Severely Ill COVID-19 Patients May Exhibit Hypercoagulability Despite Escalated Anticoagulation
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Thrombodynamics Assay
2.3. Other Assays
2.4. Materials
2.5. Outcomes
2.6. Ethics Statement
2.7. Statistical Analysis
3. Results
3.1. Heparins Treated Baseline Hypercoagulability in the Majority of Patients
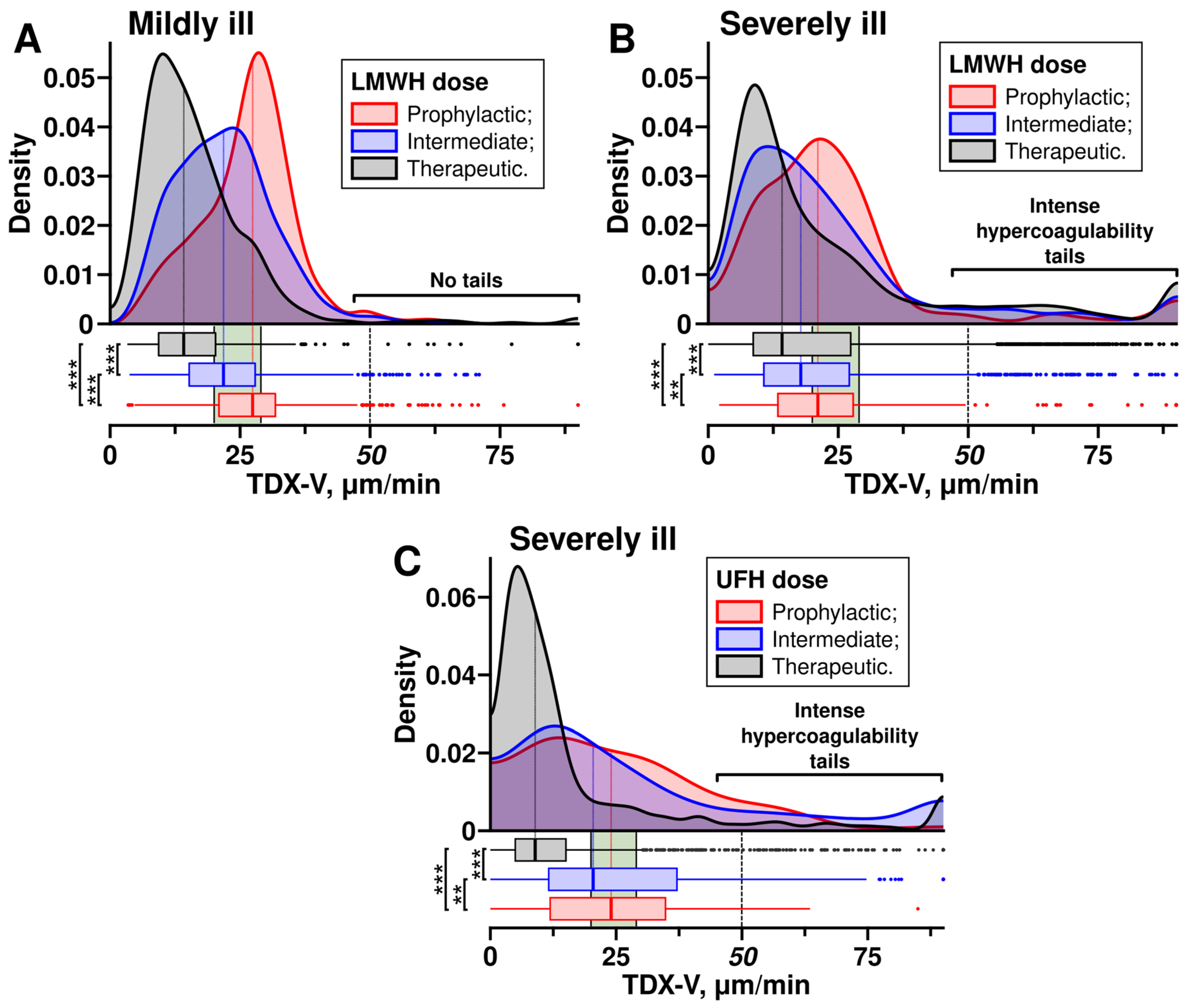
3.2. Escalated Doses of Heparins Did Not Prevent Intense Hypercoagulability in Severely Ill Patients
3.3. Intense Hypercoagulability Was Temporary
3.4. Severely Ill Patients with Intense Hypercoagulability Had Higher Levels of D-Dimer, Inflammation Markers and Better Glomerular Filtration Rates
3.5. Intense Hypercoagulability Was a Risk Factor for Thrombosis and Death
3.6. Combining TDX-V and D-Dimer Assays Can Enhance the Accuracy of Predicting Thrombotic Events and Fatal Outcomes
4. Discussion
4.1. Study Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Covitro Investigators
Conflicts of Interest
References
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Comer, S.P.; Cullivan, S.; Szklanna, P.B.; Weiss, L.; Cullen, S.; Kelliher, S.; Smolenski, A.; Murphy, C.; Altaie, H.; Curran, J.; et al. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021, 19, e3001109. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, J.; Haslbauer, J.D.; Stalder, A.K.; Romanens, A.; Mertz, K.D.; Studt, J.D.; Siegemund, M.; Buser, A.; Holbro, A.; Tzankov, A. Von Willebrand factor and the thrombophilia of severe COVID-19: In situ evidence from autopsies. Res. Pract. Thromb. Haemost. 2023, 7, 100182. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil Extracellular Traps: Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef]
- Rosell, A.; Havervall, S.; von Meijenfeldt, F.; Hisada, Y.; Aguilera, K.; Grover, S.P.; Lisman, T.; Mackman, N.; Thålin, C. Patients with COVID-19 Have Elevated Levels of Circulating Extracellular Vesicle Tissue Factor Activity That Is Associated with Severity and Mortality-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 878–882. [Google Scholar] [CrossRef]
- Balbi, C.; Burrello, J.; Bolis, S.; Lazzarini, E.; Biemmi, V.; Pianezzi, E.; Burrello, A.; Caporali, E.; Grazioli, L.G.; Martinetti, G.; et al. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine 2021, 67, 103369. [Google Scholar] [CrossRef]
- Billett, H.H.; Reyes-Gil, M.; Szymanski, J.; Ikemura, K.; Stahl, L.R.; Lo, Y.; Rahman, S.; Gonzalez-Lugo, J.D.; Kushnir, M.; Barouqa, M.; et al. Anticoagulation in COVID-19: Effect of Enoxaparin, Heparin, and Apixaban on Mortality. Thromb. Haemost. 2020, 120, 1691–1699. [Google Scholar]
- Nadkarni, G.N.; Lala, A.; Bagiella, E.; Chang, H.L.; Moreno, P.R.; Pujadas, E.; Arvind, V.; Bose, S.; Charney, A.W.; Chen, M.D.; et al. Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 1815–1826. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Levy, J.H.; Ageno, W.; Connors, J.M.; Hunt, B.J.; Iba, T.; Levi, M.; Samama, C.M.; Thachil, J.; Giannis, D.; et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Baumann Kreuziger, L.; Sholzberg, M.; Cushman, M. Anticoagulation in hospitalized patients with COVID-19. Blood 2022, 140, 809–814. [Google Scholar] [CrossRef]
- Mansory, E.M.; Srigunapalan, S.; Lazo-Langner, A. Venous Thromboembolism in Hospitalized Critical and Noncritical COVID-19 Patients: A Systematic Review and Meta-analysis. TH Open 2021, 5, e286–e294. [Google Scholar] [CrossRef]
- Salah, H.M.; Naser, J.A.; Calcaterra, G.; Bassareo, P.P.; Mehta, J.L. The Effect of Anticoagulation Use on Mortality in COVID-19 Infection. Am. J. Cardiol. 2020, 134, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Maatman, T.K.; Jalali, F.; Feizpour, C.; Douglas, A., 2nd; McGuire, S.P.; Kinnaman, G.; Hartwell, J.L.; Maatman, B.T.; Kreutz, R.P.; Kapoor, R.; et al. Routine Venous Thromboembolism Prophylaxis May Be Inadequate in the Hypercoagulable State of Severe Coronavirus Disease 2019. Crit. Care Med. 2020, 48, e783–e790. [Google Scholar] [CrossRef]
- Stattin, K.; Lipcsey, M.; Andersson, H.; Pontén, E.; Bülow Anderberg, S.; Gradin, A.; Larsson, A.; Lubenow, N.; von Seth, M.; Rubertsson, S.; et al. Inadequate prophylactic effect of low-molecular weight heparin in critically ill COVID-19 patients. J. Crit. Care. 2020, 60, 249–252. [Google Scholar] [CrossRef]
