Efficacy and Safety of Photobiomodulation in MELAS: Protocol for a Series of N-of-1 Trials †
Abstract
1. Introduction
- Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) primarily affect the nervous system and muscles. MELAS presents in children or young adults as recurrent episodes of encephalopathy, myopathy with reduced exercise tolerance and fatigue, migraines, seizure disorder, and focal neurological deficits.
- Maternally inherited diabetes and deafness (MIDD) is characterised by mitochondrial diabetes, hearing impairment, and maculopathy but can have other clinical manifestations and clinical overlap with MELAS.
2. Background
The Proposed Role of PBM in MELAS
3. Objectives
- ‐
- To determine if fatigue-related factors such as depression, anxiety, stress, and sleepiness are influenced by the application of PBM in people with MELAS.
- ‐
- To determine if physical strength and activity changes in people with MELAS after PBM application to the large muscles of the lower limbs and the anterior abdomen.
- ‐
- To determine if imaging using proton (1H) MR spectroscopy can show any change in mitochondrial activity in gastrocnemius muscles of the legs in people with MELAS.
- ‐
- To determine if plasma creatine kinase (CK) and lactate are influenced by PBM application to the large muscles of the lower limbs and the anterior abdomen in people with MELAS.
- ‐
- Based on outcomes, determine the feasibility of taking this research to the next phase as a larger clinical study.
4. Materials and Methods
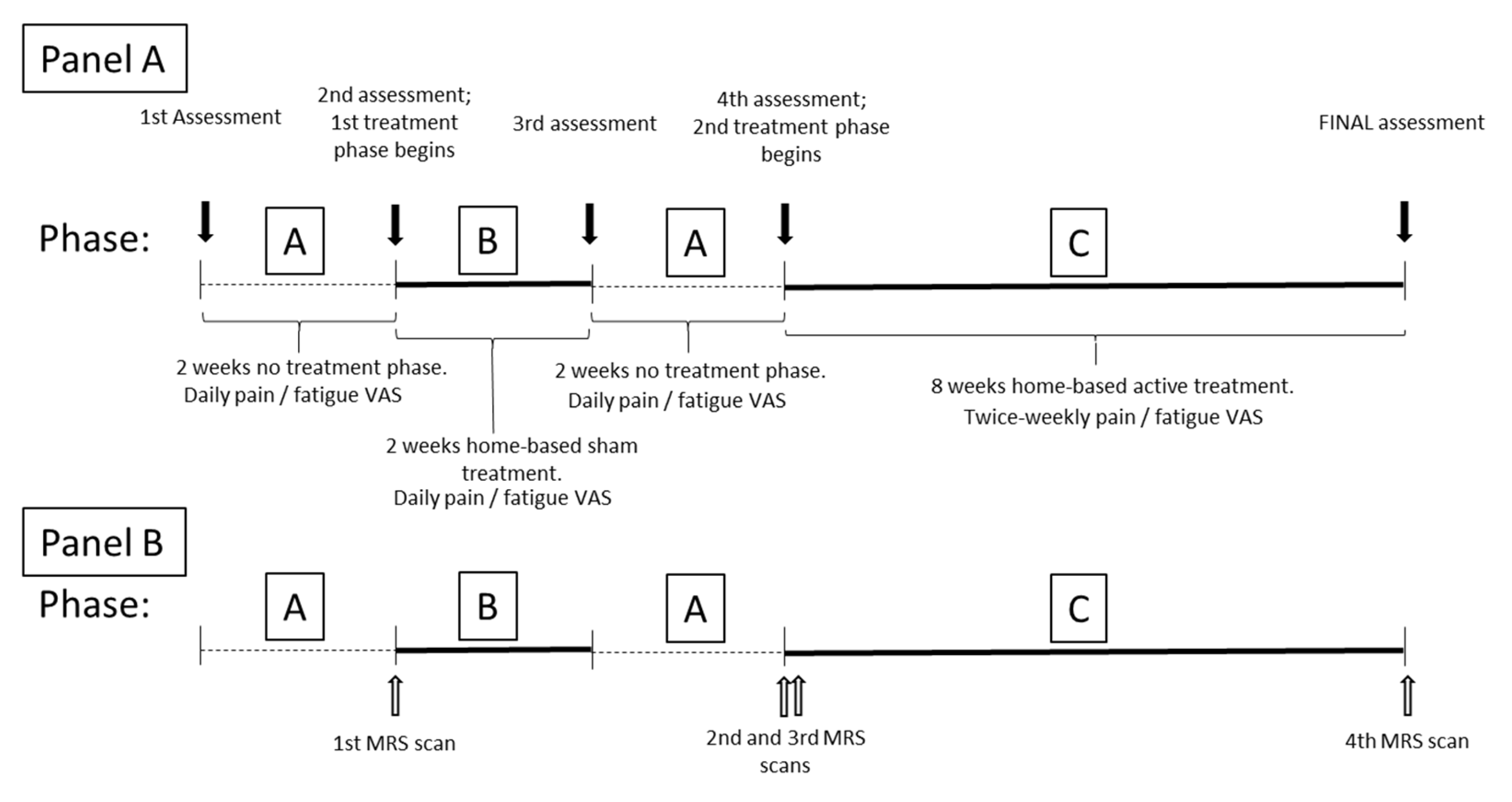
4.1. Study Design
- ‐
- A = no intervention observational period: Daily patient diary (visual analogue scale; VAS) of fatigue and pain to understand usual (baseline) behaviour of fatigue and pain symptoms, to compare with any change during/after intervention. Fourteen data points will be obtained in each non-intervention period. In this study, the repeated “A” period will also serve as a washout period after the sham phase, to account for any placebo response.
- ‐
- B = sham intervention period: Daily patient diary (VAS) of fatigue and pain symptoms will be assessed. Fourteen data points will be obtained. We have included a sham phase in this series of N-of-1 trials to satisfy the requirement to understand if there may be a placebo element to the initial application of light. To reduce the burden on participants, we have chosen a 2-week sham period which is expected to demonstrate any change from baseline. The following A phase will assist in determining the durability of any placebo effect that we may need to consider in a future study.
- ‐
