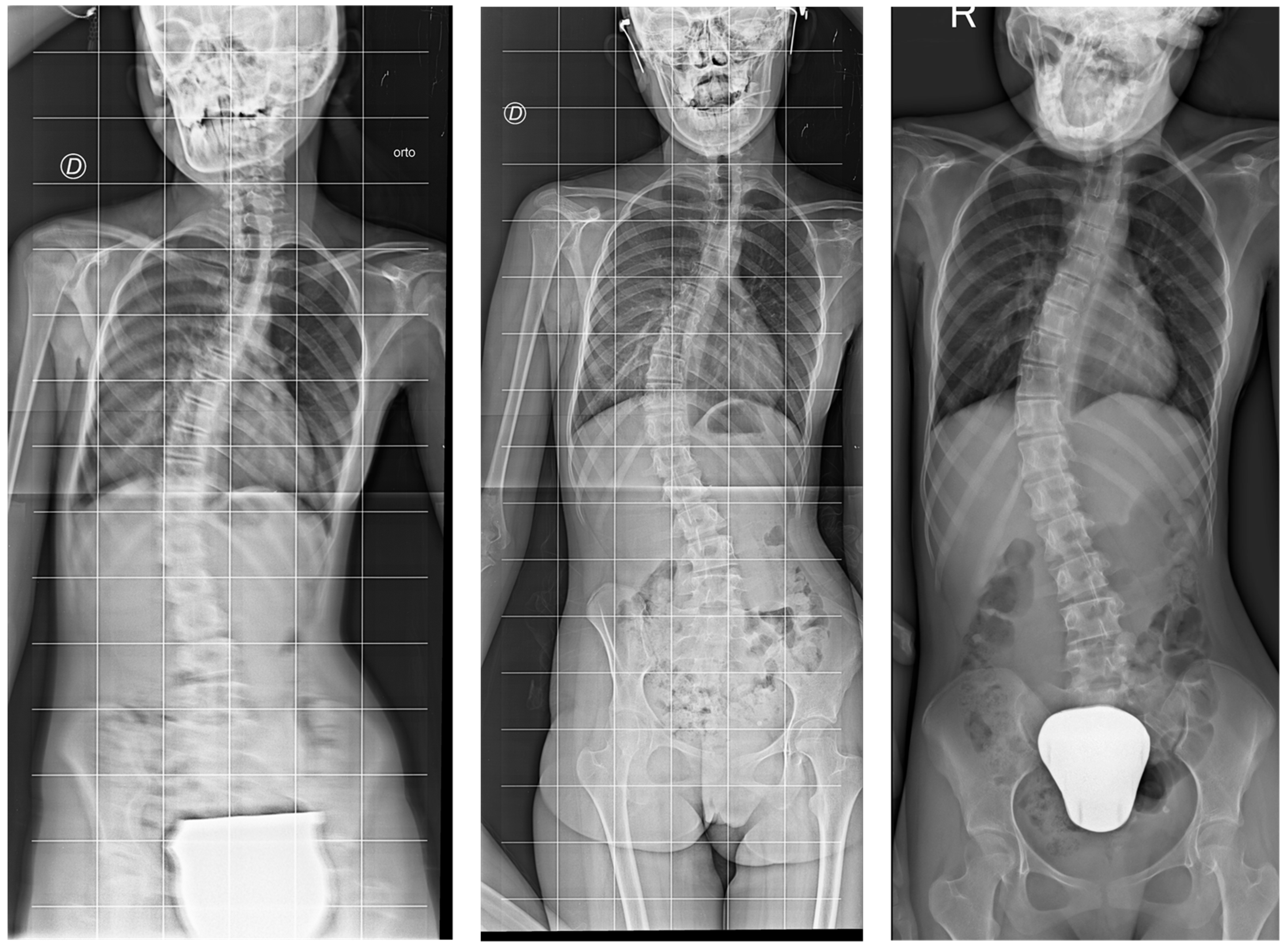
2.3.2. Scoliosis Progression
2.3.3. Gross Motor Function Level
2.4. Data Analysis
3. Results
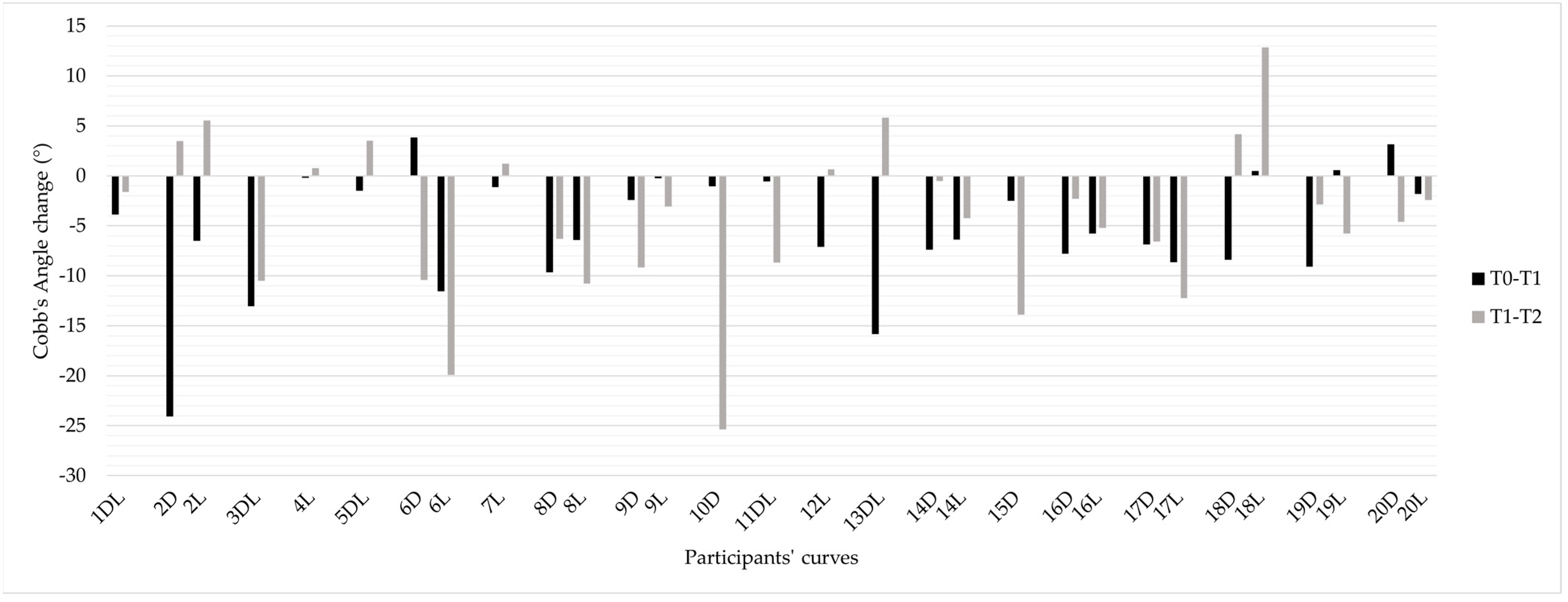
3.1. Scoliosis Characteristics and Progression
3.2. Individual Responses to Intervention
3.3. Gross Motor Function
4. Discussion
4.1. Effect of the HEP on Scoliosis Progression
4.2. Effect of the HEP on Gross Motor Function
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petriti, U.; Dudman, D.C.; Scosyrev, E.; Lopez-Leon, S. Global Prevalence of Rett Syndrome: Systematic Review and Meta-Analysis. Syst. Rev. 2023, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Zoghbi, H.Y. The Story of Rett Syndrome: From Clinic to Neurobiology. Neuron 2007, 56, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett Syndrome Is Caused by Mutations in X-Linked MECP2, Encoding Methyl-CpG-Binding Protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.E.; Johnston, M.V.; Blue, M.E. MeCP2 Expression and Function during Brain Development: Implications for Rett Syndrome’s Pathogenesis and Clinical Evolution. In Proceedings of the Brain and Development; Elsevier: Amsterdam, The Netherlands, 2005; Volume 27, pp. S77–S87. [Google Scholar]
- Einspieler, C.; Marschik, P.B. Regression in Rett Syndrome: Developmental Pathways to Its Onset. Neurosci. Biobehav. Rev. 2019, 98, 320–332. [Google Scholar] [CrossRef]
- Sai Sri Narava, S.; Kucherlapati, S.; Kumar Mugada, V.; Rao Yarguntla, S. A Review on Rett Syndrome: A Debilitating Neurodevelopmental Disorder. Res. J. Pharmacol. Pharmacodyn. 2023, 15, 159–164. [Google Scholar] [CrossRef]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett Syndrome: Revised Diagnostic Criteria and Nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef]
- Marschik, P.B.; Lemcke, S.; Einspieler, C.; Zhang, D.; Bölte, S.; Townend, G.S.; Lauritsen, M.B. Early Development in Rett Syndrome–the Benefits and Difficulties of a Birth Cohort Approach. Dev. Neurorehabil. 2018, 21, 68–72. [Google Scholar] [CrossRef]