- Bohula, E.A.; Berg, D.D.; Lopes, M.S.; Connors, J.M.; Babar, I.; Barnett, C.F.; Chaudhry, S.P.; Chopra, A.; Ginete, W.; Ieong, M.H.; et al. Anticoagulation Antiplatelet Therapy for Prevention of Venous Arterial Thrombotic Events in Critically Ill Patients with COVID-19: COVID-PACT. Circulation 2022, 146, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- INSPIRATION Investigators; Sadeghipour, P.; Talasaz, A.H.; Rashidi, F.; Sharif-Kashani, B.; Beigmohammadi, M.T.; Farrokhpour, M.; Sezavar, S.H.; Payandemehr, P.; Dabbagh, A.; et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients with COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar]
- REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators; Goligher, E.C.; Bradbury, C.A.; McVerry, B.J.; Lawler, P.R.; Berger, J.S.; Gong, M.N.; Carrier, M.; et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 777–789. [Google Scholar]
- Spyropoulos, A.C.; Goldin, M.; Giannis, D.; Diab, W.; Wang, J.; Khanijo, S.; Mignatti, A.; Gianos, E.; Cohen, M.; Sharifova, G.; et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients with COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 1612–1620, Erratum in JAMA Intern. Med. 2022, 182, 239. https://doi.org/10.1001/jamainternmed.2021.7668. [Google Scholar] [CrossRef]
- Perepu, U.S.; Chambers, I.; Wahab, A.; Ten Eyck, P.; Wu, C.; Dayal, S.; Sutamtewagul, G.; Bailey, S.R.; Rosenstein, L.J.; Lentz, S.R. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: A multi-center, open-label, randomized controlled trial. J. Thromb. Haemost. 2021, 19, 2225–2234. [Google Scholar] [CrossRef]
- Blondon, M.; Cereghetti, S.; Pugin, J.; Marti, C.; Darbellay Farhoumand, P.; Reny, J.L.; Calmy, A.; Combescure, C.; Mazzolai, L.; Pantet, O.; et al. Therapeutic anticoagulation to prevent thrombosis, coagulopathy, and mortality in severe COVID-19: The Swiss COVID-HEP randomized clinical trial. Res. Pract. Thromb. Haemost. 2022, 6, e12712. [Google Scholar] [CrossRef]
- Zuily, S.; Lefèvre, B.; Sanchez, O.; Empis de Vendin, O.; de Ciancio, G.; Arlet, J.B.; Khider, L.; Terriat, B.; Greigert, H.; Robert, C.S.; et al. Effect of weight-adjusted intermediate-dose versus fixed-dose prophylactic anticoagulation with low-molecular-weight heparin on venous thromboembolism among noncritically and critically ill patients with COVID-19: The COVI-DOSE trial, a multicenter, randomised, open-label, phase 4 trial. EClinicalMedicine 2023, 60, 102031. [Google Scholar]
- Labbé, V.; Contou, D.; Heming, N.; Megarbane, B.; Razazi, K.; Boissier, F.; Ait-Oufella, H.; Turpin, M.; Carreira, S.; Robert, A.; et al. Effects of Standard-Dose Prophylactic, High-Dose Prophylactic, and Therapeutic Anticoagulation in Patients with Hypoxemic COVID-19 Pneumonia: The ANTICOVID Randomized Clinical Trial. JAMA Intern. Med. 2023, 183, 520–531. [Google Scholar] [CrossRef]
- Wu, M.A.; Del GIovane, C.; Colombo, R.; Dolci, G.; Arquati, M.; Vicini, R.; Russo, U.; Ruggiero, D.; Coluccio, V.; Taino, A.; et al. Low-molecular-weight heparin for the prevention of clinical worsening in severe non-critically ill COVID-19 patients: A joint analysis of two randomized controlled trials. Intern. Emerg. Med. 2024, 19, 71–79. [Google Scholar] [CrossRef]
- Joshi, D.; Manohar, S.; Goel, G.; Saigal, S.; Pakhare, A.P.; Goyal, A. Adequate Antithrombin III Level Predicts Survival in Severe COVID-19 Pneumonia. Cureus. 2021, 13, e18538. [Google Scholar] [CrossRef]
- Chen-Goodspeed, A.; Dronavalli, G.; Zhang, X.; Podbielski, J.M.; Patel, B.; Modis, K.; Cotton, B.A.; Wade, C.E.; Cardenas, J.C. Antithrombin Activity Is Associated with Persistent Thromboinflammation and Mortality in Patients with Severe COVID-19 Illness. Acta Haematol. 2023, 146, 117–124. [Google Scholar] [CrossRef]
- Cuker, A.; Tseng, E.K.; Schünemann, H.J.; Angchaisuksiri, P.; Blair, C.; Dane, K.; DeSancho, M.T.; Diuguid, D.; Griffin, D.O.; Kahn, S.R.; et al. American Society of Hematology living guidelines on the use of anticoagulation for thromboprophylaxis for patients with COVID-19: March 2022 update on the use of anticoagulation in critically ill patients. Blood Adv. 2022, 6, 4975–4982. [Google Scholar] [CrossRef]
- ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators; Lawler, P.R.; Goligher, E.C.; Berger, J.S.; Neal, M.D.; McVerry, B.J.; Nicolau, J.C.; Gong, M.N.; et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar]
- Sholzberg, M.; Tang, G.H.; Rahhal, H.; AlHamzah, M.; Kreuziger, L.B.; Áinle, F.N.; Alomran, F.; Alayed, K.; Alsheef, M.; AlSumait, F.; et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021, 375, n2400. [Google Scholar] [CrossRef]
- Qiu, X.; Nair, M.G.; Jaroszewski, L.; Godzik, A. Deciphering Abnormal Platelet Subpopulations in COVID-19, Sepsis and Systemic Lupus Erythematosus through Machine Learning and Single-Cell Transcriptomics. Int. J. Mol. Sci. 2024, 25, 5941. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Panteleev, M.A.; Sveshnikova, A.N.; Shakhidzhanov, S.S.; Zamaraev, A.V.; Ataullakhanov, F.I.; Rumyantsev, A.G. The Ways of the Virus: Interactions of Platelets and Red Blood Cells with SARS-CoV-2, and Their Potential Pathophysiological Significance in COVID-19. Int. J. Mol. Sci. 2023, 24, 17291. [Google Scholar] [CrossRef]
- Barrett, T.J.; Bilaloglu, S.; Cornwell, M.; Burgess, H.M.; Virginio, V.W.; Drenkova, K.; Ibrahim, H.; Yuriditsky, E.; Aphinyanaphongs, Y.; Lifshitz, M.; et al. Platelets contribute to disease severity in COVID-19. J. Thromb. Haemost. 2021, 19, 3139–3153. [Google Scholar] [CrossRef] [PubMed]
- Althaus, K.; Marini, I.; Zlamal, J.; Pelzl, L.; Singh, A.; Häberle, H.; Mehrländer, M.; Hammer, S.; Schulze, H.; Bitzer, M.; et al. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood 2021, 137, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Martyanov, A.A.; Boldova, A.E.; Stepanyan, M.G.; An, O.I.; Gur’ev, A.S.; Kassina, D.V.; Volkov, A.Y.; Balatskiy, A.V.; Butylin, A.A.; Karamzin, S.S.; et al. Longitudinal multiparametric characterization of platelet dysfunction in COVID-19: Effects of disease severity, anticoagulation therapy and inflammatory status. Thromb. Res. 2022, 211, 27–37. [Google Scholar] [CrossRef]
- Schrottmaier, W.C.; Pirabe, A.; Pereyra, D.; Heber, S.; Hackl, H.; Schmuckenschlager, A.; Brunnthaler, L.; Santol, J.; Kammerer, K.; Oosterlee, J.; et al. Platelets and Antiplatelet Medication in COVID-19-Related Thrombotic Complications. Front. Cardiovasc. Med. 2022, 8, 802566. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022, 399, 143–151. [Google Scholar]
- Berger, J.S.; Kornblith, L.Z.; Gong, M.N.; Reynolds, H.R.; Cushman, M.; Cheng, Y.; McVerry, B.J.; Kim, K.S.; Lopes, R.D.; Atassi, B.; et al. Effect of P2Y12 Inhibitors on Survival Free of Organ Support Among Non-Critically Ill Hospitalized Patients with COVID-19: A Randomized Clinical Trial. JAMA 2022, 327, 227–236. [Google Scholar] [CrossRef]
- de Maistre, E.; Savard, P.; Guinot, P.G. COVID-19 and the Concept of Thrombo-Inflammation: Review of the Relationship between Immune Response, Endothelium and Coagulation. J. Clin. Med. 2023, 12, 7245. [Google Scholar] [CrossRef]
- Sinauridze, E.I.; Vuimo, T.A.; Tarandovskiy, I.D.; Ovsepyan, R.A.; Surov, S.S.; Korotina, N.G.; Serebriyskiy, I.I.; Lutsenko, M.M.; Sokolov, A.L.; Ataullakhanov, F.I. Thrombodynamics, a new global coagulation test: Measurement of heparin efficiency. Talanta 2018, 180, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Soshitova, N.P.; Karamzin, S.S.; Balandina, A.N.; Fadeeva, O.A.; Kretchetova, A.V.; Galstian, G.M.; Panteleev, M.A.; Ataullakhanov, F.I. Predicting prothrombotic tendencies in sepsis using spatial clot growth dynamics. Blood Coagul. Fibrinolysis 2012, 23, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Balandina, A.N.; Serebriyskiy, I.I.; Poletaev, A.V.; Polokhov, D.M.; Gracheva, M.A.; Koltsova, E.M.; Vardanyan, D.M.; Taranenko, I.A.; Krylov, A.Y.; Urnova, E.S.; et al. Thrombodynamics-A new global hemostasis assay for heparin monitoring in patients under the anticoagulant treatment. PLoS ONE 2018, 13, e0199900. [Google Scholar] [CrossRef]
- Rochon, J.; Gondan, M.; Kieser, M. To test or not to test: Preliminary assessment of normality when comparing two independent samples. BMC Med. Res. Methodol. 2012, 12, 81. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 March 2025).
