Diagnosing Heart Failure with Preserved Ejection Fraction in Obese Patients
Abstract
1. Introduction
2. HFpEF Diagnosis Based on Guidelines
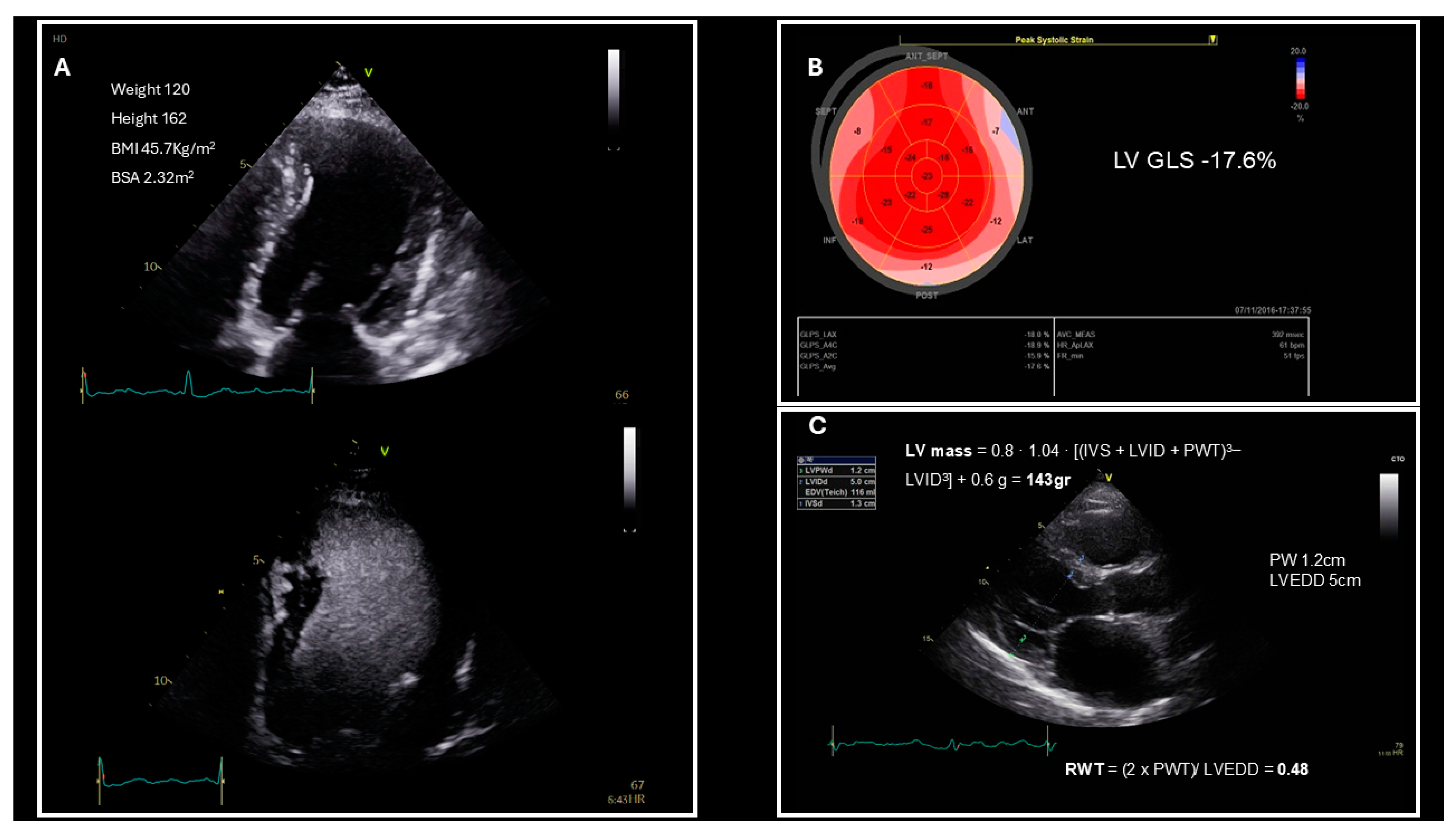
3. Left Ventricular Structure and Systolic Function Evaluation in Patients Who Are Obese
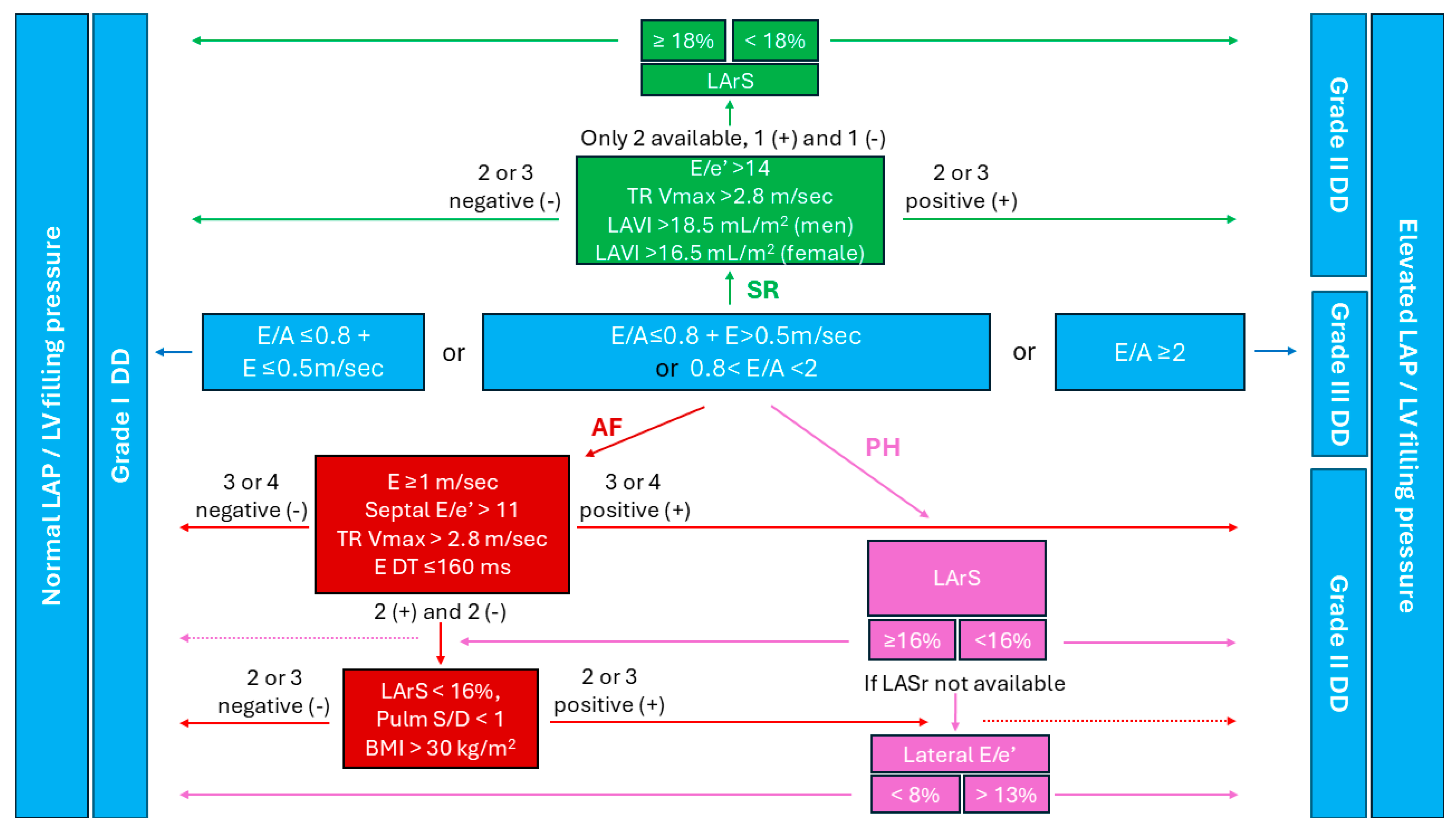
4. Diastolic Dysfunction Assessment in Individuals Who Are Obese in Sinus Rhythm
5. Diastolic Dysfunction Assessment in Individuals Who Are Obese in Atrial Fibrillation
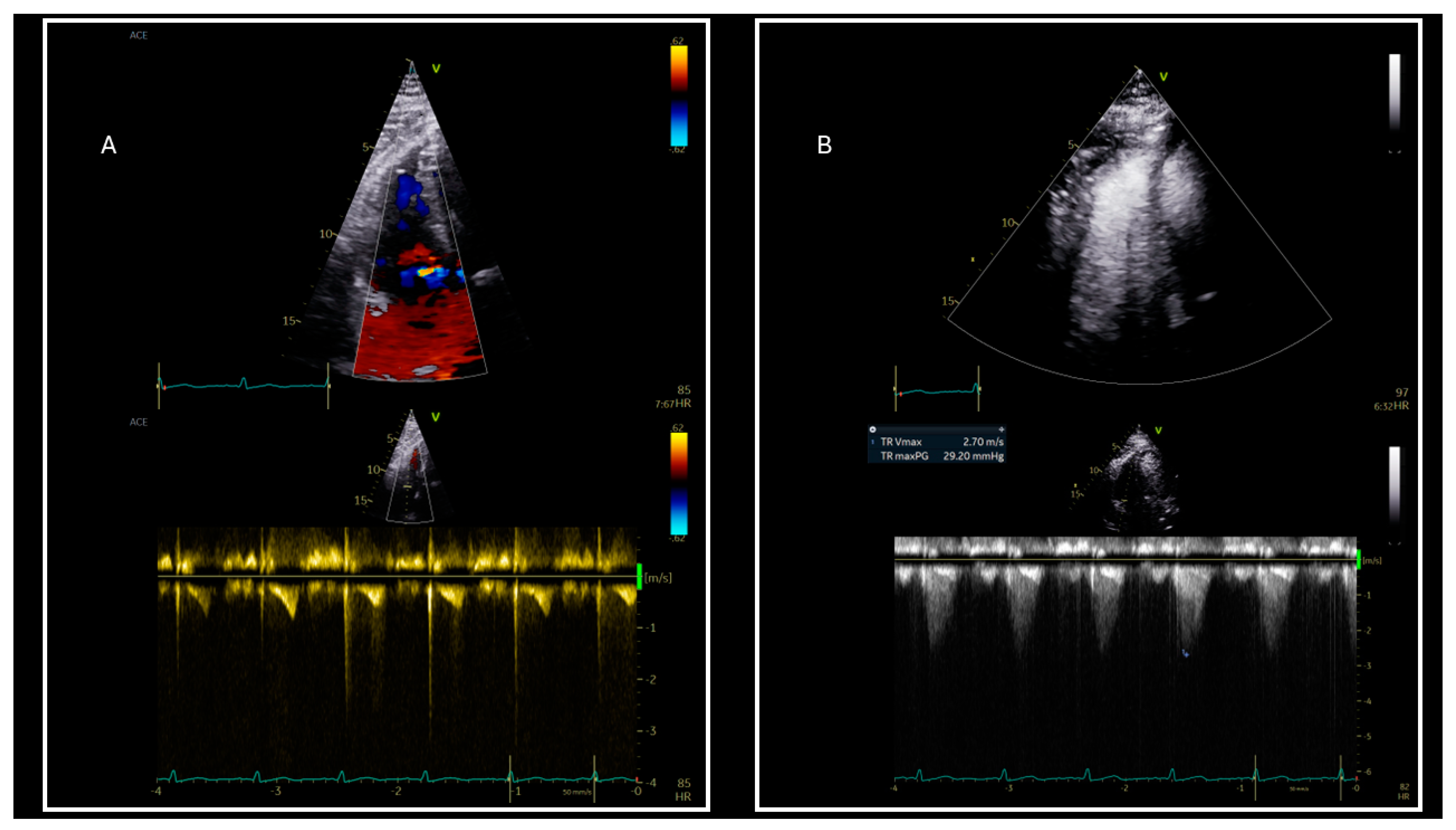
6. Pulmonary Hypertension and Diastolic Dysfunction in Obese
7. Applying Clinical Algorithms in Patients Who Are Obese for HFpEF Diagnosis
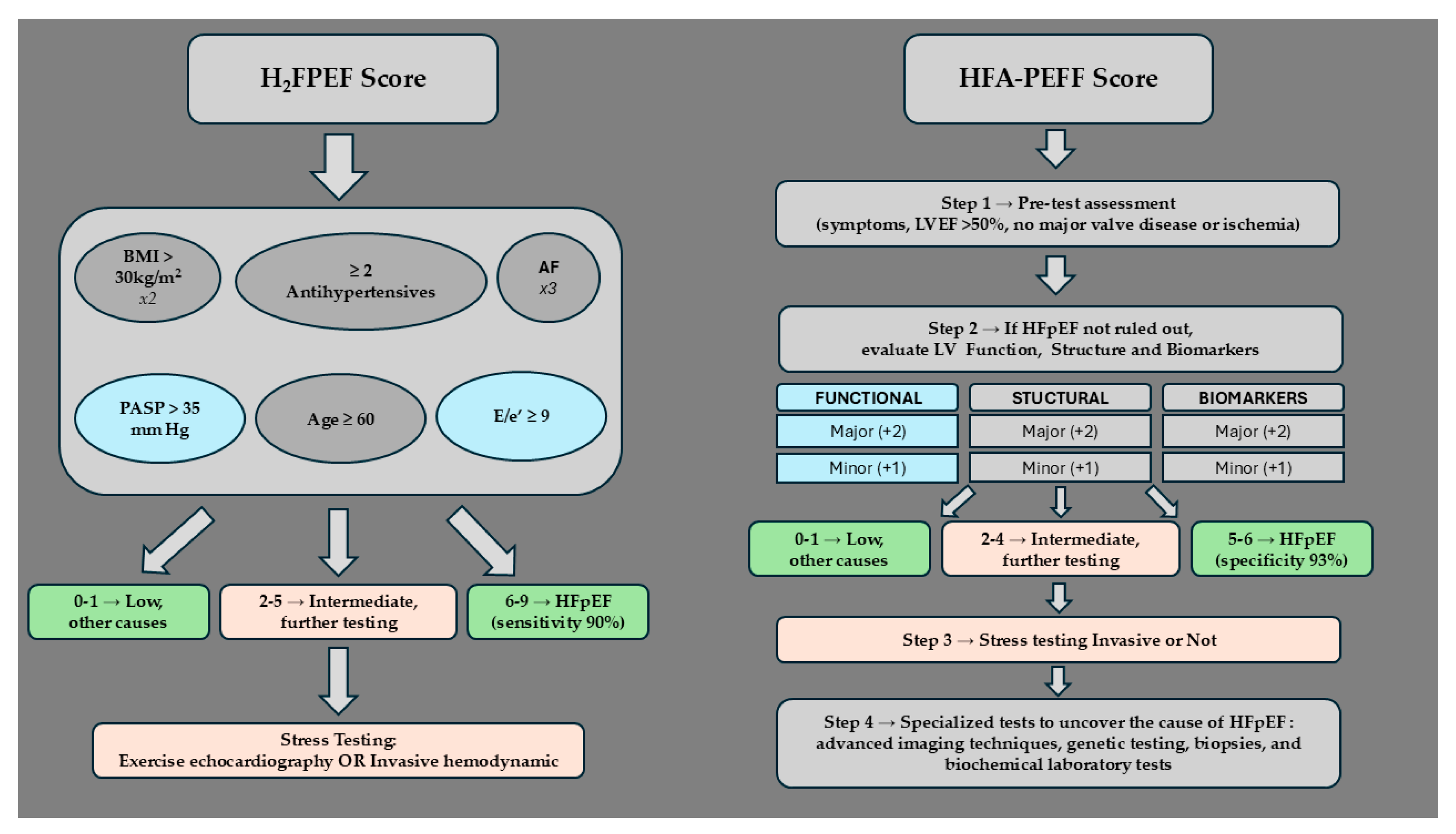
7.1. H2FPEF Diagnostic Algorithm
7.2. HFA-PEFF Diagnostic Algorithm
7.3. Accuracy and Limitations of the Diagnostic Algorithms
8. Advancing HFpEF Diagnosis in Patients Who Are Obese: Key Areas for Future Research
8.1. Multimodality Assessment in Patients Who Are Obese
8.2. Gender Differences in Patients Who Are Obese
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| BMI | Body mass index |
| BNP | B-type natriuretic peptide |
| BSA | Body Surface Area |
| CCTA | Cardiac computed tomographic angiography |
| CMR | Cardiac magnetic resonance |
| H2FPEF | Heavy, Hypertension, Atrial Fibrillation, Pulmonary Hypertension, Elder, Filling Pressures |
| HF | Heart failure |
| HFA-PEFF | Heart Failure Association Pretest Probability of Heart Failure with Preserved Ejection Fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| LA | Left atrium |
| LAVI | Left atrial volume index |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| LVMI | Left ventricular mass index |
| NP | Natriuretic peptide |
| NT-proBNP | N-terminal fragment of proBNP |
| PH | Pulmonary hypertension |
| RWT | Relative Wall Thickness |
| TR Vmax | Tricuspid regurgitation maximum velocity |
References
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22 (Suppl. S7), s176–s185. [Google Scholar] [PubMed]
- Koskinas, K.C.; Van Craenenbroeck, E.M.; Antoniades, C.; Blüher, M.; Gorter, T.M.; Hanssen, H.; Marx, N.; McDonagh, T.A.; Mingrone, G.; Rosengren, A.; et al. Obesity and cardiovascular disease: An ESC clinical consensus statement. Eur. Heart J. 2024, 45, 4063–4098. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart 2003, 89, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Jensen, M.D.; Kitzman, D.W.; Lam, C.S.P.; Obokata, M.; Rider, O.J. Obesity and heart failure with preserved ejection fraction: New insights and pathophysiological targets. Cardiovasc. Res. 2022, 118, 3434–3450. [Google Scholar] [CrossRef]
- Litwin, S.E. Cardiac Remodeling in Obesity. JACC Cardiovasc. Imaging 2010, 3, 275–277. [Google Scholar] [CrossRef]
- Kruszewska, J.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Remodeling and Fibrosis of the Cardiac Muscle in the Course of Obesity-Pathogenesis and Involvement of the Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 4195. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Q.; Kong, W. Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol. 2018, 68–69, 490–506. [Google Scholar] [CrossRef]
- de Simone, G.; Izzo, R.; Luca, N.D.; Gerdts, E. Left ventricular geometry in obesity: Is it what we expect? Nutr. Metab. Cardiovasc. Dis. 2013, 23, 905–912. [Google Scholar] [CrossRef]
- Anthony, S.R.; Guarnieri, A.R.; Gozdiff, A.; Helsley, R.N.; Owens, A.P.; Tranter, M. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin. Sci. 2019, 133, 2329–2344. [Google Scholar] [CrossRef]
