Comparison of Tricuspid Annular Plane Systolic Excursion to Pulmonary Artery Systolic Pressure Ratio Measured by Transthoracic Echocardiography and Right Heart Catheterization in Pulmonary Arterial Hypertension: Prognostic Implications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Primary and Secondary Endpoints
- To analyze whether the TAPSE/PASP ratio, with PASP estimated via TTE, correlates with the TAPSE/PASP ratio when PASP is measured via RHC.
- To evaluate the prognostic impact of TAPSE/PASP, as measured based on both TTE and RHC, in predicting clinical worsening—defined as lung transplantation and/or death—using the risk cutoff values recommended by the ESC/ERS guidelines [10].
- To assess the prognostic significance of the TAPSE/PASP ratio, as measured via RHC, in the subgroup of patients for whom PASP could not be estimated via TTE.
- Comparing PASP measurements obtained via RHC and TTE.
- Assessing right atrial pressure (RAP) as measured via RHC and estimated via TTE.
- Investigating whether the accuracy of TTE-derived estimates and their correlations are influenced by the presence of significant TR.
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Risk Stratification
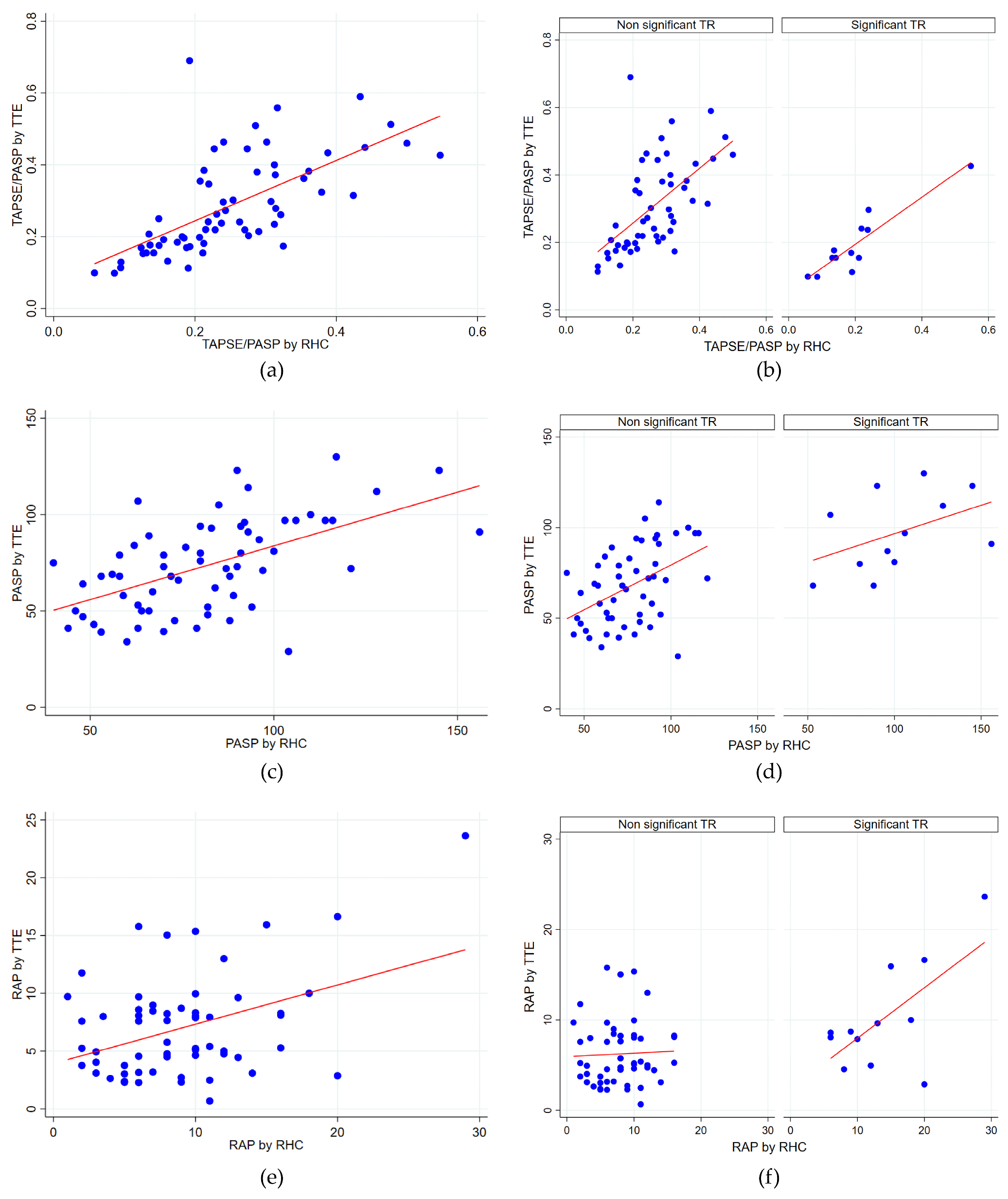
3.2. Hemodynamic Parameter Correlations with Transthoracic Echocardiography
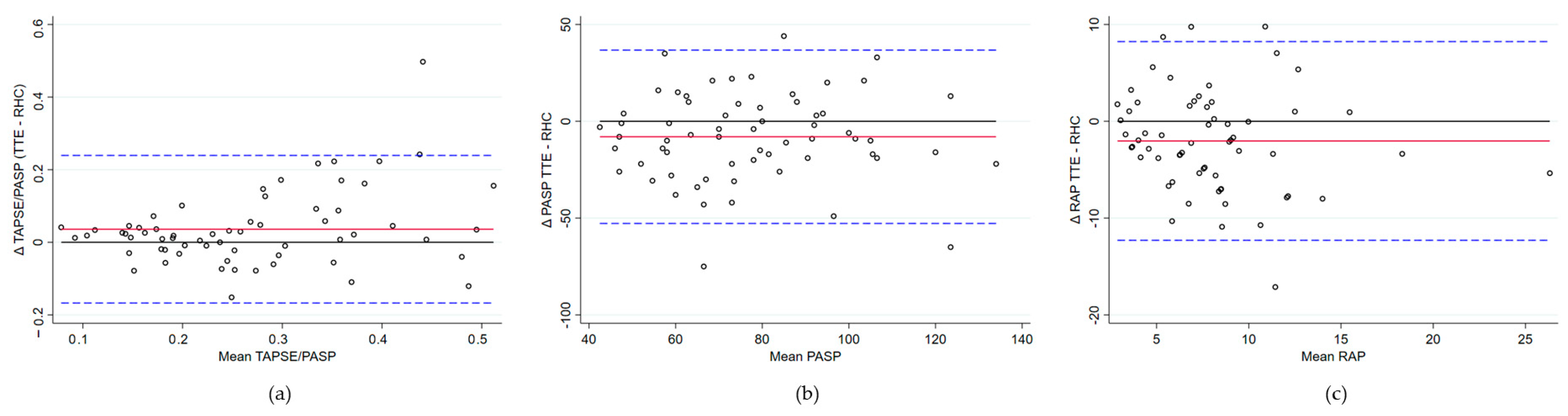