- C = active intervention (PBM application) period: Twice-weekly patient diary of fatigue and pain symptoms will be assessed. Sixteen data points will be obtained over an 8-week period of treatment. The intervention period is based on exercise science findings demonstrating increased mitochondrial activity with endurance exercise [24] and mitochondrial biogenesis after 4–6 weeks of interval training in healthy individuals [24,25]. If PBM influences muscle mitochondrial function, we expect that 8 weeks of home-based intervention may be required to demonstrate effects in a clinical population that would normally struggle to participate in exercise programs.
4.2. Participants
4.3. Intervention
- ‐
- Six days/week with one day rest each week, alternating each application site (e.g., ten minutes to the abdomen on Monday and Thursday, ten minutes to each thigh on Tuesday and Friday, and ten minutes to each calf on Wednesday and Saturday)
- ‐
- Three days/week across all application sites (e.g., Monday, Wednesday, and Friday each week, the device is applied consecutively to the abdomen, the thighs, and calves for ten minutes at each site. The applications can be done in one sitting or spread across the day in separate ten-minute sittings).
4.4. Measures of Outcome
- DASS-21 to measure depression, anxiety, and stress (21 questions taking about three minutes to complete). All three scales of the DASS have been shown to have high internal consistency and meaningful discriminations in a variety of clinical and research settings. The short version, DASS-21, will be used in the study as it has shown validity comparable with the long version and will pose a lesser burden to complete for the participants [29].
- The Epworth Sleepiness Scale (ESS) is a self-administered questionnaire with eight questions. Respondents are asked to rate, on a four-point scale (0–3), their potential for dozing off or falling asleep during eight different activities. The ESS score (the sum of eight item scores, 0–3) can range from 0 to 24. A higher ESS score indicates a greater inclination towards ‘daytime sleepiness’. The questionnaire takes no more than two or three minutes to answer and is available in many languages. The ESS has strong external criterion validity and a high level of internal consistency (Cronbach’s alpha = 0.88) [30].
- To assess for any change in physical activity level, we will use the self-reported Habitual Activity Estimation Scale (HAES). The questionnaire is used to record time spent during a typical weekday and weekend, being “inactive” (lying down), “somewhat inactive” (sitting), “somewhat active” (standing or walking), and “very active” (sweating or breathing hard). The HAES is suitable for this study as it distinguishes between weight-bearing and non-weight-bearing activities, is feasible in clinical populations, sensitive to change, reliable and valid, has strong agreement with instrumented measures of physical activity, and high utility in a wide age range of free-living clinical populations [31].
