Electroencephalography in Autism Spectrum Disorder
Abstract
:1. Introduction
Key Points
- Routine EEG examinations in children with autism spectrum disorder (ASD) without a history of seizures often reveal both epileptiform and non-epileptiform abnormalities.
- A literature review on the relationship between observable EEG changes and the occurrence of disorders in several areas—such as the severity of autistic features, development, behavior, sleep, and movement disorders—does not allow for drawing definitive conclusions about the impact of these changes on the occurrence of the disorders.
- Some reports indicate that sodium valproate, levetiracetam, lamotrigine, and even corticosteroids have demonstrated efficacy in enhancing fundamental clinical functions; however, no studies that meet adequate statistical criteria have been conducted on this topic. There is a lack of precise data supporting the rationale for treating children with EEG changes in the absence of clinical seizures.
- Given the lack of clear evidence linking EEG findings to the progression of ASD and the absence of strong indications for treating EEG abnormalities without seizures, routine EEG testing in all children with autism appears unnecessary, except when epilepsy is suspected.
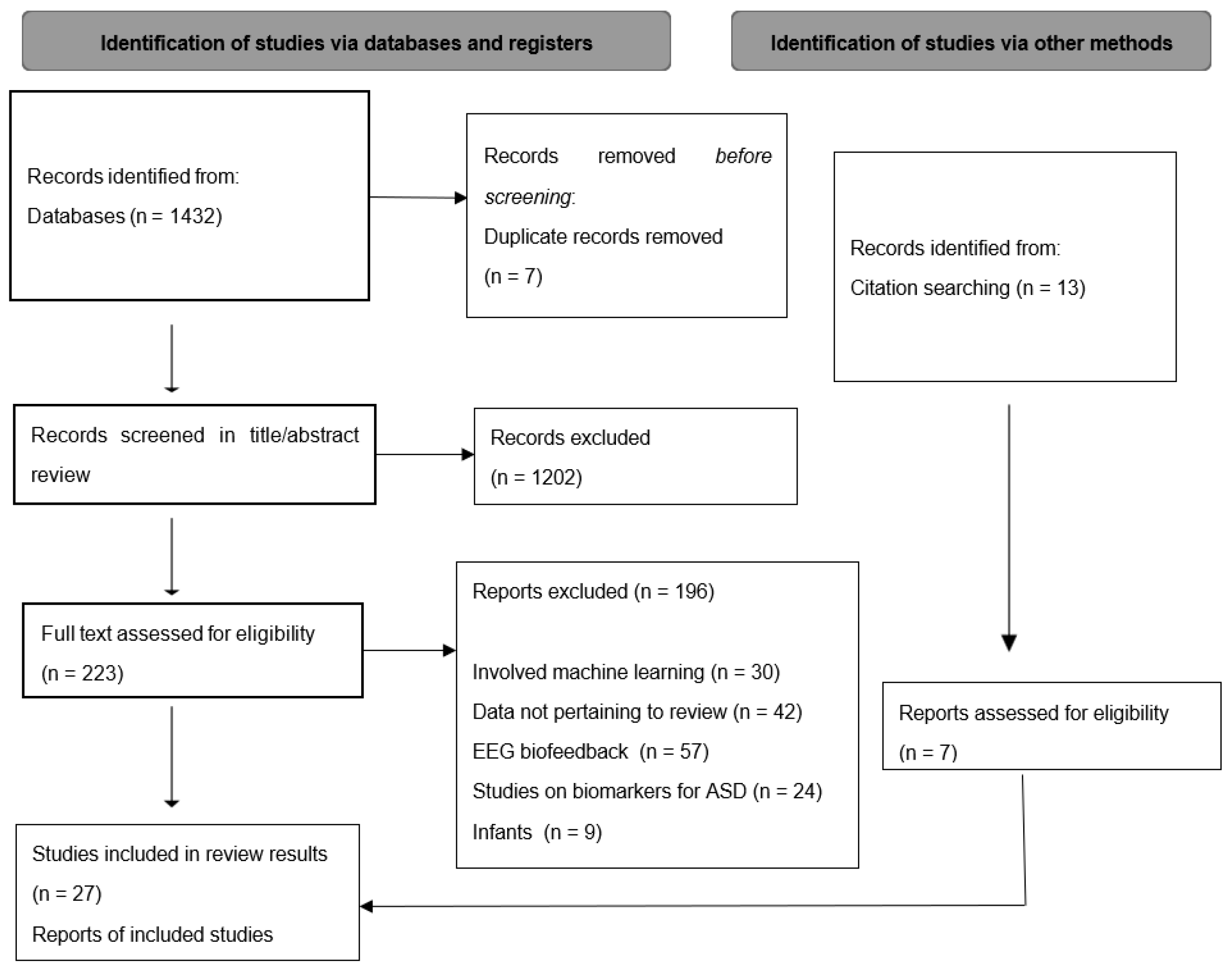
2. Methodology
2.1. Search Strategy
2.2. Inclusion Criteria and Study Selection
3. Results
3.1. Type of EEG Abnormalities in ASD
3.2. EEG and Severity of Autistic Features
3.3. EEG and Cognitive Skills
3.4. EEG and Speech Development
3.5. EEG and Behavioral Disorders
3.6. EEG and Sleep
3.7. EEG and Movements Disorders
3.8. EEG and Epilepsy
3.9. Pharmacological Treatment of Patients with ASD and EEG Abnormalities Without Seizures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), Text Revision; American Psychiatric Press Inc.: Washington, DC, USA, 2000. [Google Scholar]
- Bhat, S.; Acharya, U.R.; Adeli, H.; Bairy, G.M.; Adeli, A. Autism: Cause factors, early diagnosis and therapies. Rev. Neurosci. 2014, 25, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Eikeseth, S. Outcome of comprehensive psycho-educational interventions for young children with autism. Res. Dev. Disabil. 2009, 30, 158–178. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, A.V.; DeLucia, E.; McDonnell, C.G.; Scarpa, A. A systematic review of technological approaches for autism spectrum disorder assessment in children: Implications for the COVID-19 pandemic. Res. Dev. Disabil. 2021, 109, 103852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zwaigenbaum, L.; Penner, M. Autism spectrum disorder: Advances in diagnosis and evaluation. BMJ 2018, 361, k1674. [Google Scholar] [CrossRef] [PubMed]
- Khodatars, M.; Shoeibi, A.; Sadeghi, D.; Ghaasemi, N.; Jafari, M.; Moridian, P.; Khadem, A.; Alizadehsani, R.; Zare, A.; Kong, Y.; et al. Deep learning for neuroimaging-based diagnosis and rehabilitation of Autism Spectrum Disorder: A review. Comput. Biol. Med. 2021, 139, 104949. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wen, G.; Cao, P.; Yang, J.; Zaiane, O.R. Collaborative learning of graph generation, clustering and classification for brain networks diagnosis. Comput. Methods Programs Biomed. 2022, 219, 106772. [Google Scholar] [CrossRef] [PubMed]