- Bigdelou, B.; Sepand, M.R.; Najafikhoshnoo, S.; Negrete, J.A.T.; Sharaf, M.; Ho, J.Q.; Sullivan, I.; Chauhan, P.; Etter, M.; Shekarian, T.; et al. COVID-19 and Preexisting Comorbidities: Risks, Synergies, and Clinical Outcomes. Front. Immunol. 2022, 13, 890517. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Saricaoglu, E.M.; Coskun, B.; Ayhan, M.; Akinci, E.; Kayaaslan, B.; Aypak, A.; Tekce, A.Y.T.; Hasanoglu, I.; Kaya, A.; Eser, F.; et al. A New Laboratory Tool for COVID-19 Severity Prediction, CENIL Score. Diagnostics 2024, 14, 2557. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021, 76, 970–979. [Google Scholar] [CrossRef]
- Jiménez, D.; García-Sanchez, A.; Rali, P.; Muriel, A.; Bikdeli, B.; Ruiz-Artacho, P.; Le Mao, R.; Rodríguez, C.; Hunt, B.J.; Monreal, M. Incidence of VTE and Bleeding Among Hospitalized Patients with Coronavirus Disease 2019: A Systematic Review and Meta-analysis. Chest 2021, 159, 1182–1196. [Google Scholar] [CrossRef]
- Fournier, M.; Faille, D.; Dossier, A.; Mageau, A.; Nicaise Roland, P.; Ajzenberg, N.; Borie, R.; Bouadma, L.; Bunel, V.; Castier, Y.; et al. Arterial Thrombotic Events in Adult Inpatients with COVID-19. Mayo Clin. Proc. 2021, 96, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Candeloro, M.; Schulman, S. Arterial Thrombotic Events in Hospitalized COVID-19 Patients: A Short Review and Meta-Analysis. Semin. Thromb Hemost. 2023, 49, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.S.; Kunichoff, D.; Adhikari, S.; Ahuja, T.; Amoroso, N.; Aphinyanaphongs, Y.; Cao, M.; Goldenberg, R.; Hindenburg, A.; Horowitz, J.; et al. Prevalence and Outcomes of D-Dimer Elevation in Hospitalized Patients with COVID-19. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2539–2547. [Google Scholar] [CrossRef]
- Johnson, E.D.; Schell, J.C.; Rodgers, G.M. The D-dimer assay. Am. J. Hematol. 2019, 94, 833–839. [Google Scholar] [CrossRef]
- Nougier, C.; Benoit, R.; Simon, M.; Desmurs-Clavel, H.; Marcotte, G.; Argaud, L.; David, J.S.; Bonnet, A.; Negrier, C.; Dargaud, Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020, 18, 2215–2219. [Google Scholar] [CrossRef]
- Lipets, E.N.; Ataullakhanov, F.I. Global assays of hemostasis in the diagnostics of hypercoagulation and evaluation of thrombosis risk. Thromb. J. 2015, 13, 4. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Yassen, K.A.; Shahwar, D.I.; Alrasasi, A.Q.; Aldandan, F.; Alali, D.S.; Almuslem, M.Y.; Hassanein, N.; Khan, I.; Görlinger, K. Viscoelastic Hemostatic Testing as a Diagnostic Tool for Hypercoagulability in Liver Transplantation: A Narrative Review. J. Clin. Med. 2024, 13, 6279. [Google Scholar] [CrossRef]
- Duarte, R.C.F.; Rios, D.R.A.; Leite, P.M.; Alves, L.C.; Magalhães, H.P.B.; Carvalho, M.D.G. Thrombin generation test for evaluating hemostatic effects of Brazilian snake venoms. Toxicon 2019, 163, 36–43. [Google Scholar] [CrossRef]
- Abd El-Lateef, A.E.; Alghamdi, S.; Ebid, G.; Khalil, K.; Kabrah, S.; Abdel Ghafar, M.T. Coagulation Profile in COVID-19 Patients and its Relation to Disease Severity and Overall Survival: A Single-Center Study. Br. J. Biomed. Sci. 2022, 79, 10098. [Google Scholar] [CrossRef]
- Citu, C.; Burlea, B.; Gorun, F.; Motoc, A.; Gorun, O.M.; Malita, D.; Ratiu, A.; Margan, R.; Grigoras, M.L.; Bratosin, F.; et al. Predictive Value of Blood Coagulation Parameters in Poor Outcomes in COVID-19 Patients: A Retrospective Observational Study in Romania. J. Clin. Med. 2022, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Henry, B.M.; Lippi, G. Is Lupus Anticoagulant a Significant Feature of COVID-19? A Critical Appraisal of the Literature. Semin. Thromb. Hemost. 2022, 48, 55–71. [Google Scholar] [CrossRef]