- Gallo, G.; Desideri, G.; Savoia, C. Update on Obesity and Cardiovascular Risk: From Pathophysiology to Clinical Management. Nutrients 2024, 16, 2781. [Google Scholar] [CrossRef]
- Clerico, A.; Zaninotto, M.; Passino, C.; Plebani, M. Obese phenotype and natriuretic peptides in patients with heart failure with preserved ejection fraction. Clin. Chem. Lab. Med. CCLM 2018, 56, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Shah, S.J. The HFpEF Obesity Phenotype. J. Am. Coll. Cardiol. 2016, 68, 200–203. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef] [PubMed]
- Kosyakovsky, L.B.; Liu, E.E.; Wang, J.K.; Myers, L.; Parekh, J.K.; Knauss, H.; Lewis, G.D.; Malhotra, R.; Nayor, M.; Robbins, J.M.; et al. Uncovering Unrecognized Heart Failure with Preserved Ejection Fraction Among Individuals with Obesity and Dyspnea. Circ. Heart Fail. 2024, 17, e011366. [Google Scholar] [CrossRef]
- Seidell, J.C.; Flegal, K.M. Assessing obesity: Classification and epidemiology. Br. Med. Bull. 1997, 53, 238–252. [Google Scholar] [CrossRef]
- El Hajj, M.C.; Litwin, S.E. Echocardiography in the Era of Obesity. J. Am. Soc. Echocardiogr. 2020, 33, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, K.; Jeyaprakash, P.; Sivapathan, S.; Sangha, S.; Kitley, J.; Darshni, A.; Chen, D.; Negishi, K.; Pathan, F. The Effect of Obesity on Echocardiographic Image Quality. Heart Lung Circ. 2022, 31, 207–215. [Google Scholar] [CrossRef]
- Singh, M.; Sethi, A.; Mishra, A.K.; Subrayappa, N.K.; Stapleton, D.D.; Pellikka, P.A. Echocardiographic Imaging Challenges in Obesity: Guideline Recommendations and Limitations of Adjusting to Body Size. J. Am. Heart Assoc. 2020, 9, e014609. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef]
- Oktay, A.A.; Rich, J.D.; Shah, S.J. The Emerging Epidemic of Heart Failure with Preserved Ejection Fraction. Curr. Heart Fail. Rep. 2013, 10, 401–410. [Google Scholar] [CrossRef]
- Vasan, R.S.; Xanthakis, V.; Lyass, A.; Andersson, C.; Tsao, C.; Cheng, S.; Aragam, J.; Benjamin, E.J.; Larson, M.G. Epidemiology of Left Ventricular Systolic Dysfunction and Heart Failure in the Framingham Study. JACC Cardiovasc. Imaging 2018, 11, 1–11. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Rescaldani, M.; Sala, C.; Grassi, G. Left-ventricular hypertrophy and obesity: A systematic review and meta-analysis of echocardiographic studies. J. Hypertens. 2014, 32, 16. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R.; Waggoner, A.D.; Schechtman, K.B.; Meyer, T.; Gropler, R.J.; Barzilai, B.; Dávila-Román, V.G. Alterations in left ventricular structure and function in young healthy obese women: Assessment by echocardiography and tissue Doppler imaging. J. Am. Coll. Cardiol. 2004, 43, 1399–1404. [Google Scholar] [CrossRef]
- Powell, B.D.; Redfield, M.M.; Bybee, K.A.; Freeman, W.K.; Rihal, C.S. Association of Obesity with Left Ventricular Remodeling and Diastolic Dysfunction in Patients Without Coronary Artery Disease. Am. J. Cardiol. 2006, 98, 116–120. [Google Scholar] [CrossRef]
- von Jeinsen, B.; Vasan, R.S.; McManus, D.D.; Mitchell, G.F.; Cheng, S.; Xanthakis, V. Joint influences of obesity, diabetes, and hypertension on indices of ventricular remodeling: Findings from the community-based Framingham Heart Study. PLoS ONE 2020, 15, e0243199. [Google Scholar] [CrossRef]