- Nogay, H.S.; Adeli, H. Machine learning (ML) for the diagnosis of autism spectrum disorder (ASD) using brain imaging. Rev. Neurosci. 2020. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bajestani, G.S.; Behrooz, M.; Khani, A.G.; Nouri-Baygi, M.; Mollaei, A. Diagnosis of autism spectrum disorder based on complex network features. Comput. Methods Programs Biomed. 2019, 177, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Capal, J.K.; Jeste, S.S. Autism and Epilepsy. Pediatr. Clin. N. Am. 2024, 71, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Trauner, D.A. Behavioral correlates of epileptiform abnormalities in autism. Epilepsy Behav. 2015, 47, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Capal, J.K.; Carosella, C.; Corbin, E.; Horn, P.S.; Caine, R.; Manning-Courtney, P. EEG endophenotypes in autism spectrum disorder. Epilepsy Behav. 2018, 88, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Veerappan, V.D.; Sweetha, B.; Kavitha, H.R.; Sivalingam, D.; Nambi, S.; Pauline, L. Two-Year Follow-up of Isolated Epileptiform Discharges in Autism: An Endophenotypic Biomarker? Indian J. Psychol. Med. 2018, 40, 219–224, Erratum in Indian J. Psychol. Med. 2018, 40, 602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santarone, M.E.; Zambrano, S.; Zanotta, N.; Mani, E.; Minghetti, S.; Pozzi, M.; Villa, L.; Molteni, M.; Zucca, C. EEG Features in Autism Spectrum Disorder: A Retrospective Analysis in a Cohort of Preschool Children. Brain Sci. 2023, 13, 345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romero-González, M.; Navas-Sánchez, P.; Marín-Gámez, E.; Barbancho-Fernández, M.A.; Fernández-Sánchez, V.E.; Lara-Muñoz, J.P.; Guzmán-Parra, J. EEG abnormalities and clinical phenotypes in pre-school children with autism spectrum disorder. Epilepsy Behav. 2022, 129, 108619. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.K.; Trauner, D.A. Incidence and behavioral correlates of epileptiform abnormalities in autism spectrum disorders. J. Autism Dev. Disord. 2014, 44, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ghacibeh, G.A.; Fields, C. Interictal epileptiform activity and autism. Epilepsy Behav. 2015, 47, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Nicotera, A.G.; Hagerman, R.J.; Catania, M.V.; Buono, S.; Di Nuovo, S.; Liprino, E.M.; Stracuzzi, E.; Giusto, S.; Di Vita, G.; Musumeci, S.A. EEG Abnormalities as a Neurophysiological Biomarker of Severity in Autism Spectrum Disorder: A Pilot Cohort Study. J. Autism Dev. Disord. 2019, 49, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Shefa, J.; Mannan, M. EEG changes and their relationship with intellectual disability in children with autism spectrum disorders in a tertiary care hospital. J. Int. Child Neurol. Assoc. 2021, 1. [Google Scholar] [CrossRef]
- Carson, A.M.; Salowitz, N.M.; Scheidt, R.A.; Dolan, B.K.; Van Hecke, A.V. Electroencephalogram coherence in children with and without autism spectrum disorders: Decreased interhemispheric connectivity in autism. Autism Res. 2014, 7, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Vitale, A.; Federici, A.; Pini, E.; Molteni, M.; Casartelli, L. Altered neural oscillations and connectivity in the beta band underlie detail-oriented visual processing in autism. Neuroimage Clin. 2020, 28, 102484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larrain-Valenzuela, J.; Zamorano, F.; Soto-Icaza, P.; Carrasco, X.; Herrera, C.; Daiber, F.; Aboitiz, F.; Billeke, P. Theta and Alpha Oscillation Impairments in Autistic Spectrum Disorder Reflect Working Memory Deficit. Sci. Rep. 2017, 7, 14328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neuhaus, E.; Santhosh, M.; Kresse, A.; Aylward, E.; Bernier, R.; Bookheimer, S.; Jeste, S.; Jack, A.; McPartland, J.C.; Naples, A.; et al. Frontal EEG alpha asymmetry in youth with autism: Sex differences and social-emotional correlates. Autism Res. 2023, 16, 2364–2377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finn, C.E.; Han, G.T.; Naples, A.J.; Wolf, J.M.; McPartland, J.C. Development of peak alpha frequency reflects a distinct trajectory of neural maturation in autistic children. Autism Res. 2023, 16, 2077–2089. [Google Scholar] [CrossRef] [PubMed]