- Martín-Rojas, R.M.; Pérez-Rus, G.; Delgado-Pinos, V.E.; Domingo-González, A.; Regalado-Artamendi, I.; Alba-Urdiales, N.; Demelo-Rodríguez, P.; Monsalvo, S.; Rodríguez-Macías, G.; Ballesteros, M.; et al. COVID-19 coagulopathy: An in-depth analysis of the coagulation system. Eur. J. Haematol. 2020, 105, 741–750. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kanno, H.; Zhou, Y.; Xiao, T.H.; Suzuki, T.; Ibayashi, Y.; Harmon, J.; Takizawa, S.; Hiramatsu, K.; Nitta, N.; et al. Massive image-based single-cell profiling reveals high levels of circulating platelet aggregates in patients with COVID-19. Nat. Commun. 2021, 12, 7135. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.A.; Al Nozha, O.M. Developing a COVID-19 Mortality Prediction (CoMPred) Indicator for ICU Diabetic Patients Treated with Tocilizumab in Saudi Arabia: A Proof-of-Concept Study. Biomedicines 2023, 11, 2649. [Google Scholar] [CrossRef]
- Gorgojo-Galindo, Ó.; Martín-Fernández, M.; Peñarrubia-Ponce, M.J.; Álvarez, F.J.; Ortega-Loubon, C.; Gonzalo-Benito, H.; Martínez-Paz, P.; Miramontes-González, J.P.; Gómez-Sánchez, E.; Poves-Álvarez, R.; et al. Predictive Modeling of Poor Outcome in Severe COVID-19: A Single-Center Observational Study Based on Clinical, Cytokine and Laboratory Profiles. J. Clin. Med. 2021, 10, 5431. [Google Scholar] [CrossRef]
- Qian, F.H.; Cao, Y.; Liu, Y.X.; Huang, J.; Zhu, R.H. A predictive model to explore risk factors for severe COVID-19. Sci. Rep. 2024, 14, 18197. [Google Scholar] [CrossRef]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef]
- Sayed, A.A. Assessing the Diagnostic Values of the Neutrophil-to-Lymphocyte Ratio (NLR) and Systematic Immunoinflammatory Index (SII) as Biomarkers in Predicting COVID-19 Severity: A Multicentre Comparative Study. Medicina 2024, 60, 602. [Google Scholar] [CrossRef]
- Bulanov, A.Y.; Bulanova, E.L.; Simarova, I.B.; Bovt, E.A.; Eliseeva, O.O.; Shakhidzhanov, S.S.; Panteleev, M.A.; Roumiantsev, A.G.; Ataullakhanov, F.I.; Karamzin, S.S. Integral assays of hemostasis in hospitalized patients with COVID-19 on admission and during heparin thromboprophylaxis. PLoS ONE 2023, 18, e0282939. [Google Scholar] [CrossRef]
- Buffart, B.; Demulder, A.; Fangazio, M.; Rozen, L. Global Hemostasis Potential in COVID-19 Positive Patients Performed on St-Genesia Show Hypercoagulable State. J. Clin. Med. 2022, 11, 7255. [Google Scholar] [CrossRef] [PubMed]
- Campello, E.; Bulato, C.; Spiezia, L.; Boscolo, A.; Poletto, F.; Cola, M.; Gavasso, S.; Simion, C.; Radu, C.M.; Cattelan, A.; et al. Thrombin generation in patients with COVID-19 with and without thromboprophylaxis. Clin. Chem. Lab. Med. 2021, 59, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Lim, W. Low-molecular-weight heparin in patients with chronic renal insufficiency. Intern. Emerg. Med. 2008, 3, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Vahtera, A.; Vaara, S.; Pettilä, V.; Kuitunen, A. Plasma anti-FXa level as a surrogate marker of the adequacy of thromboprophylaxis in critically ill patients: A systematic review. Thromb. Res. 2016, 139, 10–16. [Google Scholar] [CrossRef]
- Dibiasi, C.; Gratz, J.; Wiegele, M.; Baierl, A.; Schaden, E. Anti-factor Xa Activity Is Not Associated with Venous Thromboembolism in Critically Ill Patients Receiving Enoxaparin for Thromboprophylaxis: A Retrospective Observational Study. Front. Med. 2022, 9, 888451. [Google Scholar] [CrossRef]
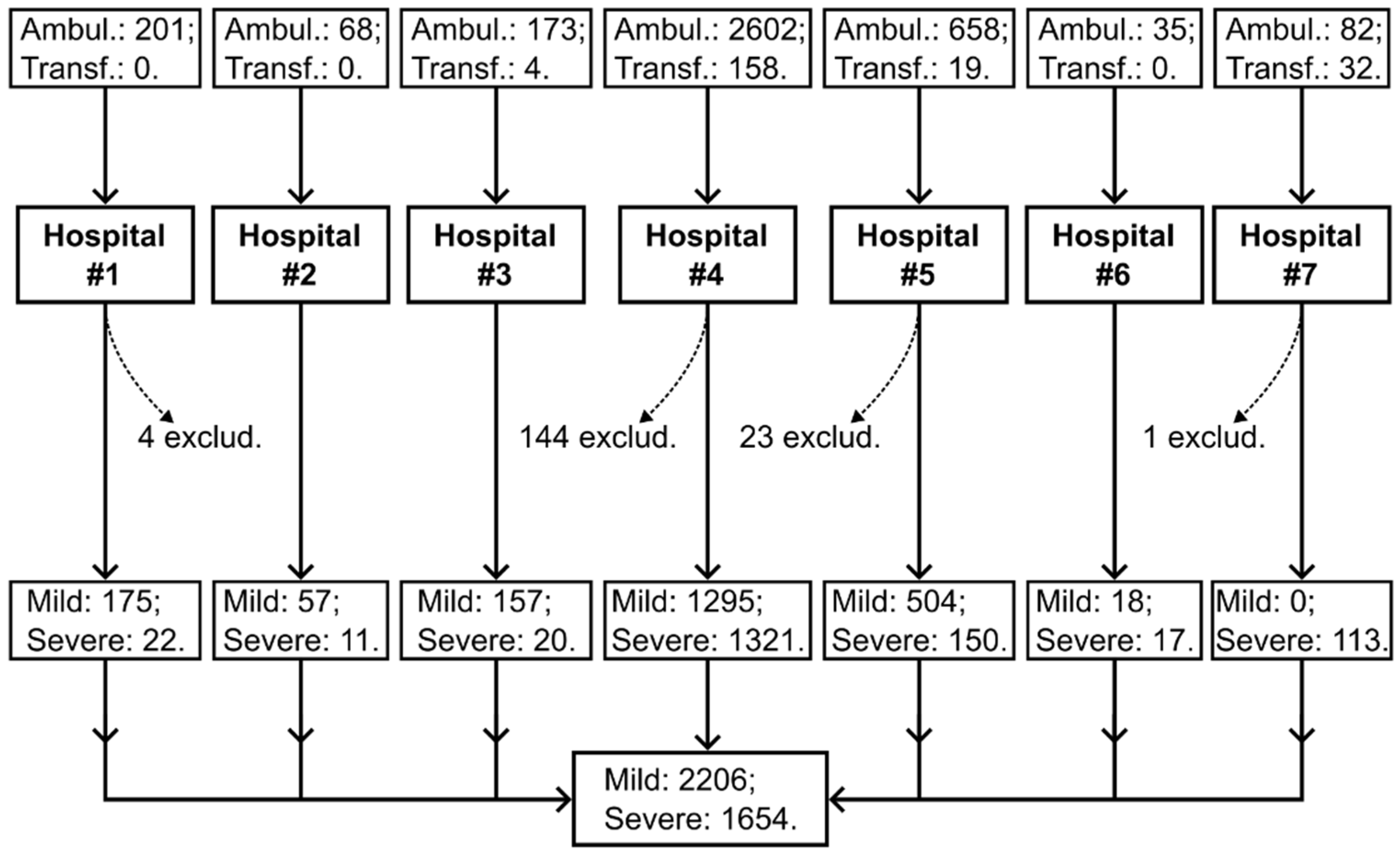




| Variable | Total (n = 3860) |
|---|---|
| Age, median (Q1–Q3) | 64 (54–74) |
| Female, % (n) | 51.1 (1974) |
| Referred from another hospital, % (n) | 5.4 (210) |
| Body mass index, median (Q1–Q3) | 28.5 (25.2–33.1) |
| Death, % (n) | 22.1 (853) |
| Admitted to ICU, % (n) | 42.8 (1654) |
| Length of stay in days, median (Q1–Q3) | 10 (7–15) |
| Vitals on admission, median (Q1–Q3) | |
| SPO2, % | 94 (92–96) |
| Respiratory rate, 1/min | 22 (20–24) |
| Systolic pressure, Hg | 130 (117–140) |
| Diastolic pressure, Hg | 80 (70–87) |
| Heart rate, 1/min | 91 (81–101) |
| Respiratory support on admission, % (n) | |