- Blood lactate and CK [32] will be analysed to determine if they are responsive to the intervention. Lactate is a major energy source for mitochondrial respiration thus an indirect indicator of the effect on mitochondrial activity. CK turns creatine into the high-energy molecule phosphocreatine, used by the body to generate energy. Any condition that interferes with muscle energy production or use increases levels of CK in the blood. These blood tests are standard and part of the routine assessment of people with mitochondrial diseases [1]. Blood tests will be required on three occasions, at intake to the study and at the start and end of active intervention Phase C.
- We will test exercise tolerance on five occasions, using the following measures which focus on the leg muscles receiving the PBM treatment:
- ○
- The 60 s sit-to-stand (60STS) test: This test is indicative of quadriceps muscle and gluteus muscle strength and endurance. Although it has not been used in mitochondrial disease, the 60STS test is simple and has been found to be sensitive in measuring the efficiency of rehabilitation in other diseases [33]. An improvement of at least three repetitions is consistent with physical benefits.
- ○
- As the gastrocnemius muscle will be the focus of MR spectroscopy, we will assess calf muscle strength. There is no validated measure of calf muscle strength [34], thus we will use a simple measure of the number of heel raises that can be repeated by participants in 60 seconds.
- ○
- If the participant uses a smart device (e.g., watch or ring), we will collect data on physical activity (step counts) across each phase of the study to assist in verifying participant activity levels as measured using the HAES.
4.5. Magnetic Resonance Spectroscopy
4.6. Research Ethics Considerations
4.7. Data Management/Analysis
5. Discussion
5.1. Limitations
5.2. Dissemination of Results
5.3. Implications for Practice
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sue, C.M.; Balasubramaniam, S.; Bratkovic, D.; Bonifant, C.; Christodoulou, J.; Coman, D.; Crawley, K.; Edema-Hildebrand, F.; Ellaway, C.; Ghaoui, R.; et al. Patient care standards for primary mitochondrial disease in Australia: An Australian adaptation of the Mitochondrial Medicine Society recommendations. Intern. Med. J. 2022, 52, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Manwaring, N.; Jones, M.M.; Wang, J.J.; Rochtchina, E.; Howard, C.; Mitchell, P.; Sue, C.M. Population prevalence of the MELAS A3243G mutation. Mitochondrion 2007, 7, 230–233. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Adesina, A.M.; Jones, J.; Scaglia, F. MELAS syndrome: Clinical manifestations, pathogenesis, and treatment options. Mol. Genet. Metab. 2015, 116, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Engelstad, B.S.; Wei, Y.; Kulikova, R.; Oskoui, M.; Sproule, D.M.; Battista, V.; Koenigsberger, D.Y.; Pascual, J.M.; Shanske, S.; et al. Natural history of MELAS associated with mitochondrial DNA m.3243A_G genotype. Neurol 2011, 77, 1965–1971. [Google Scholar] [CrossRef]
- Harrington, J.; Ryter, S.; Plataki, M.; Price, D.; Choi, A. Mitochondria in health, disease, and ageing. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondrial genetic medicine. Nat. Genet. 2018, 50, 1642–1649. [Google Scholar] [CrossRef]
- Rossignol, R.; Faustin, B.; Rocher, C.; Malgat, M.; Mazat, J.; Letellier, T. Mitochondrial threshold effects. Biochem. J. 2003, 370, 751–762. [Google Scholar] [CrossRef]
- Fernández-Vizarra, E.; Zeviani, M. Mitochondrial disorders of the OXPHOS system. FEBS Lett. 2020, 595, 1062–1106. [Google Scholar] [CrossRef]