- Arazi, A.; Meiri, G.; Danan, D.; Michaelovski, A.; Flusser, H.; Menashe, I.; Tarasiuk, A.; Dinstein, I. Reduced sleep pressure in young children with autism. Sleep 2020, 43, zsz309. [Google Scholar] [CrossRef] [PubMed]
- Rochette, A.C.; Soulières, I.; Berthiaume, C.; Godbout, R. NREM sleep EEG activity and procedural memory: A comparison between young neurotypical and autistic adults without sleep complaints. Autism Res. 2018, 11, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, T.; Carrier, J.; Godbout, R. NREM sleep EEG slow waves in autistic and typically developing children: Morphological characteristics and scalp distribution. J. Sleep. Res. 2019, 28, e12775. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, M.; Grujicic, R. Electroencephalography in Assessment of Autism Spectrum Disorders: A Review. Front. Psychiatry 2021, 12, 686021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Sun, X.; Sun, C.; Zou, M.; Chen, Y.; Huang, J.; Wu, L.; Chen, W.X. Prevalence of epilepsy in autism spectrum disorders: A systematic review and meta-analysis. Autism 2022, 26, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.W.; Holmes, G.L. Epilepsy and Autism. Cold Spring Harb. Perspect. Med. 2016, 6, a022749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, V.; Saini, A.G.; Malhi, P.; Singhi, P. Epilepsy and EEG Abnormalities in Children with Autism Spectrum Disorders. Indian. J. Pediatr. 2022, 89, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.A.; Canitano, R.; Oakley, B.; San José-Cáceres, A.; Tinelli, M.; Knapp, M.; Cusack, J.; Parellada, M.; Violland, P.; Derk Plas, J.R.; et al. Autism with co-occurring epilepsy care pathway in Europe. Eur. Psychiatry 2023, 66, e61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luz-Escamilla, L.; Morales-González, J.A. Association between Interictal Epileptiform Discharges and Autistic Spectrum Disorder. Brain Sci. 2019, 9, 185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Precenzano, F.; Parisi, L.; Lanzara, V.; Vetri, L.; Operto, F.F.; Pastorino, G.M.G.; Ruberto, M.; Messina, G.; Risoleo, M.C.; Santoro, C.; et al. Electroencephalographic Abnormalities in Autism Spectrum Disorder: Characteristics and Therapeutic Implications. Medicina 2020, 56, 419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, M.; Jiang, L.; Tang, X. Levetiracetam is associated with decrease in subclinical epileptiform discharges and improved cognitive functions in pediatric patients with autism spectrum disorder. Neuropsychiatr. Dis. Treat. 2017, 13, 2321–2326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rugino, T.A.; Samsock, T.C. Levetiracetam in Autistic Children: An Open-Label Study. J. Dev. Behav. Pediatr. 2002, 23, 225–230. [Google Scholar] [CrossRef]
