Performance of the Mayo Risk Score in Predicting Transplant and Mortality in a Single-Center U.S. Cohort of Primary Sclerosing Cholangitis
Abstract
1. Introduction
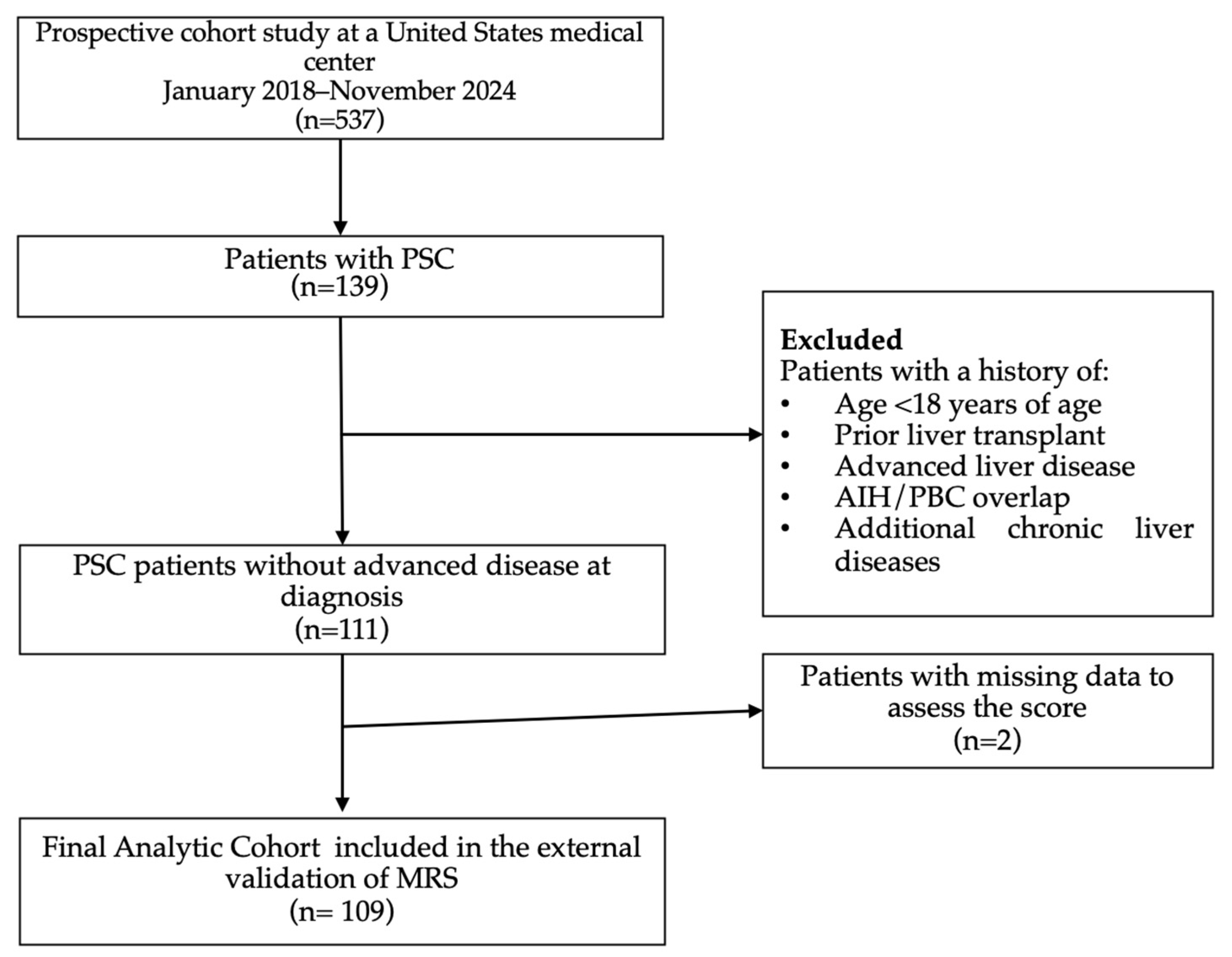
2. Materials and Methods
2.1. Study Population
2.2. Study Outcomes, Variables, and Definitions
2.3. Statistical Analysis
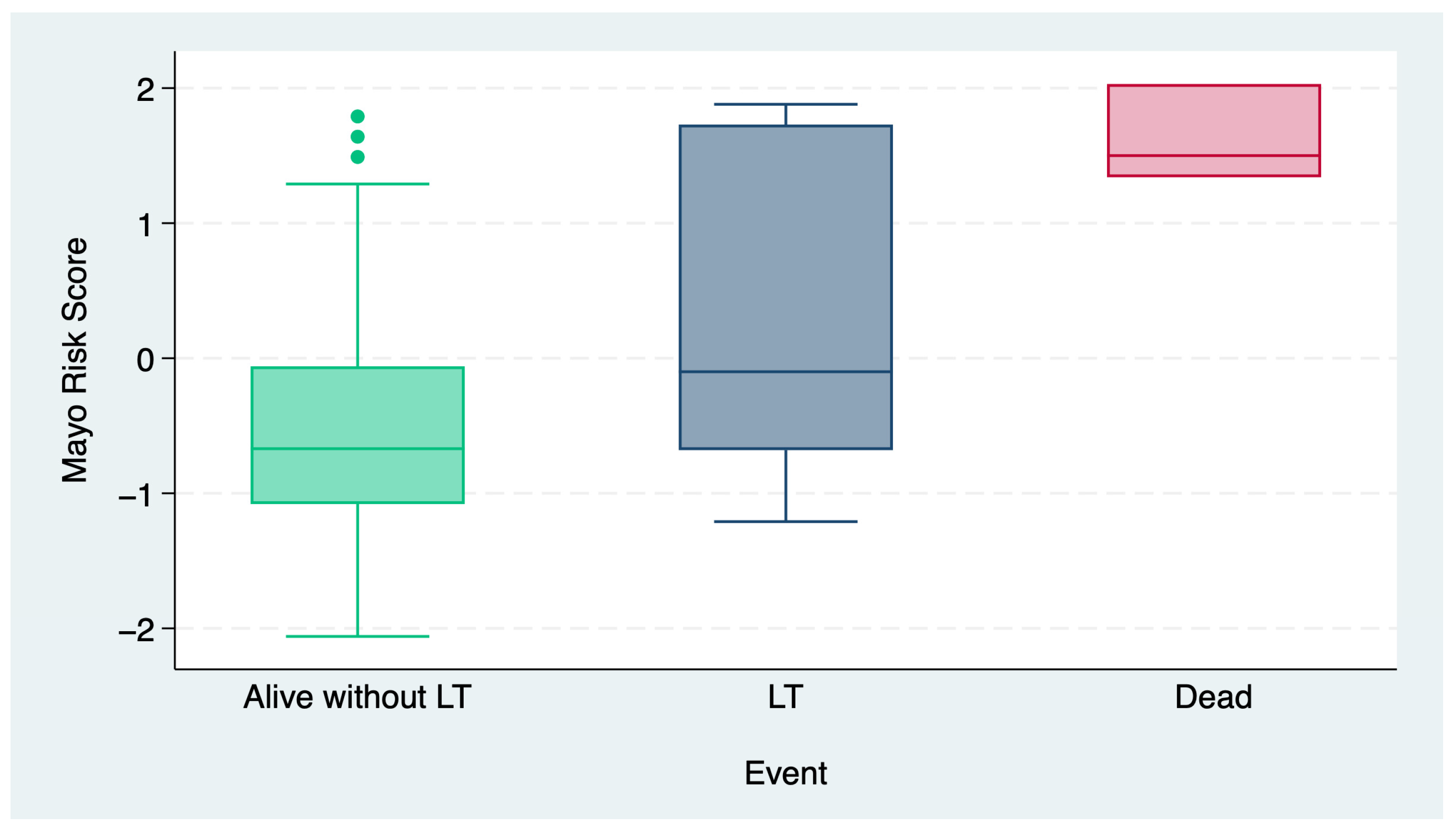
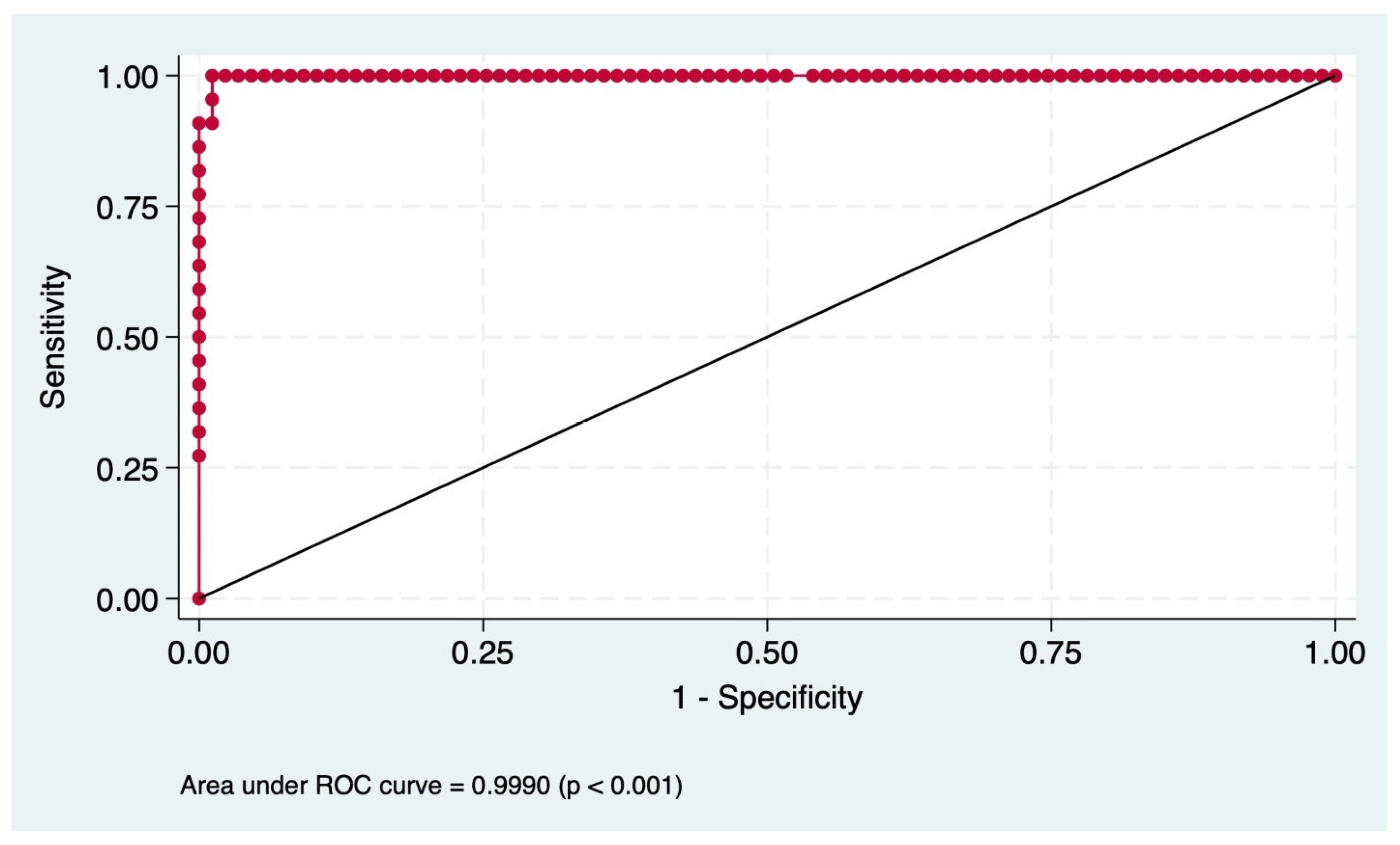
3. Results
3.1. Baseline Characteristics
3.2. Risk Classification
3.3. Predictive Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIH | Autoimmune Hepatitis |
| AILDs | Autoimmune Liver Diseases |
| ALB | Albumin |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransferase |
| AOM | Amsterdam–Oxford Model |
| AST | Aspartate Aminotransferase |
| AUROC | Area Under the Receiver Operating Characteristic Curve |
| BIDMC | Beth Israel Deaconess Medical Center |
| BMI | Body Mass Index |
| CD | Crohn’s Disease |
| CI | Confidence Interval |
| CR | Creatinine |
| ESLD | End-Stage Liver Disease |
| IBD | Inflammatory Bowel Disease |
| IQR | Interquartile Range |
| IRB | Institutional Review Board |
| LT | Liver Transplant |
| MELD | Model for End-Stage Liver Disease |
| MRS | Mayo Risk Score |
| NPV | Negative Predictive Value |
| PL | Platelet |
| PPV | Positive Predictive Value |
| OR | Odds Ratio |
| PREsTo | Primary Sclerosing Cholangitis Risk Estimate Tool |
| PSC | Primary Sclerosing Cholangitis |
| R | Risk |
| SD | Standard Deviation |
| TBIL | Total Bilirubin |
| UC | Ulcerative Colitis |
| UDCA | Ursodeoxycholic Acid |
| ULN | Upper Limit of Normal |
| UK-PSC | United Kingdom-PSC |
| VIF | Variance Inflation Factor |
| WBC | White Blood Cell |
References