- Dini, F.L.; Fabiani, I.; Miccoli, M.; Galeotti, G.G.; Pugliese, N.R.; D’Agostino, A.; Scartabelli, A.; Conte, L.; Salvetti, G.; Santini, F.; et al. Prevalence and determinants of left ventricular diastolic dysfunction in obese subjects and the role of left ventricular global longitudinal strain and mass normalized to height. Echocardiography 2018, 35, 1124–1131. [Google Scholar] [CrossRef]
- Chen, H.H.L.; Bhat, A.; Gan, G.C.H.; Khanna, S.; Ahlenstiel, G.; Negishi, K.; Tan, T.C. The impact of body mass index on cardiac structure and function in a cohort of obese patients without traditional cardiovascular risk factors. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 19, 200211. [Google Scholar] [CrossRef]
- Turkbey, E.B.; McClelland, R.L.; Kronmal, R.A.; Burke, G.L.; Bild, D.E.; Tracy, R.P.; Arai, A.E.; Lima, J.A.C.; Bluemke, D.A. The Impact of Obesity on the Left Ventricle. JACC Cardiovasc. Imaging 2010, 3, 266–274. [Google Scholar] [CrossRef]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.R.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired Systolic Function by Strain Imaging in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2014, 63, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Cannizzaro, E.; Palumbo, M.M.; Di Cesare, A.; Bruno, F.; Acanfora, C.; Arceri, A.; Evangelista, L.; Arrigoni, F.; Grassi, F.; et al. Heart Failure and Cardiomyopathies: CT and MR from Basics to Advanced Imaging. Diagnostics 2022, 12, 2298. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Chang, S.M.; Nabi, F.; Shah, D.J.; Estep, J.D. Cardiac Imaging in Patients with Heart Failure and Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2017, 10, e006547. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.; Kammerlander, A.A.; Zotter-Tufaro, C.; Aschauer, S.; Schwaiger, M.L.; Marzluf, B.A.; Bonderman, D.; Mascherbauer, J. Interstitial Fibrosis, Functional Status, and Outcomes in Heart Failure with Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2016, 9, e005277. [Google Scholar] [CrossRef] [PubMed]
- Ellims, A.H.; Shaw, J.A.; Stub, D.; Iles, L.M.; Hare, J.L.; Slavin, G.S.; Kaye, D.M.; Taylor, A.J. Diffuse Myocardial Fibrosis Evaluated by Post-Contrast T1 Mapping Correlates with Left Ventricular Stiffness. J. Am. Coll. Cardiol. 2014, 63, 1112–1118. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Kramer, C.M.; Chandrashekhar, Y. CMR Global Longitudinal Strain: A Better Tool for Unraveling the Links to Heart Failure Mortality. JACC Cardiovasc. Imaging 2018, 11, 1554–1555. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Jirataiporn, K.; Yindeengam, A.; Songsangjinda, T. Cardiac Magnetic Resonance Left Atrial Strain in the Prediction of Death, Ischemic Stroke, and Heart Failure. J. Am. Heart Assoc. 2024, 13, e034336. [Google Scholar] [CrossRef]
- Singh, R.M.; Singh, B.M.; Mehta, J.L. Role of cardiac CTA in estimating left ventricular volumes and ejection fraction. World J. Radiol. 2014, 6, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Ehara, S.; Okuyama, T.; Shirai, N.; Yamashita, H.; Sugioka, K.; Kitamura, H.; Ujino, K.; Hozumi, T.; Yoshiyama, M. Comparison of determinations of left atrial volume by the biplane area-length and Simpson’s methods using 64-slice computed tomography. J. Cardiol. 2009, 53, 257–264. [Google Scholar] [CrossRef]
- Vandenberg, B.F.; Weiss, R.M.; Kinzey, J.; Acker, M.; Stark, C.A.; Stanford, W.; Burns, T.L.; Marcus, M.L.; Kerber, R.E. Comparison off loft atrial volume by two-dimensional echocardiography and cine-computed tomography. Am. J. Cardiol. 1995, 75, 754–757. [Google Scholar] [CrossRef]