- Chez, M.G.; Chang, M.; Krasne, V.; Coughlan, C.; Kominsky, M.; Schwartz, A. Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy Behav. 2006, 8, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Hollander, E.; Dolgoff-Kaspar, R.; Cartwright, C.; Rawitt, R.; Novotny, S. An open trial of divalproex sodium in autism spectrum disorders. J. Clin. Psychiatry 2001, 62, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Hollander, E.; Soorya, L.; Wasserman, S.; Esposito, K.; Chaplin, W.; Anagnostou, E. Divalproex sodium vs. placebo in the treatment of repetitive behaviours in autism spectrum disorder. Int. J. Neuropsychopharmacol. 2006, 9, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Pressler, R.M.; Robinson, R.O.; Wilson, G.A.; Binnie, C.D. Treatment of interictal epileptiform discharges can improve behavior in children with behavioral problems and epilepsy. J. Pediatr. 2005, 146, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Veenstra-Vanderweele, J.; Hollander, E.; Kishi, T. Antiepileptic medications in autism spectrum disorder: A systematic review and meta-analysis. J. Autism Dev. Disord. 2014, 44, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Duffy, F.H.; Shankardass, A.; McAnulty, G.B.; Eksioglu, Y.Z.; Coulter, D.; Rotenberg, A.; Als, H. Corticosteroid therapy in regressive autism: A retrospective study of effects on the Frequency Modulated Auditory Evoked Response (FMAER), language, and behavior. BMC Neurol. 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wasserman, S.; Iyengar, R.; Chaplin, W.F.; Watner, D.; Waldoks, S.E.; Anagnostou, E.; Soorya, L.; Hollander, E. Levetiracetam versus placebo in childhood and adolescent autism: A double-blind placebo-controlled study. Int. Clin. Psychopharmacol. 2006, 21, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Belsito, K.M.; Law, P.A.; Kirk, K.S.; Landa, R.J.; Zimmerman, A.W. Lamotrigine therapy for autistic disorder: A randomized, double-blind, placebo-controlled trial. J. Autism Dev. Disord. 2001, 31, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Kessler, R.; Gaughan, T.; Buckley, A.W. Electroencephalogram Coherence Patterns in Autism: An Updated Review. Pediatr. Neurol. 2017, 67, 7–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shou, G.; Mosconi, M.W.; Wang, J.; Ethridge, L.E.; Sweeney, J.A.; Ding, L. Electrophysiological signatures of atypical intrinsic brain connectivity networks in autism. J. Neural Eng. 2017, 14, 046010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, J.; Chen, H.; Li, X.; Li, X. EEG entropy analysis in autistic children. J. Clin. Neurosci. 2019, 62, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.; Rieder, A.D.; Johnson, M.H. Prediction of autism in infants: Progress and challenges. Lancet Neurol. 2023, 22, 244–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hughes, R.; Poon, W.Y.; Harvey, A.S. Limited role for routine EEG in the assessment of staring in children with autism spectrum disorder. Arch. Dis. Child. 2015, 100, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Giannadou, A.; Jones, M.; Freeth, M.; Samson, A.C.; Milne, E. Investigating neural dynamics in autism spectrum conditions outside of the laboratory using mobile electroencephalography. Psychophysiology 2022, 59, e13995. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazzaq, M.M.; Ramaha, N.T.A.; Hameed, A.A.; Salman, M.; Yon, D.K.; Fitriyani, N.L.; Syafrudin, M.; Lee, S.W. Consequential Advancements of Self-Supervised Learning (SSL) in Deep Learning Contexts. Mathematics 2024, 12, 758. [Google Scholar] [CrossRef]