- PASP: A moderate positive correlation was found (rho = 0.56, p < 0.0001). The Bland–Altman analysis revealed a mean bias of −7.99 mmHg (95% CI −13.75 to −2.24), with TTE generally underestimating the PASP compared to RHC. Limits of agreement ranged from −52.76 to 36.77 mmHg, indicating substantial variability in individual measurements. Lin’s concordance correlation coefficient was 0.53, reflecting moderate agreement. No evidence of proportional bias was observed.
- RAP: The correlation was weak and not statistically significant (rho = 0.22, p = 0.0857). The Bland–Altman analysis comparing RAP revealed a mean bias of −2.03 mmHg (95% CI: −3.36 to −0.70), indicating a tendency for the underestimation of RAP via TTE. Limits of agreement ranged from −12.23 mmHg to 8.23, demonstrating considerable variability between methods. Lin’s concordance correlation coefficient was 0.36, reflecting limited agreement. No evidence of proportional bias was observed.
3.3. Stratified Analysis Based on Significant Tricuspid Regurgitation
- The TAPSE/PASP ratio showed a moderate correlation (rho = 0.65, p < 0.0001) for patients without significant TR. In patients with significant TR, the correlation was notably higher (rho = 0.82, p = 0.0012), indicating a strong and significant relationship between the two parameters.
- For the PASP, a moderate correlation was found in patients without significant TR (rho = 0.46, p = 0.0006), suggesting a significant positive relationship. In patients with significant TR, the correlation tended to be slightly stronger, although still moderate (rho = 0.54), but was marginally non-significant (p = 0.0676).
