Safety and Efficacy of TEE Guidance in Electrophysiological Procedures Without Fluoroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Periprocedural Management
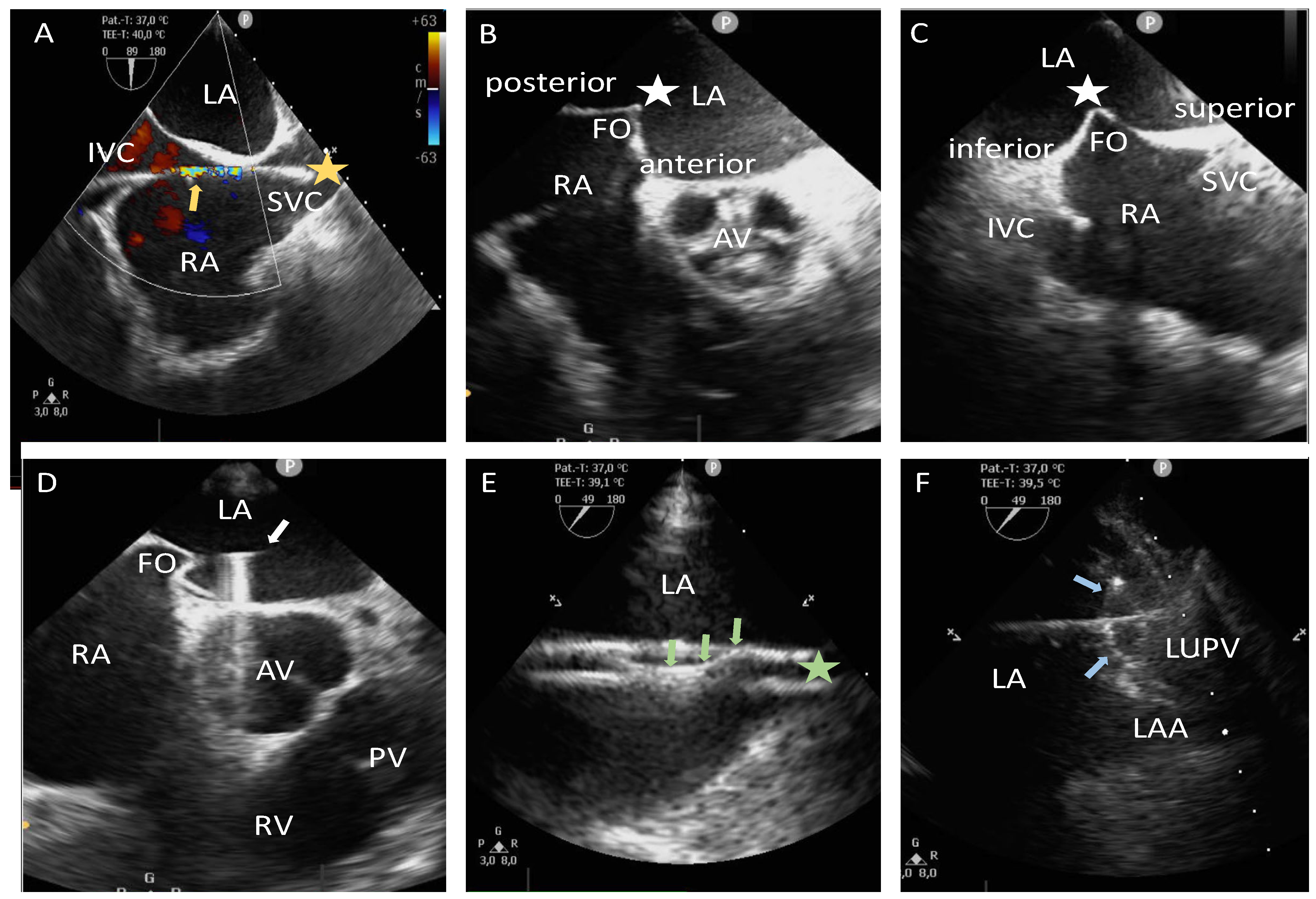
2.3. Zero-Fluoroscopy/Near-Zero-Fluoroscopy Procedure Steps (Zero-Fluro/Near-Zero-Fluoro Group)
2.4. Fluoroscopy Guidance Group (Fluoro Group)
2.5. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Procedural Characteristics
3.3. Learning Curve Effects
3.4. Reasons for Fluoroscopy Usage in the Near-Zero-Fluoroscopy Group
3.5. Transition from Zero-Fluoroscopy to Near-Zero-Fluoroscopy Approach
3.6. Procedural Complications
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3361. [Google Scholar] [PubMed]
- Shope, T.B. Radiation-Induced Skin Injuries from Fluoroscopy. Radiographics 1996, 16, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Elmaraezy, A.; Ebraheem Morra, M.; Tarek Mohammed, A.; Al-Habbaa, A.; Elgebaly, A.; Abdelmotaleb Ghazy, A.; Khalil, A.M.; Tien Huy, N.; Hirayama, K. Risk of Cataract among Interventional Cardiologists and Catheterization Lab Staff: A Systematic Review and Meta-Analysis. Catheter. Cardiovasc. Interv. 2017, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Vano, E.; Domenici, L.; Bottai, M.; Thierry-Chef, I. Cancer and Non-Cancer Brain and Eye Effects of Chronic Low-Dose Ionizing Radiation Exposure. BMC Cancer 2012, 12, 157. [Google Scholar] [CrossRef]
- Kawakami, T.; Saito, N.; Yamamoto, K.; Wada, S.; Itakura, D.; Momma, I.; Kimura, T.; Sasaki, H.; Ando, T.; Takahashi, H.; et al. Zero-fluoroscopy Ablation for Cardiac Arrhythmias: A Single-center Experience in Japan. J. Arrhythmia 2021, 37, 1488–1496. [Google Scholar] [CrossRef]
- Houmsse, M.; Daoud, E.G. Radiation Exposure: A Silent Complication of Catheter Ablation Procedures. Heart Rhythm 2012, 9, 715–716. [Google Scholar] [CrossRef]
- De Ponti, R. Reduction of Radiation Exposure in Catheter Ablation of Atrial Fibrillation: Lesson Learned. World J. Cardiol. 2015, 7, 442–448. [Google Scholar] [CrossRef]
- Preda, A.; Bonvicini, E.; Coradello, E.; Testoni, A.; Gigli, L.; Baroni, M.; Carbonaro, M.; Vargiu, S.; Varrenti, M.; Colombo, G.; et al. The Fluoroless Future in Electrophysiology: A State-of-the-Art Review. Diagnostics 2024, 14, 182. [Google Scholar] [CrossRef]
- Casella, M.; Dello Russo, A.; Pelargonio, G.; Del Greco, M.; Zingarini, G.; Piacenti, M.; Di Cori, A.; Casula, V.; Marini, M.; Pizzamiglio, F.; et al. Near zerO Fluoroscopic exPosure during Catheter ablAtion of supRavenTricular arrhYthmias: The NO-PARTY Multicentre Randomized Trial. EP Eur. 2016, 18, 1565–1572. [Google Scholar] [CrossRef]