- Downs, J.; Torode, I.; Wong, K.; Ellaway, C.; Elliott, E.J.; Christodoulou, J.; Jacoby, P.; Thomson, M.R.; Izatt, M.T.; Askin, G.N.; et al. The Natural History of Scoliosis in Females with Rett Syndrome. Spine 2016, 41, 856–863. [Google Scholar] [CrossRef]
- Downs, J.; Bergman, A.; Carter, P.; Anderson, A.; Palmer, G.M.; Roye, D.; van Bosse, H.; Bebbington, A.; Larsson, E.L.; Smith, B.G.; et al. Guidelines for Management of Scoliosis in Rett Syndrome Patients Based on Expert Consensus and Clinical Evidence. Spine 2009, 34, E607–E617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Zhang, G.; Ma, B.; Ma, Y.; Yang, Y.; Xing, S.; Kang, X.; Gao, B. Effects of Spinal Deformities on Lung Development in Children: A Review. J. Orthop. Surg. Res. 2023, 18, 246. [Google Scholar] [CrossRef]
- McPhail, G.L.; Howells, S.A.; Boesch, R.P.; Wood, R.E.; Ednick, M.; Chini, B.A.; Jain, V.; Agabegi, S.; Sturm, P.; Wall, E.; et al. Obstructive Lung Disease Is Common in Children with Syndromic and Congenital Scoliosis: A Preliminary Study. J. Pediatr. Orthop. 2013, 33, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Lidström, J.; Stokland, E.; Hagberg, B. Scoliosis in Rett Syndrome Clinical and Biological Aspects. Spine 1994, 19, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.J.; Webb, P.J. Scoliosis in the Rett Syndrome: Natural History and Treatment. Brain Dev. 1990, 12, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.J.; Lubicky, J.P.; Hammerberg, K.W. Scoliosis in Rett Syndrome. Orthop. Rev. 1994, 23, 931–937. [Google Scholar] [CrossRef]
- Keret, D.; Bassett, G.S.; Bunnell, W.P.; Marks, H.G. Scoliosis in Rett Syndrome. J. Pediatr. Orthop. 1988, 8, 138–142. [Google Scholar] [CrossRef]
- Percy, A.K.; Lee, H.S.; Neul, J.L.; Lane, J.B.; Skinner, S.A.; Geerts, S.P.; Annese, F.; Graham, J.; McNair, L.; Motil, K.J.; et al. Profiling Scoliosis in Rett Syndrome. Pediatr. Res. 2010, 67, 435–439. [Google Scholar] [CrossRef]
- Killian, J.T.; Lane, J.B.; Lee, H.S.; Skinner, S.A.; Kaufmann, W.E.; Glaze, D.G.; Neul, J.L.; Percy, A.K. Scoliosis in Rett Syndrome: Progression, Comorbidities, and Predictors. Pediatr. Neurol. 2017, 70, 20–25. [Google Scholar] [CrossRef]