| ECMO | 0.0 (1) |
| Invasive ventilation | 3.5 (122) |
| Non-invasive ventilation | 7.4 (256) |
| Nasal oxygen | 41.4 (1432) |
| No oxygen therapy | 47.7 (1649) |
| Computer tomography score on admission, % (n) | |
| CT0 | 8.1 (311) |
| CT1 | 36.0 (1388) |
| CT2 | 28.7 (1107) |
| CT3 | 20.1 (776) |
| CT4 | 7.1 (275) |
| Laboratory on admission, median (Q1–Q3) | |
| TDX-V, 20–29 µm/min | 34.8 (29.7–43.8) n = 1370 |
| TDX-TSP, >30 min | 21.9 (17.0–26.0) n = 545 |
| D-dimer, <500 ng/mL | 721 (355–1529) n = 2753 |
| APTT, 25.1–36.5 s | 30.5 (27.9–33.5) n = 3220 |
| PT, 9.4–12.5 s | 13.2 (12.2–14.6) n = 3288 |
| Fibrinogen, 2–4 g/L | 5.6 (4.4–6.9) n = 3074 |
| Hemoglobin, 120–160 g/L | 136 (124–148) n = 3624 |
| Platelet count, 180–320 × 109/L | 202 (156–259) n = 3625 |
| White blood cell count, 4–9 × 109/L | 6.4 (4.8–9.2) n = 3624 |
| Creatinine, 49–104 µmol/L | 95.3 (80.0–114.0) n = 3610 |
| Glucose, 4.1–5.9 mmol/L | 6.4 (5.5–7.8) n = 3576 |
| Alanine aminotransferase, <50 AU/L | 29.4 (19.0–48.0) n = 3581 |
| Aspartate aminotransferase, <50 AU/L | 37.0 (26.3–56.0) n = 3583 |
| Lactate dehydrogenase, <250 AU/L | 324.7 (246.4–486.4) n = 1839 |
| Bilirubin, 5–21 µmol/L | 11.1 (8.2–15.0) n = 3548 |
| C-reactive protein, <5 mg/L | 60.4 (21.0–118.9) n = 3609 |
| CKD-EPI, >60 mL × min−1 × 1.73 m−2 | 56.8 (43.5–69.4) n = 3606 |
| Medication during hospitalization, % (n) | |
| Low-molecular-weight heparins | 94.2 (3635) |
| Unfractionated heparin | 17.9 (691) |
| IL6/IL6R blockers | 13.3 (512) |
| IL17 blockers | 0.1 (4) |
| JAK inhibitors | 3.2 (125) |
| Steroids | 13.5 (520) |
| Antibiotics | 11.3 (437) |
| Statins | 10.2 (392) |
| Diuretics | 15.3 (592) |
| Antiplatelet | 3.8 (147) |
| Comorbidities, % (n) | |
| Peripheral atherosclerosis | 4.8 (187) |
| Coronary artery disease | 18.1 (700) |
| Heart failure | 21.9 (844) |
| CCI+CVD | 22.2 (856) |
| Atrial fibrillation | 14.4 (555) |
| Hypertension | 62.4 (2410) |
| Chronic kidney disease | 14.9 (575) |
| Diabetes mellitus | 23.4 (905) |
| Cancer | 8.6 (333) |
| Acute kidney injury | 10.9 (421) |
| Complications, % (n) | |
| Vein thromboembolism | |
| Deep vein thrombosis | 10.0 (385) |
| Pulmonary embolism | 2.5 (98) |
| Superficial vein thrombosis | 2.6 (99) |
| Other vein thrombosis # | 0.2 (6) |
| Arterial thromboembolism | |
| Acute ischemic stroke | 0.5 (20) |
| Mesenteric artery thrombosis | 0.4 (14) |
| Limb artery thrombosis | 0.9 (35) |
| Other artery thrombosis ## | 0.2 (7) |
| Variable | Severely Ill (n = 1654) | Mildly Ill (n = 2206) | p-Value, Severely vs Mildly Ill |
|---|---|---|---|
| Death, % (n) | 51.6 (853) | 0 (0) | <0.0001 † |
| Age, median (Q1–Q3) | 68 (58–79) | 62 (51–71) | <0.0001 |
| Female, % (n) | 50.5 (835) | 51.6 (1139) | 0.49 |
| Referred from another hospital, % (n) | 9.9 (164) | 2.1 (46) | <0.0001 |
| Body mass index, median (Q1–Q3) | 28.8 (25.3–33.7) | 28.3 (25.2–32.3) | 0.06 |
| Length of stay in days, median (Q1–Q3) | 13 (8–20) | 9 (7–12) | <0.0001 |
| Vitals on admission, median (Q1–Q3) | |||
| SPO2, % | 93 (90–95) | 94 (93–96) | <0.0001 |
| Respiratory rate, 1/min | 24 (21–26) | 22 (20–24) | <0.0001 |
| Systolic pressure, Hg | 129 (115–140) | 130 (119–140) | 0.95 |
| Diastolic pressure, Hg | 78 (70–85) | 80 (70–88) | <0.0001 |
| Heart rate, 1/min | 92 (82–103) | 91 (81–100) | 0.016 |
| Respiratory support on admission, % (n) | |||
| ECMO | 0.1 (1) | 0.0 (0) | n.a. |
| Invasive ventilation | 8.2 (122) | 0.0 (0) | <0.0001 † |
| Non-invasive ventilation | 17.0 (252) | 0.2 (4) | <0.0001 |
| Nasal oxygen | 58.6 (871) | 28.4 (561) | <0.0001 |
| No oxygen therapy | 16.2 (240) | 71.4 (1409) | <0.0001 |
| Computer tomography score on admission, % (n) | |||
| CT0 | 7.4 (123) | 8.5 (188) | 0.23 |
| CT1 | 23.7 (392) | 45.2 (996) | <0.0001 |
| CT2 | 26.0 (429) | 30.8 (678) | 0.001 |
| CT3 | 27.9 (462) | 14.2 (314) | <0.0001 |