- Zei, P.C.; Quadros, K.K.; Clopton, P.; Thosani, A.; Ferguson, J.; Brodt, C.; O’Riordan, G.; Ramsis, M.; Mitra, R.; Baykaner, T. Safety and Efficacy of Minimal-versus Zero-Fluoroscopy Radiofrequency Catheter Ablation for Atrial Fibrillation: A Multicenter, Prospective Study. J. Innov. Card. Rhythm Manag. 2020, 11, 4281–4291. [Google Scholar] [CrossRef]
- Palmeri, N.O.; Alyesh, D.; Keith, M.; Greenhaw, E.; Erickson, C.; Choe, W.; Sundaram, S. Pulsed-Field Ablation for Atrial Fibrillation without the Use of Fluoroscopy. J. Interv. Card. Electrophysiol. 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Torma, D.; Janosi, K.; Debreceni, D.; Bocz, B.; Keseru, M.; Simor, T.; Kupo, P. Initial Experience with Zero-Fluoroscopy Pulmonary Vein Isolation in Patients with Atrial Fibrillation: Single-Center Observational Trial. Sci. Rep. 2024, 14, 16332. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Sadek, M.; Hanson, M.; Yang, J.; Matos, C.D.; Neira, V.; Marchlinski, F.; Miranda-Arboleda, A.; Orellana-Cáceres, J.-J.; Alviz, I.; et al. Feasibility, Efficacy, and Safety of Fluoroless Ablation of VT in Patients with Structural Heart Disease. JACC Clin. Electrophysiol. 2024, 10, 1287–1300. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, J.; Zhang, B.; Han, J.; Zuo, C.; Lu, X.; Xuan, J.; Guo, X. Clinical Outcomes of Radiofrequency Catheter Ablation Guided by Intracardiac Echocardiography for Chinese Atrial Fibrillation Patients: A Single-Center, Retrospective Study. J. Thorac. Dis. 2024, 16, 2341–2352. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Yang, M.; Li, Z.; Zhou, R.; Lin, W.; Zheng, C.; Hu, Y.; Li, J.; Li, Y.; et al. Optimizing Transseptal Puncture Guided by Three-Dimensional Mapping: The Role of a Unipolar Electrogram in a Needle Tip. Europace 2024, 26, euae098. [Google Scholar] [CrossRef]
- Falasconi, G.; Penela, D.; Soto-Iglesias, D.; Jáuregui, B.; Chauca, A.; Antonio, R.S.; Ordoñez, A.; Teres, C.; Carreño, J.M.; Scherer, C.; et al. A Standardized Stepwise Zero-Fluoroscopy Approach with Transesophageal Echocardiography Guidance for Atrial Fibrillation Ablation. J. Interv. Card. Electrophysiol. 2022, 64, 629–639. [Google Scholar] [CrossRef]