- No significant correlation was found between RAP estimated via TTE and measured via RHC, either in patients without significant TR (rho = 0.10, p = 0.5040) or in those with significant TR (rho = 0.48, p = 0.1137), indicating a lack of relationship between the two parameters.
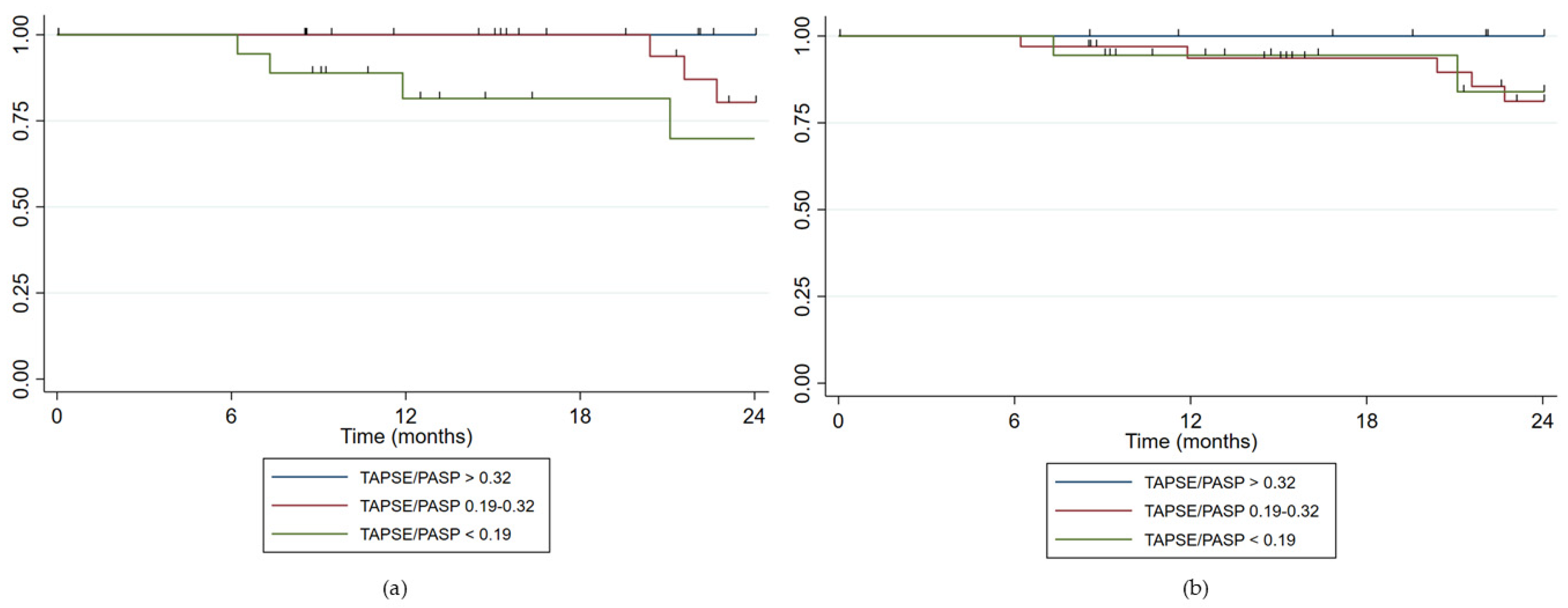
3.4. Prognostic and Predictive Value of TAPSE/PASP via Echocardiography and Right Heart Catheterization
- For TAPSE/PASP estimated via TTE (N = 63): 22 patients (34.9%) were at low risk, 23 patients (36.5%) at intermediate risk, and 18 patients (28.6%) at high risk. VA coupling measured via TTE showed appropriate risk stratification with an increasing HR as the risk category rose. For the intermediate risk group, HR = 7.03 (p = 0.076) and for the high-risk group, HR = 34.81 (p = 0.002). The Harrell’s C statistic was 0.81 (0.73–0.89), indicating good predictive ability, while the Somers’ D statistic was 0.62, suggesting a positive association between predictions and observed events.