- Eaton, J.E.; Talwalkar, J.A.; Lazaridis, K.N.; Gores, G.J.; Lindor, K.D. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 2013, 145, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Chazouilleres, O.; Beuers, U.; Bergquist, A.; Karlsen, T.H.; Levy, C.; Samyn, M.; Schramm, C.; Trauner, M. EASL clinical practice guidelines on sclerosing cholangitis. J. Hepatol. 2022, 77, 761–806. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of primary sclerosing cholangitis on risks of cancer and death in people with inflammatory bowel disease, based on sex, race, and age. Gastroenterology 2020, 159, 915–928. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.-U.; et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology 2017, 152, 1975–1984.e8. [Google Scholar] [CrossRef]
- Suri, J.; Patwardhan, V.; Bonder, A. Pharmacologic management of primary sclerosing cholangitis: What’s in the pipeline? Expert Rev. Gastroenterol. Hepatol. 2019, 13, 723–729. [Google Scholar] [CrossRef]
- Bambha, K.; Kim, W.R.; Talwalkar, J.; Torgerson, H.; Benson, J.T.; Therneau, T.M.; Loftus, E.V.; Yawn, B.P.; Dickson, E.; Melton, L. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology 2003, 125, 1364–1369. [Google Scholar] [CrossRef]
- Toy, E.; Balasubramanian, S.; Selmi, C.; Li, C.-S.; Bowlus, C.L. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol. 2011, 11, 83. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Bowlus, C.L.; Yimam, K.K.; Razavi, H.; Estes, C. Epidemiology, natural history, and outcomes of primary sclerosing cholangitis: A systematic review of population-based studies. Clin. Gastroenterol. Hepatol. 2022, 20, 1687–1700.e4. [Google Scholar] [CrossRef]
- Sirpal, S.; Chandok, N. Primary sclerosing cholangitis: Diagnostic and management challenges. Clin. Exp. Gastroenterol. 2017, 10, 265–273. [Google Scholar] [CrossRef]
- Sierra, L.; Barba, R.; Ferrigno, B.; Goyes, D.; Diaz, W.; Patwardhan, V.R.; Saberi, B.; Bonder, A. Living-donor liver transplant and improved post-transplant survival in patients with primary sclerosing cholangitis. J. Clin. Med. 2023, 12, 2807. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Neuberger, J. Liver transplantation in PBC and PSC: Indications and disease recurrence. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Cornillet, M.; Villard, C.; Rorsman, F.; Molinaro, A.; Nilsson, E.; Kechagias, S.; von Seth, E.; Bergquist, A. The Swedish initiative for the study of primary sclerosing cholangitis (SUPRIM). eClinicalMedicine 2024, 70, 102526. [Google Scholar] [CrossRef]
- Kim, W.R.; Therneau, T.M.; Wiesner, R.H.; Poterucha, J.J.; Benson, J.T.; Malinchoc, M.; Larusso, N.F.; Lindor, K.D.; Dickson, E.R. A revised natural history model for primary sclerosing cholangitis. Mayo Clin. Proc. 2000, 75, 688–694. [Google Scholar] [CrossRef]
- Khoshpouri, P.; Ameli, S.; Ghasabeh, M.A.; Pandey, A.; Zarghampour, M.; Varzaneh, F.N.; Jacob, A.; Pandey, P.; Luo, Y.; Kamel, I.R. Correlation between quantitative liver and spleen volumes and disease severity in primary sclerosing cholangitis as determined by Mayo Risk Score. Eur. J. Radiol. 2018, 108, 254–260. [Google Scholar] [CrossRef]
- Goode, E.C.; Clark, A.B.; Mells, G.F.; Srivastava, B.; Spiess, K.; Gelson, W.T.H.; Trivedi, P.J.; Lynch, K.D.; Castren, E.; Vesterhus, M.N.; et al. Factors associated with outcomes of patients with primary sclerosing cholangitis and development and validation of a risk scoring system. Hepatology 2019, 69, 2120–2135. [Google Scholar] [CrossRef]
- Trivedi, P.J. Risk stratification in primary sclerosing cholangitis: It’s time to move on from replicating imperfection and break the glass ceiling. J. Hepatol. 2019, 71, 867–870. [Google Scholar] [CrossRef]
- Schmeltzer, P.A.; Russo, M.W. Systematic review of prognostic models compared to the Mayo Risk score for primary sclerosing cholangitis. J. Clin. Med. 2021, 10, 4476. [Google Scholar] [CrossRef]
- Wiesner, R.H.; Grambsch, P.M.; Dickson, R.E.; Ludwig, J.; Maccarty, R.L.; Hunter, E.B.; Fleming, T.R.; Fisher, L.D.; Beaver, S.J.; Larusso, N.F. Primary sclerosing cholangitis: Natural history, prognostic factors and survival analysis. Hepatology 1989, 10, 430–436. [Google Scholar] [CrossRef]