- Freitas-Ferraz, A.B.; Bernier, M.; Vaillancourt, R.; Ugalde, P.A.; Nicodème, F.; Paradis, J.-M.; Champagne, J.; O’Hara, G.; Junquera, L.; del Val, D.; et al. Safety of Transesophageal Echocardiography to Guide Structural Cardiac Interventions. J. Am. Coll. Cardiol. 2020, 75, 3164–3173. [Google Scholar] [CrossRef]
- Weinmann, K.; Heudorfer, R.; Lenz, A.; Aktolga, D.; Rattka, M.; Bothner, C.; Pott, A.; Öchsner, W.; Rottbauer, W.; Dahme, T. Safety of Conscious Sedation in Electroanatomical Mapping Procedures and Cryoballoon Pulmonary Vein Isolation. Heart Vessels 2021, 36, 561–567. [Google Scholar] [CrossRef]
- Teumer, Y.; Eckart, D.; Katov, L.; Graf, M.; Bothner, C.; Rottbauer, W.; Weinmann-Emhardt, K. Ultrasound-Guided Venous Puncture Reduces Groin Complications in Electrophysiological Procedures. Biomedicines 2024, 12, 2375. [Google Scholar] [CrossRef]
- Katov, L.; Teumer, Y.; Lederbogen, K.; Melnic, R.; Rottbauer, W.; Bothner, C.; Weinmann-Emhardt, K. Transesophageal Echocardiography Improves Precision in Transseptal Puncture Compared to Fluoroscopy in Left Atrial Electrophysiological Procedures. J. Clin. Med. 2024, 13, 2476. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Wittkampf, F.H.M.; Vano, E.; Ernst, S.; Schilling, R.; Picano, E.; Mont, L.; Group, E.S.D.; Jais, P.; de Bono, J.; et al. Practical Ways to Reduce Radiation Dose for Patients and Staff during Device Implantations and Electrophysiological Procedures. Europace 2014, 16, 946–964. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Piccaluga, E.; Padovani, R.; Traino, C.A.; Andreassi, M.G.; Guagliumi, G. Risks Related to Fluoroscopy Radiation Associated with Electrophysiology Procedures. J. Atr. Fibrillation 2014, 7, 1044. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.; Miranda-Arboleda, A.F.; Velasco, A.; Varley, A.L.; Rajendra, A.; Morales, G.X.; Hoyos, C.; Matos, C.; Thorne, C.; D’Souza, B.; et al. Real-World Data of Radiofrequency Catheter Ablation in Paroxysmal Atrial Fibrillation: Short- and Long-Term Clinical Outcomes from the Prospective Multicenter REAL-AF Registry. Heart Rhythm 2024, 21, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Shao, B.; Wu, W.; Zhou, L.; Cui, J.; Chen, W.; Zhang, R.; Liu, F. Safety and Efficacy of Intracardiac Echocardiography-Guided Zero-Fluoroscopy Ablation in Atrial Fibrillation Patients: A Comparative Study of High-Power Short-Duration and Low-Power Long-Duration Strategies. Front. Cardiovasc. Med. 2024, 11, 1510889. [Google Scholar] [CrossRef]