- van Urk, P.; van den Berg, M.; Lotan, M.; Downs, J.; Royen Van, B.; Curfs, L. Profiling Scoliosis in Rett Syndrome Patients; a Retrospective Study in the Netherlands. In Proceedings of the 3rd Rett Syndrome European Conference, Maastricht, The Netherlands, 17–19 October 2013. [Google Scholar]
- Ager, S.; Fyfe, S.; Christodoulou, J.; Jacoby, P.; Schmitt, L.; Leonard, H. Predictors of Scoliosis in Rett Syndrome. J. Child. Neurol. 2006, 21, 809–813. [Google Scholar] [CrossRef]
- Rodocanachi Roidi, M.L.; Cozzi, F.; Isaias, I.U.; Grange, F.; Ferrari, E.P.; Ripamonti, E. Clinical and Genetic Correlations of Scoliosis in Rett Syndrome. Eur. Spine J. 2022, 31, 2987–2993. [Google Scholar] [CrossRef]
- Rodocanachi Roidi, M.L.; Isaias, I.U.; Cozzi, F.; Grange, F.; Scotti, F.M.; Gestra, V.F.; Gandini, A.; Ripamonti, E. A New Scale to Evaluate Motor Function in Rett Syndrome: Validation and Psychometric Properties. Pediatr. Neurol. 2019, 100, 80–86. [Google Scholar] [CrossRef]
- Downs, J.; Torode, I.; Wong, K.; Ellaway, C.; Elliott, E.J.; Izatt, M.T.; Askin, G.N.; Mcphee, B.I.; Cundy, P.; Leonard, H.; et al. Surgical Fusion of Early Onset Severe Scoliosis Increases Survival in Rett Syndrome: A Cohort Study. Dev. Med. Child. Neurol. 2016, 58, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.; Young, D.; de Klerk, N.; Bebbington, A.; Baikie, G.; Leonard, H. Impact of Scoliosis Surgery on Activities of Daily Living in Females with Rett Syndrome. J. Pediatr. Orthop. 2009, 29, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Ager, S.; Downs, J.; Fyfe, S.; Leonard, H. Parental Experiences of Scoliosis Management in Rett Syndrome. Disabil. Rehabil. 2009, 31, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Charles, Y.P.; Daures, J.P.; De Rosa, V.; Diméglio, A. Progression Risk of Idiopathic Juvenile Scoliosis during Pubertal Growth. Spine 2006, 31, 1933–1942. [Google Scholar] [CrossRef]
- Lotan, M.; Ippolito, E.; Favetta, M.; Romano, A. Skype Supervised, Individualized, Home-Based Rehabilitation Programs for Individuals with Rett Syndrome and Their Families—Parental Satisfaction and Point of View. Front. Psychol. 2021, 12, 3995. [Google Scholar] [CrossRef]