- Mazhar, A.; Russo, M.W. Systematic review: Non-invasive prognostic tests for primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2021, 53, 774–783. [Google Scholar] [CrossRef]
- Tischendorf, J.J.; Hecker, H.; Krüger, M.; Manns, M.P.; Meier, P.N. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am. J. Gastroenterol. 2007, 102, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.W.; Poterucha, J.J.; Wiesner, R.H.; LaRusso, N.F.; Lindor, K.D.; Petz, J.; Therneau, T.M.; Malinchoc, M.; Dickson, R.E. The relative role of the Child-Pugh classification and the Mayo natural history model in the assessment of survival in patients with primary sclerosing cholangitis. Hepatology 1999, 29, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Fossdal, G.; Mjelle, A.B.; Wiencke, K.; Bjørk, I.; Gilja, O.H.; Folseraas, T.; Karlsen, T.H.; Rosenberg, W.; Giil, L.M.; Vesterhus, M. Fluctuating biomarkers in primary sclerosing cholangitis: A longitudinal comparison of alkaline phosphatase, liver stiffness, and Elf. Jhep Rep. 2021, 3, 100328. [Google Scholar] [CrossRef]
- de Vries, E.M.; Wang, J.; Leeflang, M.M.; Boonstra, K.; Weersma, R.K.; Beuers, U.H.; Geskus, R.B.; Ponsioen, C.Y. Alkaline phosphatase at diagnosis of primary sclerosing cholangitis and 1 year later: Evaluation of prognostic value. Liver Int. 2016, 36, 1867–1875. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary sclerosing cholangitis—A comprehensive review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef]
- Boberg, K.M.; Rocca, G.; Egeland, T.; Bergquist, A.; Broomé, U.; Caballeria, L.; Chapman, R.; Hultcrantz, R.; Mitchell, S.; Pares, A.; et al. Time-dependent cox regression model is superior in prediction of prognosis in primary sclerosing cholangitis. Hepatology 2002, 35, 652–657. [Google Scholar] [CrossRef]
- Dickson, E.R.; Murtaugh, P.A.; Wiesner, R.H.; Grambsch, P.M.; Fleming, T.R.; Ludwig, J.; LaRusso, N.F.; Malinchoc, M.; Chapman, R.W.; Kaplan, M.M.; et al. Primary sclerosing cholangitis: Refinement and validation of Survival Models. Gastroenterology 1992, 103, 1893–1901. [Google Scholar] [CrossRef]