- Wang, R.; Fang, Z.; Wang, H.; Schoepf, U.J.; Emrich, T.; Giovagnoli, D.; Biles, E.; Zhou, Z.; Du, Z.; Liu, T.; et al. Quantitative analysis of three-dimensional left ventricular global strain using coronary computed tomography angiography in patients with heart failure: Comparison with 3T cardiac MR. Eur. J. Radiol. 2021, 135, 109485. [Google Scholar] [CrossRef]
- Xie, W.-H.; Chen, L.-J.; Hu, L.-W.; Ouyang, R.-Z.; Guo, C.; Sun, A.-M.; Wang, Q.; Qiu, H.-S.; Zhang, Y.-Q.; Zhang, H.; et al. Cardiac Computed Tomography-Derived Left Atrial Strain and Volume in Pediatric Patients with Congenital Heart Disease: A Comparative Analysis with Transthoracic Echocardiography. Front. Cardiovasc. Med. 2022, 9, 870014. [Google Scholar] [CrossRef]
- Szilveszter, B.; Nagy, A.I.; Vattay, B.; Apor, A.; Kolossváry, M.; Bartykowszki, A.; Simon, J.; Drobni, Z.D.; Tóth, A.; Suhai, F.I.; et al. Left ventricular and atrial strain imaging with cardiac computed tomography: Validation against echocardiography. J. Cardiovasc. Comput. Tomogr. 2020, 14, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Kawakami, H.; Tanabe, Y.; Fukuyama, N.; Yoshida, K.; Ohara, K.; Kitamura, T.; Kawaguchi, N.; Kido, T.; Nagai, T.; et al. Left atrial strain assessment using cardiac computed tomography in patients with hypertrophic cardiomyopathy. Jpn. J. Radiol. 2023, 41, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Shah, A.M.; Cikes, M.; Prasad, N.; Li, G.; Getchevski, S.; Claggett, B.; Rizkala, A.; Lukashevich, I.; O’Meara, E.; Ryan, J.J.; et al. Echocardiographic Features of Patients with Heart Failure and Preserved Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2019, 74, 2858–2873. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Liang, C.; Gopal, D.M.; Ayalon, N.; Donohue, C.; Santhanakrishnan, R.; Sandhu, H.; Perez, A.J.; Downing, J.; Gokce, N.; et al. Preclinical Systolic and Diastolic Dysfunctions in Metabolically Healthy and Unhealthy Obese Individuals. Circ. Heart Fail. 2015, 8, 897–904. [Google Scholar] [CrossRef]
- Rozenbaum, Z.; Topilsky, Y.; Khoury, S.; Pereg, D.; Laufer-Perl, M. Association of body mass index and diastolic function in metabolically healthy obese with preserved ejection fraction. Int. J. Cardiol. 2019, 277, 147–152. [Google Scholar] [CrossRef]
- Russo, C.; Jin, Z.; Homma, S.; Rundek, T.; Elkind, M.S.V.; Sacco, R.L.; Di Tullio, M.R. Effect of Obesity and Overweight on Left Ventricular Diastolic Function: A Community-based Study in an Elderly Cohort. J. Am. Coll. Cardiol. 2011, 57, 1368–1374. [Google Scholar] [CrossRef]
- Byrd, B.F.; O’Kelly, B.F.; Schiller, N.B. Contrast echocardiography enhances tricuspid but not mitral regurgitation. Clin. Cardiol. 1991, 14 (Suppl. S5), V10–V14. [Google Scholar] [CrossRef]
- Waggoner, A.D.; Barzilai, B.; Pérez, J.E. Saline contrast enhancement of tricuspid regurgitant jets detected by Doppler color flow imaging. Am. J. Cardiol. 1990, 65, 1368–1371. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Wang, T.K.M.; Klein, A.L.; Nagueh, S.F. Left ventricular diastolic dysfunction in non-myocardial disorders. Eur. Heart J.