- Edwards, M.; Bushong, S.C.; Dalrymple, G.V.; Kereiakes, J.G.; Gibbs, S.J. Report No. 107—Implementation of the Principle of as Low as Reasonably Achievable (ALARA) for Medical and Dental Personnel; National Council on Radiation Protection and Measurements (NCRP): Bethesda, MD, USA, 1990. [Google Scholar]
- Shuang, T.; Kong, L.; Cheng, F.; Wang, X. Prevalence, Predictors and Mechanisms of Steam Pops in Ablation Index-Guided High-Power Pulmonary Vein Isolation. J. Cardiovasc. Dev. Dis. 2022, 9, 441. [Google Scholar] [CrossRef]
- Chen, S.; Schmidt, B.; Bordignon, S.; Urbanek, L.; Tohoku, S.; Bologna, F.; Angelkov, L.; Garvanski, I.; Tsianakas, N.; Konstantinou, A.; et al. Ablation Index-Guided 50 W Ablation for Pulmonary Vein Isolation in Patients with Atrial Fibrillation: Procedural Data, Lesion Analysis, and Initial Results from the FAFA AI High Power Study. J. Cardiovasc. Electrophysiol. 2019, 30, 2724–2731. [Google Scholar] [CrossRef]
- du Fay de Lavallaz, J.; Badertscher, P.; Ghannam, M.; Oral, H.; Jongnarangsin, K.; Boveda, S.; Madeira, M.; Gupta, D.; Ding, W.Y.; Providencia, R.; et al. Severe Periprocedural Complications After Ablation for Atrial Fibrillation: An International Collaborative Individual Patient Data Registry. JACC Clin. Electrophysiol. 2024, 10, 1353–1364. [Google Scholar] [CrossRef]
- Steinbeck, G.; Sinner, M.F.; Lutz, M.; Müller-Nurasyid, M.; Kääb, S.; Reinecke, H. Incidence of Complications Related to Catheter Ablation of Atrial Fibrillation and Atrial Flutter: A Nationwide in-Hospital Analysis of Administrative Data for Germany in 2014. Eur. Heart J. 2018, 39, 4020–4029. [Google Scholar] [CrossRef]
- Shimamoto, K.; Yamagata, K.; Wakamiya, A.; Ueda, N.; Kamakura, T.; Wada, M.; Inoue-Yamada, Y.; Miyamoto, K.; Nagase, S.; Kusano, K.F. Zero-Fluoroscopy Ablation in Patients with Cardiac Electronic Implantable Devices. J. Cardiovasc. Electrophysiol. 2022, 33, 423–429. [Google Scholar] [CrossRef]
- Bigelow, A.M.; Clark, J.M. Learning Curve of Zero Fluoroscopy. In Cardiac Electrophysiology Without Fluoroscopy; Proietti, R., Wang, Y., Yao, Y., Zhong, G.Q., Wu, S.L., Ayala-Paredes, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 65–77. ISBN 978-3-030-16992-3. [Google Scholar]
- Purtell, C.S.; Kipp, R.T.; Eckhardt, L.L. Into a Fluoroless Future: An Appraisal of Fluoroscopy-Free Techniques in Clinical Cardiac Electrophysiology. Curr. Cardiol. Rep. 2021, 23, 28. [Google Scholar] [CrossRef]