| CT4 | 14.9 (247) | 1.3 (28) | <0.0001 |
| Laboratory on admission, median (Q1–Q3) | |||
| TDX-V, 20–29 µm/min | 35.3 (29.1–44.6) n = 241 | 34.7 (29.8–43.7) n = 1129 | 0.84 |
| TDX-TSP, >30 min | 22.1 (16.4–26.3) n = 103 | 21.9 (17.4–25.9) n = 442 | 0.80 |
| D-dimer, <500 ng/mL | 1256 (686–2954) n = 1145 | 487 (277–906) n = 1608 | <0.0001 |
| APTT, 25.1–36.5 s | 30.2 (27.0–33.6) n = 1497 | 30.7 (28.5–33.4) n = 1723 | 0.0002 |
| PT, 9.4–12.5 s | 13.5 (12.4–14.9) n = 1508 | 13.0 (12.0–14.3) n = 1780 | <0.0001 |
| Fibrinogen, 2–4 g/L | 6.0 (4.5–7.4) n = 1441 | 5.3 (4.3–6.6) n = 1633 | <0.0001 |
| Haemoglobin, 120–160 g/L | 134 (119–147) n = 1571 | 138 (127–149) n = 2053 | <0.0001 |
| Platelet count, 180–320 × 109/L | 191 (146–253) n = 1571 | 207 (165–264) n = 2054 | <0.0001 |
| White blood cell count, 4–9 × 109/L | 7.6 (5.4–11.4) n = 1571 | 5.8 (4.5–7.8) n = 2053 | <0.0001 |
| Creatinine, 49–104 µmol/L | 99.0 (81.7–123.8) n = 1586 | 93.0 (79.3–110.0) n = 2024 | <0.0001 |
| Glucose, 4.1–5.9 mmol/L | 7.0 (6.0–9.1) n = 1563 | 6.0 (5.3–7.0) n = 2013 | <0.0001 |
| Alanine aminotransferase, <50 AU/L | 31.0 (20.0–52.0) n = 1573 | 28.0 (18.3–45) n = 2008 | <0.0001 |
| Aspartate aminotransferase, <50 AU/L | 45.7 (31.0–68.8) n = 1575 | 32.0 (25.0–47.0) n = 2008 | <0.0001 |
| Lactate dehydrogenase, <250 AU/L | 431.2, (298.9–610.0) n = 946 | 266.7, (219.8–344.0) n = 893 | <0.0001 |
| Bilirubin, 5–21 µmol/L | 11.5 (8.4–16.3) n = 1568 | 10.8 (8.1–14.1) n = 1980 | <0.0001 |
| C-reactive protein, <5 mg/L | 98.8 (41.1–170.1) n = 1579 | 40.1 (14.7–80.6) n = 2030 | <0.0001 |
| CKD-EPI, >60 mL × min−1 × 1.73 m−2 | 53.4 (38.7–67.1) n = 1584 | 59.3 (47.3–71.1) n = 2022 | <0.0001 |
| Medication during hospitalization, % (n) | |||
| Low-molecular-weight heparins | 93.1 (1540) | 95.0 (2095) | 0.72 |
| Unfractionated heparin | 39.4 (652) | 1.8 (39) | <0.0001 |
| IL6/IL6R blockers | 25.3 (418) | 4.3 (94) | <0.0001 |
| IL17 blockers | 0.0 (0) | 0.2 (4) | n.a. |
| JAK inhibitors | 2.4 (39) | 3.9 (86) | 0.008 |
| Steroids | 25.6 (424) | 4.4 (96) | <0.0001 |
| Antibiotics | 14.8 (244) | 8.7 (193) | <0.0001 |
| Statins | 14.7 (243) | 6.8 (149) | <0.0001 |
| Diuretics | 20.6 (341) | 11.4 (251) | <0.0001 |
| Antiplatelet | 5.6 (93) | 2.4 (54) | <0.0001 |
| Comorbidities, % (n) | |||
| Peripheral atherosclerosis | 7.7 (128) | 2.7 (59) | <0.0001 |
| Coronary artery disease | 27.9 (461) | 10.8 (239) | <0.0001 |
| Heart failure | 33.1 (548) | 13.4 (296) | <0.0001 |
| CCI+CVD | 37.2 (615) | 10.9 (241) | <0.0001 |
| Atrial fibrillation | 20.8 (344) | 9.6 (211) | <0.0001 |
| Hypertension | 78.1 (1291) | 50.7 (1119) | <0.0001 |
| Chronic kidney disease | 23.6 (390) | 8.4 (185) | <0.0001 |
| Diabetes mellitus | 30.8 (509) | 18.0 (396) | <0.0001 |
| Cancer | 12.0 (198) | 6.1 (135) | <0.0001 |
| Acute kidney injury | 25.3 (419) | 0.2 (5) | <0.0001 |
| Complications, % (n) | |||
| Vein thromboembolism | |||
| Deep vein thrombosis | 20.1 (332) | 2.4 (53) | <0.0001 |
| Pulmonary embolism | 5.7 (94) | 0.2 (4) | <0.0001 |
| Superficial vein thrombosis | 5.1 (84) | 0.7 (15) | <0.0001 |
| Other vein thrombosis # | 0.3 (5) | 0.0 (1) | 0.09 |
| Arterial thromboembolism | |||
| Acute ischemic stroke | 1.2 (20) | 0.0 (0) | <0.0001 † |
| Mesenteric artery thrombosis | 0.8 (14) | 0.0 (0) | <0.0001 † |
| Limb artery thrombosis | 1.9 (32) | 0.1 (3) | <0.0001 |
| Other artery thrombosis ## | 0.4 (7) | 0.0 (0) | n.a. |
| Patients | Time | % of Patients with TDX-V | n | ||
|---|---|---|---|---|---|
| >29 µm/min | 20–29 µm/min | <20 µm/min | |||
| Severely ill | At admission # | 75.6 | 17.3 | 7.1 | 241 |
| By day 2 ## | 28.0 | 18.6 | 53.4 | 724 | |
| Average in the next days ## | 25.3 ± 4.4 | 14.6 ± 1.3 | 60.1 ± 5.4 | 2692 | |
| Mildly ill | At admission # | 79.6 | 14.3 | 6.1 | 1129 |
| By day 2 ## | 25.6 | 32.3 | 42.1 | 726 | |
| Average in the next days ## | 26.8 ± 2.3 | 33.9 ± 5.3 | 39.3 ± 5.9 | 1917 | |