- Narumi, S.; Roberts, J.P.; Emond, J.C.; Lake, J.; Ascher, N. Liver transplantation for sclerosing cholangitis. Hepatology 1995, 22, 451–457. [Google Scholar] [CrossRef]
- Brandsæter, B.; Friman, S.; Broomé, U.; Isoniemi, H.; Olausson, M.; Bäckman, L.; Hansen, B.; Schrumpf, E.; Oksanen, A.; Ericzon, B.; et al. Outcome following liver transplantation for primary sclerosing cholangitis in the Nordic countries. Scand. J. Gastroenterol. 2003, 38, 1176–1183. [Google Scholar] [CrossRef]
| Variable | Baseline (n = 109) |
|---|---|
| Age at diagnosis, mean (SD) | 45 (15) |
| Gender, female n (%) | 56 (51) |
| Race/ethnicity, n (%) | |
| White Caucasian | 86 (79) |
| Hispanic | 7 (6) |
| Asian | 2 (2) |
| African American | 10 (9) |
| Indian American | 1 (1) |
| Unknown | 3 (3) |
| BMI, mean (SD) | 27 (6) |
| Concomitant IBD, n (%) | |
| Ulcerative colitis (UC) | 50 (45) |
| Crohn’s disease (CD) | 12 (11) |
| Bile duct involvement, n (%) | |
| Small duct | 28 (26) |
| Large duct | 55 (51) |
| Dominant stricture | 26 (24) |
| UDCA treatment, n (%) | 61 (57) |
| UDCA dose, mg/kg/day | 13 (4) |
| Liver biopsy at diagnosis, n (%) | 45 (41) |
| Laboratory values at diagnosis, median (IQR) | |
| ALP | 154 (100–301) |
| AST | 40 (23–67) |
| ALT | 49 (25–88) |
| TBIL | 0.5 (0.4–0.8) |
| ALB | 4.3 (4–4.6) |
| PL | 265 (208–338) |
| INR | 1 (1–1.1) |
| CR | 0.8 (0.7–0.9) |
| WBC | 6.6 (5.3–8.05) |
| Median follow-up, years mean (SD) | 4.63 (3.58) |
| Outcome at follow-up, n (%) | |
| Alive without transplant | 93 (85) |
| Liver transplant | 13 (12) |
| Death | 3 (3) |
| MRS, median (IQR) | −0.5 (−1.03–−0.02) |
| Risk classification Mayo Score, n (%) | |
| Low (R < 0) | 87 (80) |
| Intermediate (0 ≤ R < 2) | 19 (18) |
| High (R ≥ 2) | 3 (3) |
| Variable | Low Risk (n = 87) | Intermediate Risk (n = 19) | High Risk (n = 3) | p Value |