- Baskerville, R.; Krijgsveld, N.; Esser, P.; Jeffery, G.; Poulton, J. The effect of photobiomodulation on the treatment of hereditary mitochondrial diseases. J. Lasers Med. Sci. 2023, 14, e41. [Google Scholar] [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases primer. Nat. Rev. 2016, 2, 16080. [Google Scholar]
- Pek, N.M.Q.; Phua, Q.H.; Ho, B.X.; Pang, J.K.S.; Hor, J.-H.; An, O.; Yang, H.H.; Yu, Y.; Fan, Y.; Ng, S.-Y.; et al. Mitochondrial 3243A > G mutation confers pro-atherogenic and pro-inflammatory properties in MELAS iPS derived endothelial cells. Cell Death Dis. 2019, 10, 802. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Tanaka, M.; Nishikimi, M.; Suzuki, H.; Ozawa, T.; Kobayashi, M.; Wada, Y. Deficiency of subunits of complex I and mitochondrial encephalomyopathy. Ann. Neurol. 1988, 23, 287–294. [Google Scholar] [CrossRef]
- Carroll, J.D.; Milward, M.R.; Cooper, P.R.; Hadis, M.; Palin, W.M. Developments in low level light therapy (LLLT) for dentistry. Dent. Mater. J. 2014, 30, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of Photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Silveira, P.C.L.; Ferreira, G.K.; Zaccaron, R.P.; Glaser, V.; Remor, A.P.; Mendes, C.; Pinho, R.A.; Latini, A. Effects of photobiomodulation on mitochondria of brain, muscle, and C6 astroglioma cells. Med. Eng. Phys. 2019, 71, 108–113. [Google Scholar] [CrossRef]
- Serrage, H.J.; Joanisse, S.; Cooper, P.R.; Palin, W.; Hadis, M.; Darch, O.; Philp, A.; Milward, M.R. Differential responses of myoblasts and myotubes to photobiomodulation are associated with mitochondrial number. J. Biophotonics 2019, 12, e2018004112019. [Google Scholar] [CrossRef] [PubMed]
- Nampo, F.K.; Cavalheri, V.; Ramos, S.P.; Camargo, E.A. Effect of low-level phototherapy on delayed onset muscle soreness: A systematic review and meta-analysis. Laser Med. Sci. 2016, 31, 165–177. [Google Scholar] [CrossRef]
- Vanin, A.A.; Verhagen, E.; Barboza, S.D.; Costa, L.O.P.; Leal-Junior, E.C.P. Photobiomodulation therapy for the improvement of muscular performance and reduction of muscular fatigue associated with exercise in healthy people: A systematic review and meta-analysis. Laser Med. Sci. 2018, 33, 181–214. [Google Scholar] [CrossRef]
- Miranda, E.F.; de Oliveira, L.V.F.; Antonialli, F.C.; Vanin, A.A.; de Carvalho, P.T.C.; Leal-Junior, E.C.P. Phototherapy with combination of super-pulsed laser and light-emitting diodes is beneficial in improvement of muscular performance (strength and muscular endurance), dyspnea, and fatigue sensation in patients with chronic obstructive pulmonary disease. Laser Med. Sci. 2015, 30, 437–443. [Google Scholar] [CrossRef]
- Toma, L.T.; Oliveira, M.X.; Renno, A.C.M.; Laakso, E.L. Photobiomodulation (PBM) therapy at 904 nm mitigates effects of exercise-induced skeletal muscle fatigue in young women. Laser Med. Sci. 2018, 33, 1197–1205. [Google Scholar] [CrossRef]
- Oliveira, M.X.; Toma, R.L.; Jones, B.J.L.; Cyprien, T.P.; Tier, M.; Wallace, C.A.; Renno, A.C.M.; Sabapathy, S.; Laakso, E.L. Effects of photobiomodulation therapy (pulsed LASER 904 nm) on muscle oxygenation and performance in exercise-induced skeletal muscle fatigue in young women: A pilot study. In Proceedings of the SPIE Mechanisms of Photobiomodulation Therapy XII, San Francisco, CA, USA, 28 January–2 February 2017; Volume 10048. [Google Scholar]
- Shamseer, L.; Sampson, M.; Bukutu, C.; Schmid, C.H.; Nikles, J.; Tate, R.; Johnston, B.C.; Zucker, D.; Shadish, W.R.; Kravitz, R.; et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015: Explanation and elaboration. BMJ 2015, 350, h1793. [Google Scholar] [CrossRef] [PubMed]
- Krasny-Pacini, A.; Evans, J. Single-case experimental designs to assess intervention effectiveness in rehabilitation: A practical guide. Ann. Phys. Rehabil. Med. 2018, 61, 164–179. [Google Scholar] [CrossRef]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2021, 599, 803–817. [Google Scholar] [CrossRef]