-Cardiovasc. Imaging 2024, 25, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Aga, Y.S.; Kroon, D.; Snelder, S.M.; Biter, L.U.; De Groot-de Laat, L.E.; Zijlstra, F.; Brugts, J.J.; Van Dalen, B.M. Decreased left atrial function in obesity patients without known cardiovascular disease. Int. J. Cardiovasc. Imaging 2022, 39, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sezenöz, B.; Ünlü, S.; Yalçın, Y.; Yamak, B.A.; Yazgan, E.; Türkoğlu, S.; Taçoy, G. The effect of body weight on left atrial function determined by longitudinal strain analysis in young adults. Int. J. Cardiovasc. Imaging 2023, 40, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Sardana, M.; Satija, V.; Gillebert, T.C.; De Buyzere, M.L.; Chahwala, J.; De Bacquer, D.; Segers, P.; Rietzschel, E.R. Effect of Obesity on Left Atrial Strain in Persons Aged 35–55 Years (The Asklepios Study). Am. J. Cardiol. 2019, 123, 854–861. [Google Scholar] [CrossRef]
- Law, W.-Y.; Huang, G.-L.; Yang, C.-C. Effect of Body Mass Index in Coronary CT Angiography Performed on a 256-Slice Multi-Detector CT Scanner. Diagnostics 2022, 12, 319. [Google Scholar] [CrossRef]
- Pan, Y.-N.; Li, A.-J.; Chen, X.-M.; Wang, J.; Ren, D.-W.; Huang, Q.-L. Coronary Computed Tomographic Angiography at Low Concentration of Contrast Agent and Low Tube Voltage in Patients with Obesity:: A Feasibility Study. Acad. Radiol. 2016, 23, 438–445. [Google Scholar] [CrossRef]
- Bianchettin, R.G.; Lavie, C.J.; Lopez-Jimenez, F. Challenges in Cardiovascular Evaluation and Management of Obese Patients. J. Am. Coll. Cardiol. 2023, 81, 490–504. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the Risk of New-Onset Atrial Fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef]
- Lavie, C.J.; Pandey, A.; Lau, D.H.; Alpert, M.A.; Sanders, P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Petraglia, L.; Cabaro, S.; Valerio, V.; Poggio, P.; Pilato, E.; Attena, E.; Russo, V.; Ferro, A.; Formisano, P.; et al. Epicardial Adipose Tissue and Cardiac Arrhythmias: Focus on Atrial Fibrillation. Front. Cardiovasc. Med. 2022, 9, 932262. [Google Scholar] [CrossRef] [PubMed]
- Alpert, M.A.; Karthikeyan, K.; Abdullah, O.; Ghadban, R. Obesity and Cardiac Remodeling in Adults: Mechanisms and Clinical Implications. Prog. Cardiovasc. Dis. 2018, 61, 114–123. [Google Scholar] [CrossRef]
- Stockand, J.D.; Meszaros, J.G. Aldosterone stimulates proliferation of cardiac fibroblasts by activating Ki-RasA and MAPK1/2 signaling. Am. J. Physiol.-Heart Circ. Physiol. 2003, 284, H176–H184. [Google Scholar] [CrossRef]
- Verdecchia, P.; Reboldi, G.; Gattobigio, R.; Bentivoglio, M.; Borgioni, C.; Angeli, F.; Carluccio, E.; Sardone, M.G.; Porcellati, C. Atrial Fibrillation in Hypertension. Hypertension 2003, 41, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Goudis, C.A.; Korantzopoulos, P.; Ntalas, I.V.; Kallergis, E.M.; Ketikoglou, D.G. Obesity and atrial fibrillation: A comprehensive review of the pathophysiological mechanisms and links. J. Cardiol. 2015, 66, 361–369. [Google Scholar] [CrossRef]
- Khan, F.H.; Zhao, D.; Ha, J.-W.; Nagueh, S.F.; Voigt, J.-U.; Klein, A.L.; Gude, E.; Broch, K.; Chan, N.; Quill, G.M.; et al. Evaluation of left ventricular filling pressure by echocardiography in patients with atrial fibrillation. Echo Res. Pract. 2024, 11, 14. [Google Scholar] [CrossRef]
- Frank, R.C.; Min, J.; Abdelghany, M.; Paniagua, S.; Bhattacharya, R.; Bhambhani, V.; Pomerantsev, E.; Ho, J.E. Obesity Is Associated with Pulmonary Hypertension and Modifies Outcomes. J. Am. Heart Assoc. 2020, 9, e014195. [Google Scholar] [CrossRef]
- Romero-Corral, A.; Caples, S.M.; Lopez-Jimenez, F.; Somers, V.K. Interactions Between Obesity and Obstructive Sleep Apnea. Chest 2010, 137, 711–719. [Google Scholar] [CrossRef]