| Model | n | Variable | Base Model HR (95% CI), p-Value | Model Adjusted for Age, Sex and BMI HR (95% CI), p-Value |
|---|---|---|---|---|
| Death | 393 | Intense hypercoagulability (IH) # | 1.72 (1.38–2.14), *** | 1.68 (1.35–2.10), *** |
| Age | - | 1.54 (1.32–1.80), *** | ||
| Male | - | 1.19 (0.90–1.57), ns | ||
| BMI | - | 1.20 (1.05–1.38), ** | ||
| Thrombosis | 318 | Intense hypercoagulability (IH) # | 3.16 (2.29–4.37), *** | 3.19 (2.31–4.41), *** |
| Age | - | 0.94 (0.71–1.25), ns | ||
| Male | - | 1.05 (0.62–1.77), ns | ||
| BMI | - | 0.94 (0.71–1.26), ns |
| Guideline | Mildly Ill Inpatients | Severely Ill Inpatients |
|---|---|---|
| ISTH | Recommend therapeutic anticoagulation in select patients. | Intermediate- or therapeutic-dose anticoagulation not recommended over prophylactic-dose heparin. |
| NIH COVID-19 Guideline | Recommend therapeutic-dose heparin for patients who have a D-dimer above the upper limit of normal, require low-flow oxygen, and have no increased bleeding risk. | Recommend prophylactic-dose heparin. Recommends against the use of intermediate-dose (e.g., enoxaparin 1 mg/kg daily) and therapeutic-dose anticoagulation for VTE prophylaxis, except in a clinical trial. |
| World Health Organization | Suggest standard thromboprophylaxis dosing. | Suggest standard thromboprophylaxis dosing. |
| Temporal Guidelines of the Ministry of Health of Russian Federation, v. 12 | LMWH or UFH is recommended in at least prophylactic doses for all hospitalized patients until discharge. Dose escalation to intermediate or therapeutic levels may be considered for high D-dimer levels or additional thrombotic risk factors. | Routine escalation to intermediate/therapeutic doses in ICU patients does not improve outcomes. Therapeutic doses are used for confirmed thrombotic events. |
| Current Study Findings | Consistent with current guidelines. Escalated anticoagulation reduced the risk of hypercoagulability, thereby decreasing the likelihood of thrombotic events. | Both standard and escalated anticoagulation did not prevent intense hypercoagulability in some patients. TDX-V values exceeding 50 μm/min could serve as a clinical decision point for switching to UFH infusion with the rate adjusted using APTT or Thrombodynamics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakhidzhanov, S.; Filippova, A.; Bovt, E.; Gubkin, A.; Sukhikh, G.; Tsarenko, S.; Spiridonov, I.; Protsenko, D.; Zateyshchikov, D.; Vasilieva, E.; et al. Severely Ill COVID-19 Patients May Exhibit Hypercoagulability Despite Escalated Anticoagulation. J. Clin. Med. 2025, 14, 1966. https://doi.org/10.3390/jcm14061966
Shakhidzhanov S, Filippova A, Bovt E, Gubkin A, Sukhikh G, Tsarenko S, Spiridonov I, Protsenko D, Zateyshchikov D, Vasilieva E, et al. Severely Ill COVID-19 Patients May Exhibit Hypercoagulability Despite Escalated Anticoagulation. Journal of Clinical Medicine. 2025; 14(6):1966. https://doi.org/10.3390/jcm14061966
Chicago/Turabian StyleShakhidzhanov, Soslan, Anna Filippova, Elizaveta Bovt, Andrew Gubkin, Gennady Sukhikh, Sergey Tsarenko, Ilya Spiridonov, Denis Protsenko, Dmitriy Zateyshchikov, Elena Vasilieva, and et al. 2025. "Severely Ill COVID-19 Patients May Exhibit Hypercoagulability Despite Escalated Anticoagulation" Journal of Clinical Medicine 14, no. 6: 1966. https://doi.org/10.3390/jcm14061966
APA StyleShakhidzhanov, S., Filippova, A., Bovt, E., Gubkin, A., Sukhikh, G., Tsarenko, S., Spiridonov, I., Protsenko, D., Zateyshchikov, D., Vasilieva, E., Kalinskaya, A., Dukhin, O., Novichkova, G., Karamzin, S., Serebriyskiy, I., Lipets, E., Kopnenkova, D., Morozova, D., Melnikova, E., ... Ataullakhanov, F., for the COVITRO study group. (2025). Severely Ill COVID-19 Patients May Exhibit Hypercoagulability Despite Escalated Anticoagulation. Journal of Clinical Medicine, 14(6), 1966. https://doi.org/10.3390/jcm14061966