- Andrew, S.; Abdelmonem, M.R.; Kohli, S.; Dabke, H. Evaluation of Back Pain and Lead Apron Use Among Staff at a District General Hospital. Cureus 2021, 13, e18859. [Google Scholar] [CrossRef]
- Hout, J.D.; Ryu, J. The Association between Musculoskeletal Disorders and Lead Apron Use in Healthcare Workers: A Systematic Review and Meta-Analysis. Saf. Sci. 2025, 181, 106669. [Google Scholar] [CrossRef]

| Baseline Characteristics | All Patients (n = 142) | Zero-Fluoro/Near-Zero-Fluoro Group (n = 73) | Fluoro Group (n = 69) | p-Value |
|---|---|---|---|---|
| Age [years], median (IQR) | 73.0 (64.0; 79.0) | 70.5 (62.2; 77.7) | 72.0 (67.0; 79.5) | 0.05 |
| Female, n (%) | 58.0 (40.8) | 29.0 (39.7) | 29.0 (42.0) | 0.78 |
| BMI [kg/m2], median (IQR) | 27.2 (24.7; 30.4) | 27.4 (25.0; 29.7) | 26.9 (24.7; 30.6) | 0.68 |
| CHA2DS2-VA score, median (IQR) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | 0.46 |
| LVEF (%), median (IQR) | 54.0 (40.2; 60.0) | 56.0 (41.7; 60.7) | 50.5 (38.5; 60.0) | 0.36 |
| LA diameter [cm], median (IQR) | 4.4 (3.7; 5.2) | 4.2 (3.5; 5.2) | 4.6 (3.9; 5.3) | 0.20 |
| Arterial hypertension, n (%) | 100.0 (70.4) | 53.0 (72.6) | 47.0 (68.1) | 0.56 |
| Diabetes mellitus, n (%) | 23.0 (16.2) | 13.0 (17.8) | 10.0 (14.5) | 0.59 |
| Coronary artery disease, n (%) | 61.0 (43.0) | 29.0 (39.7) | 32.0 (46.4) | 0.42 |
| OSA, n (%) | 16.0 (11.3) | 7.0 (9.6) | 9.0 (13.0) | 0.51 |
| COPD, n (%) | 6.0 (4.2) | 1.0 (1.4) | 5.0 (7.2) | 0.08 |
| Positive family history for vascular or cardiac events, n (%) | 21.0 (14.8) | 10.0 (13.7) | 11.0 (15.9) | 0.71 |
| Former or current nicotine abuse, n (%) | 35.0 (24.6) | 15.0 (20.5) | 20.0 (29.0) | 0.44 |
| Procedural Characteristics | All Patients (n = 142) | Zero-Fluoro Group (n = 53) | Near-Zero-Fluoro Group (n = 20) | Combined Zero-Fluoro/Near-Zero-Fluoro Group (n = 73) | Fluoro Group (n = 69) | p-Value |
|---|---|---|---|---|---|---|
| Procedure duration [minutes], median (IQR) | 132.5 (107.5; 160.2) | 130.0 (107.0; 157.0) | 140.5 (127.0; 165.5) | 132.0 (110.0; 157.0) | 133.0 (101.5; 161.5) | 0.52 |
| Fluoroscopy time [minutes], median (IQR) | 2.3 (0.0; 9.6) | 0 | 0.4 (0; 1.4) | 0 | 9.7 (5.9; 15.3) | <0.001 |
| Fluoroscopy dose [cGy·cm2], median (IQR) | 95.4 (0.0; 740.1) | 0 | 16.9 (5.5; 51.8) | 0.0 (0.0; 0.3) | 755.8 (519.0; 1467.9) | <0.001 |
| Procedures | All Patients (n = 142) | Zero-Fluoro Group (n = 53) | Near-Zero-Fluoro Group (n = 20) | Combined Zero-Fluoro/Near-Zero-Fluoro Group (n = 73) | Fluoro Group (n = 69) | p-Value |
|---|---|---|---|---|---|---|