- Imran, S.M.A.; Saleem, M.W.; Hameed, M.T.; Hussain, A.; Naqvi, R.A.; Lee, S.W. Feature preserving mesh network for semantic segmentation of retinal vasculature to support ophthalmic disease analysis. Front. Med. 2023, 9, 1040562. [Google Scholar] [CrossRef] [PubMed]
- Abidin, Z.U.; Naqvi, R.A.; Haider, A.; Kim, H.S.; Jeong, D.; Lee, S.W. Recent deep learning-based brain tumor segmentation models using multi- modality magnetic resonance imaging: A prospective survey. Front. Bioeng. Biotechnol. 2024, 12, 1392807. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.U.; Kim, K.S.; Khalid, M.; Farrash, M.; Zafar, A.; Lee, S.W. Enhancing Alzheimer’s disease diagnosis and staging: A multistage CNN framework using MRI. Front. Psychiatry 2024, 15, 1395563. [Google Scholar] [CrossRef] [PubMed]
| Study | EEG Abnormalities—Type/Specification | EEG Abnormalities—Localisation | Percentage of Patients with Abnormal EEG |
|---|---|---|---|
| Epileptiform Abnormalities | |||
| Capal et al. [12] | - | 83% * focal—most commonly left temporal 17% * diffuse | 67.4% |
| Veerappan et al. [13] | sharp waves (33%) and other abnormal wave patterns (9%) | - | 42% |
| Santarone et al. [14] | paroxysmal slowing and interictal epileptiform discharges (95.5% *—during sleep, 4.8% *—wakefulness and sleep) | 37.7% * focal: (48.4% central 32.3% temporal 19.3% frontal) 62.7% * diffuse | 28.4% |
| Romero-González et al. [15] | - | 66.7% * focal (50% temporal-parietal 33.3% right temporal 16.7% central-temporal) 33.3% * diffuse | 13% |
| Mulligan et al. [16] | 56.4% rare 27.3% recurrent 16.4% frequent (3.6% * during wakefulness, 58.9% * during sleep, 37.5% *—wakefulness and sleep) | - | 59.4% |
| Nicotera et al. [18] | spike, sharp waves, slow spike, and wave complexes (72% * during sleep, 27% *—wakefulness and sleep) | 55.5% * focal (70% anterior areas 30% e posterior areas) 44.4% * diffuse (widespread anomalies and/or multifocal) | 26.8% |
| Akhter et al. [19] | 89% spike–wave complexes | 33% * focal (50% temporal 40% frontal 7.4% multifocal sharp waves) 37% * diffuse | 70.3% (36.5% of all participants) |
| Non-Epileptiform Abnormalities | |||
| Capal et al. [12] | background slowing (most common) | 47% focal slowing 65% generalized slowing | 36.8% |
| Mulligan et al. [16] | slowing | - | 21.8% |
| Nicotera et al. [18] | slowing and/or irregularity of the background rhythm | - | 13.04% |
| Akhter et al. [19] | theta/delta slowing, excessive beta activity, or asymmetry | - | 29.6% (15.4% of all participants) |
| Carson et al. [20] | alpha frequency interhemispheric coherence | - | - |
| Ronconi et al. [21] | atypical oscillatory beta band activity (15–30 Hz) | - | - |
| Larrain-Valenzuela et al. [22] | theta and alpha oscillation impairments | - | - |
| Neuhaus et al. [23] | frontal alpha asymmetry (FAA) | - | - |
| Development Area | Study | Results of the Study |
|---|---|---|
| Severity of autistic features | Veerappan et al. (2018) [13] Ghacibeh et al. (2015) [17] | Association between sharp waves and epileptiform dischargesand more severe autistic features. |
| Romero-González et al. (2022) [15] | No significant differences. | |
| Mulligan et al. (2014) [16] | Frequency of epileptiform discharges:
| |
| Nicotera et al. (2019) [18] | Severe form of autistic features:
| |
| Cognitive skills | Santaroe et al. (2023) [14] | Association between abnormal background activity during sleep and developmental delay. |
| Nicotera et al. (2019) [18] |
| |
| Akhter et al. (2021) [19] |
| |
| Finn et al. (2023) [24] | Children with ASD show atypical age-dependent rise in PAF values. | |