- Friedman, S.E.; Andrus, B.W. Obesity and Pulmonary Hypertension: A Review of Pathophysiologic Mechanisms. J. Obes. 2012, 2012, 505274. [Google Scholar] [CrossRef]
- Abenhaim, L.; Moride, Y.; Brenot, F.; Rich, S.; Benichou, J.; Kurz, X.; Higenbottam, T.; Oakley, C.; Wouters, E.; Aubier, M.; et al. Appetite-Suppressant Drugs and the Risk of Primary Pulmonary Hypertension. N. Engl. J. Med. 1996, 335, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Gibbs, J.S.R.; Wachter, R.; De Marco, T.; Vonk-Noordegraaf, A.; Vachiéry, J.-L. Left ventricular heart failure and pulmonary hypertension. Eur. Heart J. 2016, 37, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Paulus, W.J. H2FPEF Score. Circulation 2018, 138, 871–873. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Cediel, G.; Domingo, M.; Codina, P.; Santiago, E.; Lupón, J. Biomarkers in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2022, 8, e20. [Google Scholar] [CrossRef]
- Morfino, P.; Aimo, A.; Castiglione, V.; Vergaro, G.; Emdin, M.; Clerico, A. Biomarkers of HFpEF: Natriuretic Peptides, High-Sensitivity Troponins and Beyond. J. Cardiovasc. Dev. Dis. 2022, 9, 256. [Google Scholar] [CrossRef]
- Khan, A.M.; Cheng, S.; Magnusson, M.; Larson, M.G.; Newton-Cheh, C.; McCabe, E.L.; Coviello, A.D.; Florez, J.C.; Fox, C.S.; Levy, D.; et al. Cardiac natriuretic peptides, obesity, and insulin resistance: Evidence from two community-based studies. J. Clin. Endocrinol. Metab. 2011, 96, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, C.; Alhosaini, H.; Sumida, A.; Runge, M.S. Obesity and natriuretic peptides, BNP and NT-proBNP: Mechanisms and diagnostic implications for heart failure. Int. J. Cardiol. 2014, 176, 611–617. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Wilson, P.W.F.; Vasan, R.S. Impact of Obesity on Plasma Natriuretic Peptide Levels. Circulation 2004, 109, 594–600. [Google Scholar] [CrossRef]
- Keyzer, J.M.; Hoffmann, J.J.; Ringoir, L.; Nabbe, K.C.; Widdershoven, J.W.; Pop, V.J. Age- and gender-specific brain natriuretic peptide (BNP) reference ranges in primary care. Clin. Chem. Lab. Med. 2014, 52, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Cediel, G.; Codina, P.; Spitaleri, G.; Domingo, M.; Santiago-Vacas, E.; Lupón, J.; Bayes-Genis, A. Gender-Related Differences in Heart Failure Biomarkers. Front. Cardiovasc. Med. 2021, 7, 617705. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Coresh, J.; Lazo, M.; Hoogeveen, R.C.; Blumenthal, R.S.; Folsom, A.R.; Selvin, E.; Ballantyne, C.M.; Nambi, V. Obesity, Subclinical Myocardial Injury, and Incident Heart Failure. JACC Heart Fail. 2014, 2, 600–607. [Google Scholar] [CrossRef]
- Florido, R.; Kwak, L.; Echouffo-Tcheugui, J.B.; Zhang, S.; Michos, E.D.; Nambi, V.; Goldberg, R.B.; Hoogeveen, R.C.; Lazo, M.; Gerstenblith, G.; et al. Obesity, Galectin-3, and Incident Heart Failure: The ARIC Study. J. Am. Heart Assoc. 2022, 11, e023238. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Myhre, P.L.; Vaduganathan, M.; Claggett, B.L.; Matsushita, K.; Kitzman, D.W.; Borlaug, B.A.; Shah, A.M.; Solomon, S.D. Application of Diagnostic Algorithms for Heart Failure with Preserved Ejection Fraction to the Community. JACC Heart Fail. 2020, 8, 640–653. [Google Scholar] [CrossRef]
- Sepehrvand, N.; Alemayehu, W.; Dyck, G.J.B.; Dyck, J.R.B.; Anderson, T.; Howlett, J.; Paterson, I.; McAlister, F.A.; Ezekowitz, J.A.; on behalf of the Alberta HEART Investigators. External Validation of the H2F-PEF Model in Diagnosing Patients with Heart Failure and Preserved Ejection Fraction. Circulation 2019, 139, 2377–2379. [Google Scholar] [CrossRef]