- Fonzo, M.; Sirico, F.; Corrado, B. Evidence-based Physical Therapy for Individuals with Rett Syndrome: A Systematic Review. Brain Sci. 2020, 10, 410. [Google Scholar] [CrossRef]
- Lotan, M.; Merrick, J.; Carmeli, E. Managing Scoliosis in a Young Child with Rett Syndrome: A Case Study. Sci. World J. 2005, 5, 264–273. [Google Scholar] [CrossRef]
- Romano, A.; Ippolito, E.; Risoli, C.; Malerba, E.; Favetta, M.; Sancesario, A.; Lotan, M.; Moran, D.S. Intensive Postural and Motor Activity Program Reduces Scoliosis Progression in People with Rett Syndrome. J. Clin. Med. 2022, 11, 559. [Google Scholar] [CrossRef]
- Fabio, R.A.; Martinazzoli, C.; Antonietti, A. Development and Standardization of the “Rars”(Rett Assessment Rating Scale). Life Span. Disabil. 2005, 8, 257–281. [Google Scholar]
- Negrini, S.; Donzelli, S.; Aulisa, A.G.; Czaprowski, D.; Schreiber, S.; de Mauroy, J.C.; Diers, H.; Grivas, T.B.; Knott, P.; Kotwicki, T.; et al. 2016 SOSORT Guidelines: Orthopaedic and Rehabilitation Treatment of Idiopathic Scoliosis during Growth. Scoliosis Spinal Disord 2018, 13. [Google Scholar] [CrossRef]
- Romano, A.; Caprì, T.; Semino, M.; Bizzego, I.; Di Rosa, G.; Fabio, R.A. Gross Motor, Physical Activity and Musculoskeletal Disorder Evaluation Tools for Rett Syndrome: A Systematic Review. Dev. Neurorehabil. 2020, 23, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, M.; Tomczak, E. The Need to Report Effect Size Estimates Revisited. An Overview of Some Recommended Measures of Effect Size. Trends Sport. Sci. 2014, 1, 19–25. [Google Scholar]
- King, B.M.; Rosopa, P.; Minium, E.W. Statistical Reasoning in the Behavioral Sciences, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; ISBN 9780470134870/0470134879. [Google Scholar]