- Sorriento, D.; Di Vaia, E.; Iaccarino, G. Physical exercise: A novel tool to protect mitochondrial health. Front. Physiol. 2021, 12, 660068. [Google Scholar] [CrossRef] [PubMed]
- Van de Loo, K.F.; van Zeijl, N.T.; Custers, J.A.; Janssen, M.C.; Verhaak, C.M. A conceptual disease model for quality of life in mitochondrial disease. Orphanet J. Rare Dis. 2022, 17, 263. [Google Scholar] [CrossRef]
- Beurskens, A.J.H.M.; Bültmann, U.; Kant, I.J.; Vercoulen, J.H.M.M.; Bleijenberg, G.; Swaen, G.M.H. Fatigue among working people: Validity of a questionnaire measure. Occup. Environ. Med. 2000, 57, 353–357. [Google Scholar] [CrossRef]
- Worm-Smeitink, M.; Gielissen, M.; Bloot, L.; Van Laarhoven, H.W.M.; Van Engelen, B.G.M.; Van Riel, P.; Bleijenberg, G.; Nikolaus, S.; Knoop, H. The assessment of fatigue: Psychometric qualities and norms for the Checklist individual strength. J. Psychosom. Res. 2017, 98, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.M.; Bieling, P.J.; Cox, B.J.; Enns, M.W.; Swinson, R.P. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol. Assess. 1998, 10, 176–181. [Google Scholar] [CrossRef]
- Johns, M.W. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep 1992, 15, 376–381. [Google Scholar] [CrossRef]
- Hay, J.A.; Cairney, J. Development of the Habitual Activity Estimation Scale for clinical research: A systematic approach. Pediatr. Exerc. Sci. 2006, 18, 193–202. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Almannai, M.; Scaglia, F. MELAS. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2018. [Google Scholar]
- McDonald, O.; Perraton, L.; Osadnik, C. Validity and clinical applicability of the 60-second sit-to-stand test in people with acute exacerbations of COPD. Respir. Med. 2023, 2023, 107264. [Google Scholar] [CrossRef] [PubMed]
- Hébert-Losier, K.; Newsham-West, R.J.; Schneiders, A.G.; Sullivan, S.J. Raising the standards of the calf-raise test: A systematic review. J. Sci. Med. Sport. 2009, 12, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Aeles, J.; Bolsterlee, B.; Kelp, N.Y.; Dick, T.J.M.; Hug, F. Regional variation in lateral and medial gastrocnemius muscle fibre lengths obtained from diffusion tensor imaging. J. Anat. 2022, 240, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; McDonald, S.; Araújo-Soares, V.; Sniehotta, F.; Henderson, R. Dynamic modelling of n-of-1 data: Powerful and flexible data analytics applied to individualised studies. Health Psychol. Rev. 2017, 11, 222–234. [Google Scholar] [CrossRef]
- Passarella, S.; Perlino, E.; Quagliariello, E.; Baldassarre, L.; Catalano, I.M.; Cingolani, A. Evidence of changes, induced by HeNe laser irradiation, in the optical and biochemical properties of rat liver mitochondria. J. Electroanal. Chem. Interf. Electrochem. 1983, 155, 185–198. [Google Scholar] [CrossRef]
- Karu, T.I. Ten Lectures on Basic Science of Laser Phototherapy; Prima Books: Grängesberg, Sweden, 2007. [Google Scholar]
- Hamblin, M.R. Mechanisms and mitochondrial redox signaling in photobiomodulation. J. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Eells, J.T.; Wong-Riley, M.T.; VerHoeve, J.; Henry, M.; Buchman, E.V.; Kane, M.P.; Gould, L.J.; Das, R.; Jett, M.; Hodgson, B.D.; et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 2004, 4, 559–567. [Google Scholar] [CrossRef]
- Da Silva Neto Trajano, L.A.; Siqueira, P.B.; Rodrigues, M.M.D.S.; Pires, B.R.B.; da Fonseca, A.D.S.; Mencalha, A.L. Does photobiomodulation alter mitochondrial dynamics? J. Photochem. Photobiol. 2024, 101, 21–37. [Google Scholar] [CrossRef]