- For TAPSE/PASP estimated via RHC in those patients with PASP estimated via TTE (N = 63): 12 patients (19.1%) were at low risk, 33 patients (52.4%) at intermediate risk, and 18 patients (28.6%) at high risk. VA coupling using PASP measured via RHC did not show a relationship with clinical worsening (HR 1.13, p = 0.877 for intermediate risk and HR 2.3, p = 0.315 for high risk). The Harrell’s C concordance statistic for the model was 0.59 (0.46–0.71) suggesting limited predictive ability, while Somers’ D statistic was 0.17 suggesting a low association with the events.
- When analyzing all patients, including those without PASP estimated via TTE (N = 90), no relationship with clinical worsening was found either.
3.5. Subgroup Analysis of Patients with Non-Estimable PASP via Echocardiography
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C-statistics | Harrell’s C-statistics (a measure of model discrimination) |
| CI | Confidence Interval |
| C-index | Concordance Index |
| CW | Clinical Worsening |
| ESC/ERS | European Society of Cardiology/European Respiratory Society |
| HR | Hazard Ratio |
| IQR | Interquartile Range |
| Lin’s CCC | Lin’s Concordance Correlation Coefficient |
| mPAP | Mean Pulmonary Artery Pressure |
| PASP | Pulmonary Artery Systolic Pressure |
| PH | Pulmonary Hypertension |
| PVR | Pulmonary Vascular Resistance |
| RAP | Right Atrial Pressure |
| RHC | Right Heart Catheterization |
| REVEAL Lite 2.0 | Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) abridged version risk calculator. |
| Spearman’s rho | Spearman’s Rank Correlation Coefficient |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| TTE | Transthoracic Echocardiography |
| TR | Tricuspid Regurgitation |
| VA | Ventricular-arterial |
| WHO | World Health Organization |
References
- Sanz, J.; Sánchez-Quintana, D.; Bossone, E.; Bogaard, H.J.; Naeije, R. Anatomy, Function, and Dysfunction of the Right Ventricle: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1463–1482. [Google Scholar] [CrossRef]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, A.; Rauwolf, T.; Groscheck, T.; Kropf, S.; Luani, B.; Tanev, I.; Hansen, M.; Meißler, S.; Steendijk, P.; Braun-Dullaeus, R.C. Pressure-Volume Loop Validation of TAPSE/PASP for Right Ventricular Arterial Coupling in Heart Failure with Pulmonary Hypertension. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP Ratio in Pulmonary Arterial Hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef]
- Kazimierczyk, R.; Kazimierczyk, E.; Knapp, M.; Sobkowicz, B.; Malek, L.A.; Blaszczak, P.; Ptaszynska-Kopczynska, K.; Grzywna, R.; Kaminski, K.A. Echocardiographic Assessment of Right Ventricular-Arterial Coupling in Predicting Prognosis of Pulmonary Arterial Hypertension Patients. J. Clin. Med. 2021, 10, 2995. [Google Scholar] [CrossRef] [PubMed]
- Bragança, B.; Trêpa, M.; Santos, R.; Silveira, I.; Fontes-Oliveira, M.; Sousa, M.J.; Reis, H.; Torres, S.; Santos, M. Echocardiographic Assessment of Right Ventriculo-Arterial Coupling: Clinical Correlates and Prognostic Impact in Heart Failure Patients Undergoing Cardiac Resynchronization Therapy. J. Cardiovasc. Imaging 2020, 28, 109–120. [Google Scholar] [CrossRef]
- Brener, M.I.; Lurz, P.; Hausleiter, J.; Rodés-Cabau, J.; Fam, N.; Kodali, S.K.; Rommel, K.-P.; Muntané-Carol, G.; Gavazzoni, M.; Nazif, T.M.; et al. Right Ventricular-Pulmonary Arterial Coupling and Afterload Reserve in Patients Undergoing Transcatheter Tricuspid Valve Repair. J. Am. Coll. Cardiol. 2022, 79, 448–461. [Google Scholar] [CrossRef]