- Kinney, A.R.; Eakman, A.M.; Graham, J.E. Novel Effect Size Interpretation Guidelines and an Evaluation of Statistical Power in Rehabilitation Research. Arch. Phys. Med. Rehabil. 2020, 101, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. When to Use the Bonferroni Correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Di Rosa, G.; Tisano, A.; Fabio, R.A.; Lotan, M. Effects of a Remotely Supervised Motor Rehabilitation Program for Individuals with Rett Syndrome at Home. Disabil. Rehabil. 2021, 44, 5898–5908. [Google Scholar] [CrossRef]
- Lim, J.; Greenspoon, D.; Hunt, A.; McAdam, L. Rehabilitation Interventions in Rett Syndrome: A Scoping Review. Dev. Med. Child. Neurol. 2020, 62, 906–916. [Google Scholar] [CrossRef]
- Coillard, C.; Circo, A.; Rivard, C. Correlation between Maturation, Growth Spurt, and the Progression of Adolescent Idiopathic Scoliosis. Scoliosis 2013, 8, O12. [Google Scholar] [CrossRef]
- Brenner, P.S.; DeLamater, J.D. Social Desirability Bias in Self-Reports of Physical Activity: Is an Exercise Identity the Culprit? Soc. Indic. Res. 2014, 117, 489–504. [Google Scholar] [CrossRef]
- Lai, B.; Bond, K.; Kim, Y.; Barstow, B.; Jovanov, E.; Bickel, C.S. Exploring the Uptake and Implementation of Tele-Monitored Home-Exercise Programmes in Adults with Parkinson’s Disease: A Mixed-Methods Pilot Study. J. Telemed. Telecare 2020, 26, 53–63. [Google Scholar] [CrossRef]
- Downs, J.; Blackmore, A.M.; Wong, K.; Buckley, N.; Lotan, M.; Elefant, C.; Leonard, H.; Stahlhut, M. Can Telehealth Increase Physical Activity in Individuals with Rett Syndrome? A Multicentre Randomized Controlled Trial. Dev. Med. Child. Neurol. 2022, 65, 489–497. [Google Scholar] [CrossRef]
- Weeda, J.E.; van Kuijk, S.M.J.; van den Berg, M.P.; Bastiaenen, C.H.G.; Borst, H.E.; van Rhijn, L.W.; de Bie, R.A. Identification of Predictors for Progression of Scoliosis in Rett Syndrome. Dev. Neurorehabil 2024, 27, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Stahlhut, M.; Downs, J.; Wong, K.; Bisgaard, A.M.; Nordmark, E. Feasibility and Effectiveness of an Individualized 12-Week “Uptime” Participation (U-PART) Intervention in Girls and Women With Rett Syndrome. Phys. Ther. 2020, 100, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, P.J.A.; Hinman, R.S.; Wrigley, T.V.; Stratford, P.W.; Bennell, K.L. Self-Reported Home Exercise Adherence: A Validity and Reliability Study Using Concealed Accelerometers. J. Orthop. Sports Phys. Ther. 2018, 48, 943–950. [Google Scholar] [CrossRef] [PubMed]