- Fear, E.J.; Torkelsen, F.H.; Zamboni, E.; Chen, K.J.; Scott, M.; Jeffery, G.; Baseler, H.; Kennerley, A.J. Use of 31P magnetisation transfer magnetic resonance spectroscopy to measure ATP changes after 670 nm transcranial photobiomodulation in older adults. Aging Cell 2023, 22, e14005. [Google Scholar] [CrossRef]
- Osipov, A.N.; Machneva, T.V.; Buravlev, E.A.; Vladimirov, Y.A. Effects of laser radiation on mitochondria and mitochondrial proteins subjected to nitric oxide. Front. Med. 2018, 5, 112. [Google Scholar] [CrossRef]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.-Y.; de Sousa, M.V.P.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. J. Photochem. Photobiol. 2015, 91, 411–416. [Google Scholar] [CrossRef]
- Gavish, L.; Asher, Y.; Becker, Y.; Kleinman, Y. Low level laser irradiation stimulates mitochondrial membrane potential and disperses subnuclear promyelocytic leukemia protein. LSM 2004, 35, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Laser Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, C.; Hamblin, M.R.; Parizotto, N.A. Low-level laser (light) therapy (LLLT) on muscle tissue: Performance, fatigue and repair benefited by the power of light: Low-Level-Laser (Licht)-Therapie an Muskelgewebe—Möglichkeiten zur Verbesserung der Leistungsfähigkeit und zur Behandlung von Muskelermüdung und Muskelverletzungen. Photonics Lasers Med. 2012, 1, 267–286. [Google Scholar] [PubMed]
- Rupel, K.; Zupin, L.; Colliva, A.; Kamada, A.; Poropat, A.; Ottaviani, G.; Gobbo, M.; Fanfoni, L.; Gratton, R.; Santoro, M.; et al. Photobiomodulation at multiple wavelengths differentially modulates oxidative stress in vitro and in vivo. Oxidative Med. Cell. Longev. 2018, 1, 6510159. [Google Scholar] [CrossRef]
- Halevy, O.; Biran, I.; Rozenboim, I. Various light source treatments affect body and skeletal muscle growth by affecting skeletal muscle satellite cell proliferation in broilers. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 1998, 120, 317–323. [Google Scholar] [CrossRef]
- Monfrecola, G.; Lembo, S.; Cantelli, M.; Ciaglia, E.; Scarpato, L.; Fabbrocini, G.; Balato, A. The effect of visible blue light on the differentiation of dendritic cells in vitro. Biochimie 2014, 101, 252–255. [Google Scholar] [CrossRef]
- Becker, D.; Langer, E.; Seemann, M.; Seemann, G.; Fell, I.; Saloga, J.; Grabbe, S.; von Stebut, E. Clinical efficacy of blue light full body irradiation as treatment option for severe atopic dermatitis. PLoS ONE 2011, 6, e20566. [Google Scholar] [CrossRef]
- Ewais, T.; Hunt, M.; Laakso, E.-L. Photobiomodulation at 904nm reduces symptoms of inflammatory bowel disease: Early results from a pilot single arm feasibility study. In Proceedings of the PBM2024, London, UK, 23–25 August 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laakso, E.-L.; Ewais, T.; McMahon, K.; Forbes, J.; Phillips, L. Efficacy and Safety of Photobiomodulation in MELAS: Protocol for a Series of N-of-1 Trials. J. Clin. Med. 2025, 14, 2047. https://doi.org/10.3390/jcm14062047
Laakso E-L, Ewais T, McMahon K, Forbes J, Phillips L. Efficacy and Safety of Photobiomodulation in MELAS: Protocol for a Series of N-of-1 Trials. Journal of Clinical Medicine. 2025; 14(6):2047. https://doi.org/10.3390/jcm14062047
Chicago/Turabian StyleLaakso, E-Liisa, Tatjana Ewais, Katie McMahon, Josephine Forbes, and Liza Phillips. 2025. "Efficacy and Safety of Photobiomodulation in MELAS: Protocol for a Series of N-of-1 Trials" Journal of Clinical Medicine 14, no. 6: 2047. https://doi.org/10.3390/jcm14062047
APA StyleLaakso, E.-L., Ewais, T., McMahon, K., Forbes, J., & Phillips, L. (2025). Efficacy and Safety of Photobiomodulation in MELAS: Protocol for a Series of N-of-1 Trials. Journal of Clinical Medicine, 14(6), 2047. https://doi.org/10.3390/jcm14062047