- Lai, J.; Zhao, J.; Li, K.; Qin, X.; Wang, H.; Tian, Z.; Wang, Q.; Li, M.; Guo, X.; Liu, Y.; et al. Right Ventricle to Pulmonary Artery Coupling Predicts the Risk Stratification in Patients with Systemic Sclerosis-Associated Pulmonary Arterial Hypertension. Front. Cardiovasc. Med. 2022, 9, 872795. [Google Scholar] [CrossRef]
- Ishii, S.; Minatsuki, S.; Hatano, M.; Saito, A.; Yagi, H.; Shimbo, M.; Soma, K.; Fujiwara, T.; Itoh, H.; Konoeda, C.; et al. The Ratio of TAPSE to PASP Predicts Prognosis in Lung Transplant Candidates with Pulmonary Arterial Hypertension. Sci. Rep. 2023, 13, 3758. [Google Scholar] [CrossRef]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713; quiz 786–788. [Google Scholar] [CrossRef] [PubMed]
- Greiner, S.; Jud, A.; Aurich, M.; Hess, A.; Hilbel, T.; Hardt, S.; Katus, H.A.; Mereles, D. Reliability of Noninvasive Assessment of Systolic Pulmonary Artery Pressure by Doppler Echocardiography Compared to Right Heart Catheterization: Analysis in a Large Patient Population. J. Am. Heart Assoc. 2014, 3, e001103. [Google Scholar] [CrossRef]
- Abramson, S.V.; Burke, J.B.; Pauletto, F.J.; Kelly, J.J. Use of Multiple Views in the Echocardiographic Assessment of Pulmonary Artery Systolic Pressure. J. Am. Soc. Echocardiogr. 1995, 8, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Benza, R.L.; Kanwar, M.K.; Raina, A.; Scott, J.V.; Zhao, C.L.; Selej, M.; Elliott, C.G.; Farber, H.W. Development and Validation of an Abridged Version of the REVEAL 2.0 Risk Score Calculator, REVEAL Lite 2, for Use in Patients with Pulmonary Arterial Hypertension. Chest 2021, 159, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Badagliacca, R.; D’Alto, M.; Scelsi, L.; Argiento, P.; Brunetti, N.D.; Casu, G.; Cedrone, N.; Confalonieri, M.; Corda, M.; et al. Right Ventricular Phenotyping in Incident Patients with Idiopathic Pulmonary Arterial Hypertension. J. Heart Lung Transplant. 2024, 43, 1668–1676. [Google Scholar] [CrossRef]
- Testani, J.M.; St John Sutton, M.G.; Wiegers, S.E.; Khera, A.V.; Shannon, R.P.; Kirkpatrick, J.N. Accuracy of Noninvasively Determined Pulmonary Artery Systolic Pressure. Am. J. Cardiol. 2010, 105, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.R.; Forfia, P.R.; Chamera, E.; Housten-Harris, T.; Champion, H.C.; Girgis, R.E.; Corretti, M.C.; Hassoun, P.M. Accuracy of Doppler Echocardiography in the Hemodynamic Assessment of Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2009, 179, 615–621. [Google Scholar] [CrossRef]
- Venkateshvaran, A.; Seidova, N.; Tureli, H.O.; Kjellström, B.; Lund, L.H.; Tossavainen, E.; Lindquist, P. Accuracy of Echocardiographic Estimates of Pulmonary Artery Pressures in Pulmonary Hypertension: Insights from the KARUM Hemodynamic Database. Int. J. Cardiovasc. Imaging 2021, 37, 2637–2645. [Google Scholar] [CrossRef]