| Speech development | Mulligan et al. (2014) [16] | No significant correlation between EEG abnormalities and language skills. |
| Nicotera et al. (2019) [18] | Patients with normal EEG:
| |
| Behavioral disorders | Capal et al. (2018) [12] |
|
| Veerappan et al. (2018) [13] | Children with sharp waves had significantly more behavior problems compared to those with other waves. | |
| Romero-González et al. (2022) [15] |
| |
| Nicotera et al. (2019) [18] |
| |
| Neuhaus et al. (2023) [23] | Frontal alpha asymmetry (FAA):
| |
| Sleep | Arazi et al. (2020) [25] | ASD patients have lower slow-wave activity levels and shorter periods of slow-wave sleep. |
| Rochette et al. (2018) [26] | Atypical thalamo-cortical activity in the parieto-occipital area during NREM sleep in children with ASD. | |
| Lehoux et al. (2018) [27] | Slow waves during NREM as a potential electrophysiological indicator of altered cortical maturation in ASD. | |
| Movements disorders | Nicotera et al. (2019) [18] | Noticeable and disruptive motor stereotypies:
|
| Milovanovic et al. (2021) [28] | Epileptiform discharges were associated with lower motor skill scores on the Vineland Adaptive Behavior Scale II. |
| Drug/Method | Study | Description |
|---|---|---|
| Levetiracetam | Wang et al. (2017) [35] | Dose: 60 mg/kg/day The study demonstrated the efficacy of levetiracetam in reduction in EEG discharges in 75% cases while also improving behavioral and cognitive functions. |
| Valproic acid (VPA) | Chez et al. (2006) [37] | Dose: from 80 to 120 mg/dL A total of 46.6% of patients showed subsequent EEG normalization, 17.0% of patients exhibited improvement without achieving normalization, 36.3% of patients remained unchanged, and none deteriorated in the second overnight EEG. |
| Divalproex sodium | E. Hollander et al. (2001) [38] | Dose: 768 mg/day (average dose) Divalproex sodium has the potential to mitigate EEG abnormalities, alleviate ASD symptoms, and improve social functioning. |
| Lamotrigine | Pressler et al. (2005) [40] | Dose: 2 mg/kg/day (<12 yrs) or 150 mg/day (>12 yrs) for children with sodium valproate, 10 mg/kg/day (<12 yrs) or 300 mg/day (>12 yrs) without it Reduction in EEG discharges during the lamotrigine phase corresponded with a improvement in the global behavioral evaluation of the children. |
| Corticosteroids | Duffy et al. (2014) [42] | Dose: Oral prednisolone, 2 mg/kg/day There were no notable differences in EEG readings over time, indicating that the EEG did not reflect treatment effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hankus, M.; Ochman-Pasierbek, P.; Brzozowska, M.; Striano, P.; Paprocka, J. Electroencephalography in Autism Spectrum Disorder. J. Clin. Med. 2025, 14, 1882. https://doi.org/10.3390/jcm14061882
Hankus M, Ochman-Pasierbek P, Brzozowska M, Striano P, Paprocka J. Electroencephalography in Autism Spectrum Disorder. Journal of Clinical Medicine. 2025; 14(6):1882. https://doi.org/10.3390/jcm14061882
Chicago/Turabian StyleHankus, Magdalena, Patrycja Ochman-Pasierbek, Malwina Brzozowska, Pasquale Striano, and Justyna Paprocka. 2025. "Electroencephalography in Autism Spectrum Disorder" Journal of Clinical Medicine 14, no. 6: 1882. https://doi.org/10.3390/jcm14061882
APA StyleHankus, M., Ochman-Pasierbek, P., Brzozowska, M., Striano, P., & Paprocka, J. (2025). Electroencephalography in Autism Spectrum Disorder. Journal of Clinical Medicine, 14(6), 1882. https://doi.org/10.3390/jcm14061882