- Barandiarán Aizpurua, A.; Sanders-van Wijk, S.; Brunner-La Rocca, H.-P.; Henkens, M.; Heymans, S.; Beussink-Nelson, L.; Shah, S.J.; van Empel, V.P.M. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 413–421. [Google Scholar] [CrossRef]
- Mert, G.Ö.; Özlek, B.; Özlek, E.; Zencirkiran Ağuş, H.; Tekinalp, M.; Kahraman, S.; Çil, C.; Çelik, O.; Başaran, Ö.; Doğan, V.; et al. Comparing the Diagnostic Performance of HFA-PEFF and H2FPEF Scoring Systems in Heart Failure with Preserved Ejection Fraction Patients: Insights from the APOLLON Registry. Anatol. J. Cardiol. 2023, 27, 539–548. [Google Scholar] [CrossRef]
- Jung, M.-H.; Shin, M.-S. Obesity-related heart failure with preserved ejection fraction: Diagnostic and therapeutic challenges. Korean J. Intern. Med. 2023, 38, 157–166. [Google Scholar] [CrossRef]
- Kaur, G.; Lau, E. Sex differences in heart failure with preserved ejection fraction: From traditional risk factors to sex-specific risk factors. Women’s Health 2022, 18, 17455057221140209. [Google Scholar] [CrossRef]
- Dewan, P.; Rorth, R.; Raparelli, V.; Campbell, R.T.; Shen, L.; Jhund, P.S.; Petrie, M.C.; Anand, I.S.; Carson, P.E.; Desai, A.S.; et al. Sex-related differences in heart failure with preserved ejection fraction. Circ. Heart Fail. 2019, 12, e006539. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.L.; Nanayakkara, S.; Segan, L.; Mariani, J.A.; Maeder, M.T.; van Empel, V.; Vizi, D.; Evans, S.; Lam, C.S.P.; Kaye, D.M. Sex Differences in Heart Failure with Preserved Ejection Fraction Pathophysiology: A Detailed Invasive Hemodynamic and Echocardiographic Analysis. JACC Heart Fail. 2019, 7, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]

| Poor image quality |
| Use of contrast agents for accurate left ventricular ejection fraction estimation |
| Use of contrast agents for accurate tricuspid regurgitation maximum velocity estimation |
| Underestimation of left ventricular mass index |
| Underestimation of left atrial volume index |
| Lower natriuretic peptides levels |
| Left ventricular structure | Left ventricular mass index |
| Relative wall thickness | |
| Left ventricular systolic function | Left ventricular ejection fraction |
| Global longitudinal Strain | |
| Left ventricular diastolic function | E wave velocity |
| A wave velocity | |
| E/A ratio | |
| E/e’ ratio | |
| Tricuspid regurgitation Vmax | |
| Left atrial volume index | |
| Left atrial reservoir strain | |
| E wave deceleration time (in AF) | |
| Pulmonary vein Systolic/Diastolic velocity ratio (in AF) |
| Lean | Obese | |
|---|---|---|
| Left atrial volume index—LAVI | 34 mL/m2, index to BSA | 29 mL/m2, index to BSA |
| Left ventricular mass index—LVMI | 95 g/m2 (women) 115 g/m2 (men), index to BSA | 47 g/m2.7 (women) 50 g/m2.7 (men), index to height |
| B-type Natriuretic peptide—BNP | 80 pg/mL | 54 pg/mL |
| H2FPEF Algorithm | ||||
| Parameters | Score | |||
| BMI > 30 kg/m2 (Heavy) | 2 | |||
| Treatment with ≥2 Antihypertensives (Hypertensive) | 1 | |||
| Atrial Fibrillation (Fibrillation) | 3 | |||
| PASP > 35 mm Hg (Pulmonary Hypertension) | 1 | |||
| Age ≥ 60 years (Elder) | 1 | |||
| Elevated Filling Pressure→E/e’ ≥ 9 (Filling) | 1 | |||
| Probability of HFpEF based on Score | 0–1 → Low, explore other causes | |||
| 2–5 → Intermediate, further testing required | ||||