- Abu, T.; Levi, A.; Hasdai, D.; Kramer, M.R.; Bental, T.; Bdolah-Abram, T.; Shiyovich, A.; Samara, A.; Vaknin-Assa, H.; Perl, L.; et al. Preoperative Evaluation of Pulmonary Hypertension in Lung Transplant Candidates: Echocardiography versus Right Heart Catheterization. BMC Cardiovasc. Disord. 2022, 22, 53. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Cassandro, R.; Luisi, F.; Ferrante, D.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Harari, S. Correlation Between Doppler Echocardiography and Right Heart Catheterisation-Derived Systolic and Mean Pulmonary Artery Pressures: Determinants of Discrepancies Between the Two Methods. Heart Lung Circ. 2021, 30, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Balcı, M.K.; Arı, E.; Vayvada, M.; Salturk, C.; Aşıcıoğlu, E.; Kahveci, G.; Akbal, Ö.Y.; Kutlu, C.A. Assessment of Pulmonary Hypertension in Lung Transplantation Candidates: Correlation of Doppler Echocardiography with Right Heart Catheterization. Transplant. Proc. 2016, 48, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Cailes, J.B.; Phillips, G.D.; Wells, A.U.; Black, C.M.; Bois, R.M. Comparison of Doppler Echocardiography and Right Heart Catheterization to Assess Pulmonary Hypertension in Systemic Sclerosis. Br. J. Rheumatol. 1997, 36, 239–243. [Google Scholar] [CrossRef]
- Farber, H.W.; Foreman, A.J.; Miller, D.P.; McGoon, M.D. REVEAL Registry: Correlation of Right Heart Catheterization and Echocardiography in Patients with Pulmonary Arterial Hypertension. Congest. Heart Fail. 2011, 17, 56–64. [Google Scholar] [CrossRef] [PubMed]

| Demographic Characteristics | N = 90 |
|---|---|
| Age, y | 52 (42–63) |
| Female/male | 2.5/1 |
| BMI, kg/m2 | 26.0 (22.5–30.0) |
| Disease characteristics at baseline | |
| WHO-FC | |
| Functional Class I | 16 (17.8) |
| Functional Class II | 44 (48.9) |
| Functional Class III | 28 (31.1) |
| Functional Class IV | 2 (2.2) |
| 6MWD, m | 411 (322–495) |
| REVEAL Lite risk score | |
| ≤6 | 56 (62.2) |
| 7–8 | 20 (22.2) |
| ≥9 | 14 (15.6) |
| Time from diagnoses (months) | 2.6 (4.0–8.4) |
| PAH pathogenesis | |
| Group 1 | |
| IPAH | 32 (35.6) |
| Heritable | 12 (13.3) |
| Drugs and toxins | 4 (4.4) |
| Associated with | |
| Connective tissue disease | 16 (17.8) |
| Portal hypertension | 1 (1.1) |
| Congenital heart disease | 8 (8.9) |
| PVOD | 15 (16.7) |
| Group 5 (neurofibromatosis) | 2 (2.1) |
| Hemodynamic measures | |
| Systolic pulmonary artery pressure, mmHg | 73 (61–91) |
| Diastolic pulmonary artery pressure, mmHg | 30 (23–37) |
| Mean pulmonary artery pressure, mmHg | 48 (37–55) |
| Pulmonary arterial wedge pressure, mmHg | 10 (8–12) |
| Cardiac output, l/min | 4.6 (3.7–5.5) |
| Cardiac index, l/minxm2 | 2.7 (2.2–3.1) |
| Pulmonary vascular resistance, WU | 7.9 (5.7–11.5) |
| Right atrial pressure, mmHg | 8 (5–10) |
| Echocardiographic data | |
| Right atria area (cm2) | 19 (17–24) |
| Systolic pulmonary artery pressure, mmHg | 72 (52–93) |
| TAPSE (mm) | 19 (16–21) |
| TAPSE/PASP (mm/mmHg) | 0.24 (0.18–0.38) |
| RV/LV ratio | 1.1 (0.9–1.2) |
| Eccentricity diastolic index | 1.3 (1.1–1.5) |
| Pericardial effusion (presence) | 13 (14.4) |
| Estimated RA pressure (mmHg) | 5 (4–9) |
| Grade TR | |
| None | 27 (30.0)) |
| Mild | 30 (33.3) |
| Mild to moderate | 21 (23.3) |
| Moderate to severe | 6 (6.7) |
| Severe | 6 (6.7) |
| Treatment strategy | |
| Single therapy | 6 (6.7) |
| Dual therapy | 37 (41.1) |
| Triple oral therapy | 10 (11.1) |
| Oral therapy with systemic prostanoid | 11 (12.2) |
| Triple parenteral therapy | 25 (27.8) |
| Bland–Altman Analysis | Spearman’s Rank Correlation | ||||
|---|---|---|---|---|---|
| Bias | 95% CI | Lin’s CCC | Coefficients | p Values | |
| TAPSE/PASP | 0.04 | 0.01–0.06 | 0.60 | 0.74 | <0.0001 |
| PASP | −7.99 | −13.75–−2.24 | 0.53 | 0.56 | <0.0001 |