| First-time PVI, n (%) | 31.0 (21.8) | 7.0 (13.2) | 4.0 (20.0) | 11.0 (15.1) | 20.0 (29.0) | 0.07 |
| Redo AF ablation, n (%) | 69.0 (48.6) | 24.0 (45.3) | 9.0 (45.0) | 33.0 (45.2) | 36.0 (52.2) | 0.50 |
| Atypical LA AFL, n (%) | 27.0 (19.0) | 13.0 (24.5) | 3.0 (15.0) | 16.0 (21.9) | 11.0 (15.9) | 0.49 |
| Focal LAT, n (%) | 11.0 (7.7) | 7.0 (13.2) | 2.0 (10.0) | 9.0 (12.3) | 2.0 (2.9) | 0.07 |
| WPW syndrome, n (%) | 4.0 (2.8) | 2.0 (3.8) | 2.0 (10.0) | 4.0 (5.5) | 0 | 0.12 |
| Regression Coefficient | Standard Error | z-Value | Sig. | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| First-time PVI, n (%) | 0.5 | 0.4 | 1.1 | 0.25 | 1.7 | 0.7–4.0 |
| Redo AF ablation, n (%) | −0.5 | 0.4 | 1.1 | 0.25 | 0.6 | 0.2–1.4 |
| Atypical LA AFL, n (%) | −0.5 | 0.5 | 1 | 0.31 | 0.6 | 0.3–1.5 |
| Focal LAT, n (%) | −1.6 | 0.8 | 1.9 | 0.05 | 0.2 | 0.1–1.0 |
| WPW syndrome, n (%) | −20.4 | 7968.5 | 0 | 0.99 | N/A * | N/A * |
| Reasons for Fluoroscopy Usage | Near-Zero-Fluoroscopy Group (n = 20) |
|---|---|
| Complex CS catheter placement, n (%) | 6.0 (30.0) |
| Esophageal temperature probe not visible, n (%) | 5.0 (25.0) |
| PM/ICD leads, n (%) | 3.0 (15.0) |
| Pericardial drainage, n (%) | 3.0 (15.0) |
| Difficult TSP, n (%) | 2.0 (10.0) |
| ASD occluder, n (%) | 1.0 (5.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katov, L.; Kistner, T.; Teumer, Y.; Diofano, F.; Bothner, C.; Rottbauer, W.; Weinmann-Emhardt, K. Safety and Efficacy of TEE Guidance in Electrophysiological Procedures Without Fluoroscopy. J. Clin. Med. 2025, 14, 1917. https://doi.org/10.3390/jcm14061917
Katov L, Kistner T, Teumer Y, Diofano F, Bothner C, Rottbauer W, Weinmann-Emhardt K. Safety and Efficacy of TEE Guidance in Electrophysiological Procedures Without Fluoroscopy. Journal of Clinical Medicine. 2025; 14(6):1917. https://doi.org/10.3390/jcm14061917
Chicago/Turabian StyleKatov, Lyuboslav, Theresa Kistner, Yannick Teumer, Federica Diofano, Carlo Bothner, Wolfgang Rottbauer, and Karolina Weinmann-Emhardt. 2025. "Safety and Efficacy of TEE Guidance in Electrophysiological Procedures Without Fluoroscopy" Journal of Clinical Medicine 14, no. 6: 1917. https://doi.org/10.3390/jcm14061917
APA StyleKatov, L., Kistner, T., Teumer, Y., Diofano, F., Bothner, C., Rottbauer, W., & Weinmann-Emhardt, K. (2025). Safety and Efficacy of TEE Guidance in Electrophysiological Procedures Without Fluoroscopy. Journal of Clinical Medicine, 14(6), 1917. https://doi.org/10.3390/jcm14061917