|---|---|---|---|---|
| Age at diagnosis, mean (SD) | 46 (15) | 45 (15) | 59 (23) | 0.281 |
| BMI, median (IQR) | 25.1 (23–30) | 26.8 (25–30) | 27 (20–29) | 0.395 |
| Concomitant IBD, n (%) | 0.873 | |||
| Ulcerative colitis (UC) | 39 (45) | 10 (53) | 1 (33) | |
| Crohn’s disease (CD) | 10 (11) | 2 (11) | 0 | |
| Bile duct involvement, n (%) | 0.177 | |||
| Small duct | 24 (28) | 2 (11) | 1 (33) | |
| Large duct | 41 (47) | 13 (68) | 1 (33) | |
| Dominant stricture | 22 (25) | 3 (16) | 1 (33) | |
| UDCA treatment, n (%) | 50 (82) | 9 (15) | 2 (3) | 0.649 |
| UDCA dose, mg/kg/day median (IQR) | 13 (11–15) | 12 (11–14) | 12 (11–13) | 0.583 |
| Liver biopsy at diagnosis, n (%) | 33 (38) | 10 (53) | 2 (67) | 0.331 |
| Laboratory values at diagnosis, median (IQR) | ||||
| ALP | 139 (90–250) | 293 (116–555) | 268 (218–608) | 0.010 |
| AST | 34 (22–57) | 75 (40–116) | 93 (63–132) | 0.003 |
| ALT | 41 (23–74) | 83 (45–127) | 98 (27–192) | 0.028 |
| TBIL | 0.5 (0.4–0.7) | 0.6 (0.9–1.7) | 1.5 (0.8–16.7) | <0.001 |
| ALB | 4.4 (4–4.7) | 4 (3.8–4.4) | 3.5 (3.5–3.6) | <0.001 |
| PL | 269 (214–357) | 236 (157–349) | 177 (163–306) | 0.379 |
| INR | 1.1 (1–1.1) | 1 (1–1.1) | 1.2 (1–1.4) | 0.636 |
| CR | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.7 (0.6–1) | 0.561 |
| WBC | 7.1 (5.3–8.4) | 6 (5.4–6.6) | 4.7 (3.4–7.6) | 0.223 |
| Median follow-up, years mean (SD) | 5 (4.74–5) | 5 (4.49–5) | 2.72 (0.83–2.98) | <0.001 |
| Outcome at follow-up, n (%) | <0.001 | |||
| Alive without LT | 79 (91) | 14 (74) | 0 | |
| LT | 8 (9) | 4 (18) | 1 (33) | |
| Death | 0 | 1 (6) | 2 (66) | |
| MRS, median (IQR) | −0.8 (−1.18-−0.26) | 0.64 (0.2–1.64) | 2.03 (1.85 −2.03) | <0.001 |
| Variable | Univariate OR (95% CI) | p Value | Pseudo R2 | Variable | Multivariate OR (95% CI) | p Value | Pseudo R2 |
|---|---|---|---|---|---|---|---|
| Gender | 0.37 (0.12–1.16) | 0.089 | 0.034 | ||||
| Concomitant IBD | 1.41 (0.65–3.10) | 0.385 | 0.008 | ||||