| RAP | −2.03 | −3.36–−0.70 | 0.36 | 0. 22 | 0.0857 |
| n (%) | Death or Lung Transplantion n (%) | Cox Regression HR (p Value) | Discrimination Indices Predictive Performance | |
|---|---|---|---|---|
| TAPSE/PASP with PASP estimated by TTE (N = 63) | ||||
| TAPSE/PASP > 0.32 | 22 (34.9) | 1 (1.6) | -- | C-Harrel: 0.81 (0.73–0.89) Somers’ D: 0.62 AUC ROC: 0.75 (0.62–0.85) |
| TAPSE/PASP 0.19–0.32 | 23 (36.5) | 5 (7.9) | HR 7.03 (0.076) | |
| TAPSE/PASP < 0.19 | 18 (28.6) | 8 (12.7) | HR 34.81 (0.002) | |
| TAPSE/PASP with PASP measured by RHC in patients with PASP estimable by TTE (N = 63) | ||||
| TAPSE/PASP > 0.32 | 12 (19.1) | 2 (3.2) | -- | C-Harrel: 0.58 (0.46–0.71) Somers’ D: 0.17 AUC ROC: 0.56 (0.43–0.68) |
| TAPSE/PASP 0.19–0.32 | 33 (52.4) | 7 (11.1) | HR 1.13 (0.877) | |
| TAPSE/PASP < 0.19 | 18 (28.6) | 5 (7.9) | HR 2.33 (0.315) | |
| TAPSE/PASP with PASP measured by RHC (N = 90) | ||||
| TAPSE/PASP > 0.32 | 23 (25.6) | 3 (3.3) | -- | C Harrel: 0.57 (0.43–0.71) Somers’ D: 0.14 TAUC ROC: 0.56 (0.45–0.67) |
| TAPSE/PASP 0.19–0.32 | 45 (50.0) | 8 (8.8) | HR 1.52 (0.547) | |
| TAPSE/PASP < 0.19 | 22 (24.4) | 5 (5.5) | HR 2.60 (0.193) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Ortiz, E.; López-Guarch, C.J.; Jiménez, J.F.D.; Coronel, M.L.; Martín de Miguel, I.; Biscotti Rodil, B.; Duarte Torres, J.; Segura de la Cal, T.; Velázquez Martín, M.T.; Arribas Ynsaurriaga, F.; et al. Comparison of Tricuspid Annular Plane Systolic Excursion to Pulmonary Artery Systolic Pressure Ratio Measured by Transthoracic Echocardiography and Right Heart Catheterization in Pulmonary Arterial Hypertension: Prognostic Implications. J. Clin. Med. 2025, 14, 1968. https://doi.org/10.3390/jcm14061968
Gutiérrez-Ortiz E, López-Guarch CJ, Jiménez JFD, Coronel ML, Martín de Miguel I, Biscotti Rodil B, Duarte Torres J, Segura de la Cal T, Velázquez Martín MT, Arribas Ynsaurriaga F, et al. Comparison of Tricuspid Annular Plane Systolic Excursion to Pulmonary Artery Systolic Pressure Ratio Measured by Transthoracic Echocardiography and Right Heart Catheterization in Pulmonary Arterial Hypertension: Prognostic Implications. Journal of Clinical Medicine. 2025; 14(6):1968. https://doi.org/10.3390/jcm14061968
Chicago/Turabian StyleGutiérrez-Ortiz, Eva, Carmen Jiménez López-Guarch, Juan Francisco Delgado Jiménez, María Lorena Coronel, Irene Martín de Miguel, Belen Biscotti Rodil, Juan Duarte Torres, Teresa Segura de la Cal, María Teresa Velázquez Martín, Fernando Arribas Ynsaurriaga, and et al. 2025. "Comparison of Tricuspid Annular Plane Systolic Excursion to Pulmonary Artery Systolic Pressure Ratio Measured by Transthoracic Echocardiography and Right Heart Catheterization in Pulmonary Arterial Hypertension: Prognostic Implications" Journal of Clinical Medicine 14, no. 6: 1968. https://doi.org/10.3390/jcm14061968
APA StyleGutiérrez-Ortiz, E., López-Guarch, C. J., Jiménez, J. F. D., Coronel, M. L., Martín de Miguel, I., Biscotti Rodil, B., Duarte Torres, J., Segura de la Cal, T., Velázquez Martín, M. T., Arribas Ynsaurriaga, F., Cruz-Utrilla, A., & Escribano-Subías, P. (2025). Comparison of Tricuspid Annular Plane Systolic Excursion to Pulmonary Artery Systolic Pressure Ratio Measured by Transthoracic Echocardiography and Right Heart Catheterization in Pulmonary Arterial Hypertension: Prognostic Implications. Journal of Clinical Medicine, 14(6), 1968. https://doi.org/10.3390/jcm14061968