| Subscale (Range) | Median (IQR) | p-Value (ES) | ||
|---|---|---|---|---|
| T1 | T2 | |||
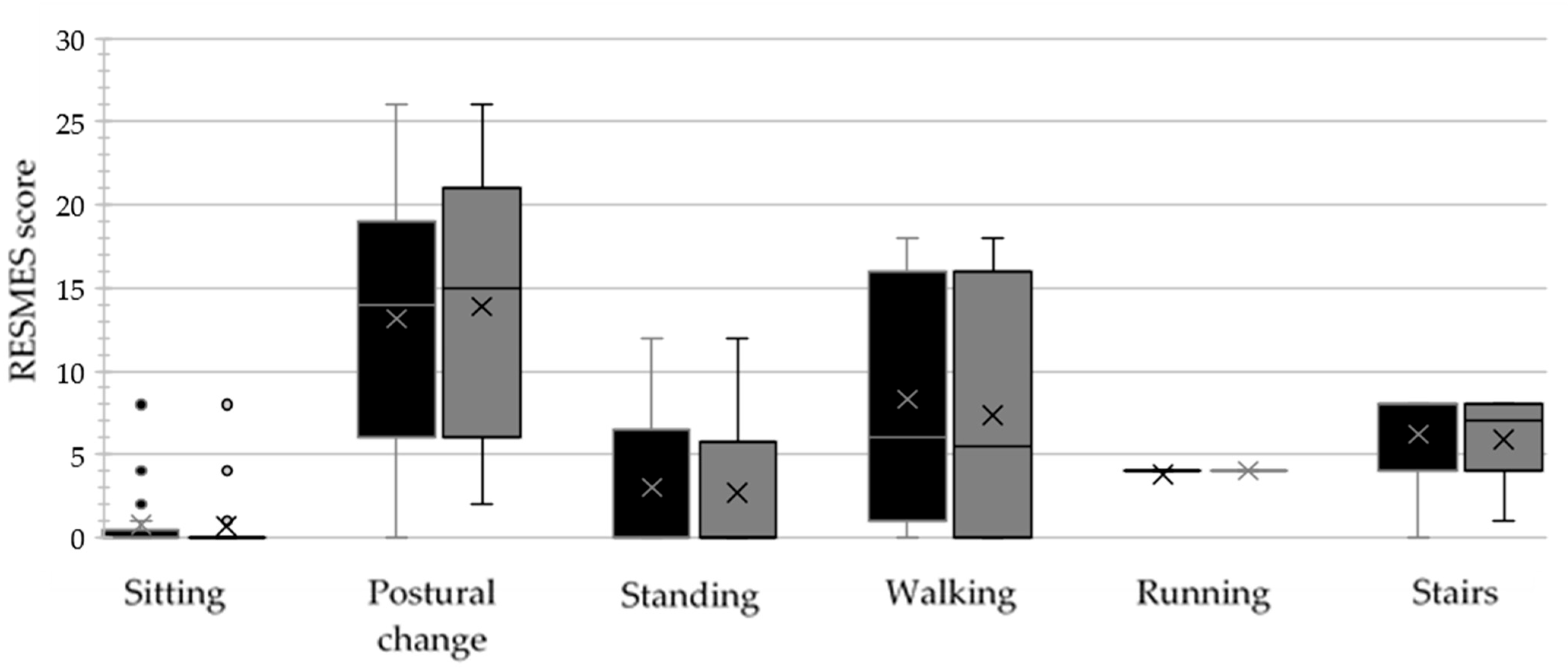
| RESMES subscales | Sitting (0–12) | 0 (0.25) | 0 (0) | 0.424 |
| Postural change (0–28) | 14.5 (11.25) | 15 (12.75) | 0.669 | |
| Standing (0–12) | 0.5 (6.25) | 0 (5.25) | 0.026 | |
| Walking (0–18) | 8 (14) | 5.5 (16) | 0.009 | |
| Running (0–4) | 4 (0) | 4 (0) | 1.000 | |
| Stairs (0–8) | 8 (4) | 7 (4) | 0.021 | |
| RESMES total score (0–82) | 33.5 (37) | 31.5 (36.25) | 0.002 * (0.850) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Rodocanachi Roidi, M.L.; Savini, M.N.; Viganò, I.; Dziubak, M.; Pietrogrande, L.; Moran, D.S.; Lotan, M. Effects of a Supervised-As-Needed Home Exercise Program on Scoliosis and Motor Function in Rett Syndrome: A Multiple-Baseline Study. J. Clin. Med. 2025, 14, 1873. https://doi.org/10.3390/jcm14061873
Romano A, Rodocanachi Roidi ML, Savini MN, Viganò I, Dziubak M, Pietrogrande L, Moran DS, Lotan M. Effects of a Supervised-As-Needed Home Exercise Program on Scoliosis and Motor Function in Rett Syndrome: A Multiple-Baseline Study. Journal of Clinical Medicine. 2025; 14(6):1873. https://doi.org/10.3390/jcm14061873
Chicago/Turabian StyleRomano, Alberto, Marina Luisa Rodocanachi Roidi, Miriam Nella Savini, Ilaria Viganò, Michal Dziubak, Luca Pietrogrande, Daniel Sender Moran, and Meir Lotan. 2025. "Effects of a Supervised-As-Needed Home Exercise Program on Scoliosis and Motor Function in Rett Syndrome: A Multiple-Baseline Study" Journal of Clinical Medicine 14, no. 6: 1873. https://doi.org/10.3390/jcm14061873
APA StyleRomano, A., Rodocanachi Roidi, M. L., Savini, M. N., Viganò, I., Dziubak, M., Pietrogrande, L., Moran, D. S., & Lotan, M. (2025). Effects of a Supervised-As-Needed Home Exercise Program on Scoliosis and Motor Function in Rett Syndrome: A Multiple-Baseline Study. Journal of Clinical Medicine, 14(6), 1873. https://doi.org/10.3390/jcm14061873