| Bile duct involvement, small duct | 0.84 (044–1.61) | 0.610 | 0.003 | MRS | 3.08 (1.71–5.58) | <0.001 | |
| UDCA treatment | 0.98 (0.34–2.88) | 0.984 | <0.001 | 0.1783 | |||
| ALP | 1.00 (0.99–1.00) | 0.283 | 0.013 | ||||
| PL | 0.99 (0.98–0.99) | 0.031 | 0.111 | ||||
| MRS | 3.00 (1.71–5.6) | <0.001 | 0.178 |
| Metric | Value (%) |
|---|---|
| Sensitivity | 95.45 |
| Specificity | 98.85 |
| PPV | 95.45 |
| NPV | 98.85 |
| Correct Classification Rate | 98.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahan, T.; Marenco-Flores, A.; Amaris, N.R.; Barba, R.; Goyes, D.; Medina-Morales, E.; Sierra, L.; Patwardhan, V.R.; Bonder, A. Performance of the Mayo Risk Score in Predicting Transplant and Mortality in a Single-Center U.S. Cohort of Primary Sclerosing Cholangitis. J. Clin. Med. 2025, 14, 2098. https://doi.org/10.3390/jcm14062098
Kahan T, Marenco-Flores A, Amaris NR, Barba R, Goyes D, Medina-Morales E, Sierra L, Patwardhan VR, Bonder A. Performance of the Mayo Risk Score in Predicting Transplant and Mortality in a Single-Center U.S. Cohort of Primary Sclerosing Cholangitis. Journal of Clinical Medicine. 2025; 14(6):2098. https://doi.org/10.3390/jcm14062098
Chicago/Turabian StyleKahan, Tamara, Ana Marenco-Flores, Natalia Rojas Amaris, Romelia Barba, Daniela Goyes, Esli Medina-Morales, Leandro Sierra, Vilas R. Patwardhan, and Alan Bonder. 2025. "Performance of the Mayo Risk Score in Predicting Transplant and Mortality in a Single-Center U.S. Cohort of Primary Sclerosing Cholangitis" Journal of Clinical Medicine 14, no. 6: 2098. https://doi.org/10.3390/jcm14062098
APA StyleKahan, T., Marenco-Flores, A., Amaris, N. R., Barba, R., Goyes, D., Medina-Morales, E., Sierra, L., Patwardhan, V. R., & Bonder, A. (2025). Performance of the Mayo Risk Score in Predicting Transplant and Mortality in a Single-Center U.S. Cohort of Primary Sclerosing Cholangitis. Journal of Clinical Medicine, 14(6), 2098. https://doi.org/10.3390/jcm14062098