| 6–9 → High, no further testing required | ||||
| HFA-PEFF | ||||
| Step 1→Pre-test assessment: HF symptoms, typical risk factors, preserved ejection fraction, no major valve disease or ischemia. Elevated natriuretic peptides support but low levels do not exclude HFpEF | ||||
| Step 2→If all the conditions of the previous step are met, then continue with evaluation of three domains: functional, structural and biomarkers | Score | |||
| Functional | Major | septal e′ < 7 cm/sec | 2 | |
| lateral e′ < 10 cm/sec | ||||
| mean septal and lateral E/e′ ratio > 15 | ||||
| TR Vmax > 2.8 m/sec or systolic pulmonary artery pressure > 35 mmHg | ||||
| Minor | mean E/e′ ratio ≥ 9 and ≤14 | 1 | ||
| LV-GLS | ||||
| Structural | Major | In SR LAVI > 34 mL/m2 (lean)/29 mL/m2 (obese) index to BSA | 2 | |
| LAVI > 40 mL/m2 for AF index to BSA | ||||
| LVMI > 50 g/m2.7(men)/>47 g/m2.7(women) index to height + RWT > 0.42 | ||||
| Minor | LAVI ≥ 29 and ≤ 34 mL/m2 for SR | 1 | ||
| LAVI ≥ 34 and ≤ 40 mL/m2 for AF | ||||
| LVMI > 115 g/m2 (men)/>95 g/m2(women) | ||||
| RWT > 0.42 | ||||
| LV wall thickness in the end of diastole ≥ 12 mm | ||||
| Biomarkers | Major | In SR, NTproBNP > 220 pg/mL | 2 | |
| In AF, NTproBNP > 660 pg/mL | ||||
| In SR, BNP > 80 pg/mL (lean)/>54 pg/mL (obese) | ||||
| In AF, BNP > 240 pg/mL | ||||
| Minor | In SR, NTproBNP ≥ 125 and ≤220 pg/mL | 1 | ||
| In AF, NTproBNP ≥ 375 and ≤660 pg/mL | ||||
| In SR, BNP ≥ 35 and ≤80 pg/mL | ||||
| In AF, BNP ≥ 105 and ≤240 pg/mL | ||||
| Probability of HFpEF based on Score | 0–1 → Low, explore other causes | |||
| 2–4 → Intermediate, further testing required | ||||
| 5–6 → High, no further testing required | ||||
| Step 3→ Stress testing, either invasive or non-invasive, is performed for scores in the intermediate range (2–4), to rule out or set the definitive diagnosis of HFpEF | ||||
| Step 4→ Uncover the underlying cause of HFpEF:Advanced imaging techniques, genetic testing, biopsies, and biochemical laboratory tests | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basha, M.; Stavropoulou, E.; Nikolaidou, A.; Dividis, G.; Peteinidou, E.; Tsioufis, P.; Kamperidis, N.; Dimitriadis, K.; Karamitsos, T.; Giannakoulas, G.; et al. Diagnosing Heart Failure with Preserved Ejection Fraction in Obese Patients. J. Clin. Med. 2025, 14, 1980. https://doi.org/10.3390/jcm14061980
Basha M, Stavropoulou E, Nikolaidou A, Dividis G, Peteinidou E, Tsioufis P, Kamperidis N, Dimitriadis K, Karamitsos T, Giannakoulas G, et al. Diagnosing Heart Failure with Preserved Ejection Fraction in Obese Patients. Journal of Clinical Medicine. 2025; 14(6):1980. https://doi.org/10.3390/jcm14061980
Chicago/Turabian StyleBasha, Marino, Evdoxia Stavropoulou, Anastasia Nikolaidou, Georgios Dividis, Emmanouela Peteinidou, Panagiotis Tsioufis, Nikolaos Kamperidis, Kyriakos Dimitriadis, Theodoros Karamitsos, George Giannakoulas, and et al. 2025. "Diagnosing Heart Failure with Preserved Ejection Fraction in Obese Patients" Journal of Clinical Medicine 14, no. 6: 1980. https://doi.org/10.3390/jcm14061980
APA StyleBasha, M., Stavropoulou, E., Nikolaidou, A., Dividis, G., Peteinidou, E., Tsioufis, P., Kamperidis, N., Dimitriadis, K., Karamitsos, T., Giannakoulas, G., Tsioufis, K., Ziakas, A., & Kamperidis, V. (2025). Diagnosing Heart Failure with Preserved Ejection Fraction in Obese Patients. Journal of Clinical Medicine, 14(6), 1980. https://doi.org/10.3390/jcm14061980










