Safety and Efficacy of Stem Cell Therapy in Ischemic Stroke: A Comprehensive Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
3. Study Selection
Data Extraction
4. Statistical Analysis
5. Heterogeneity Exploration
6. Results
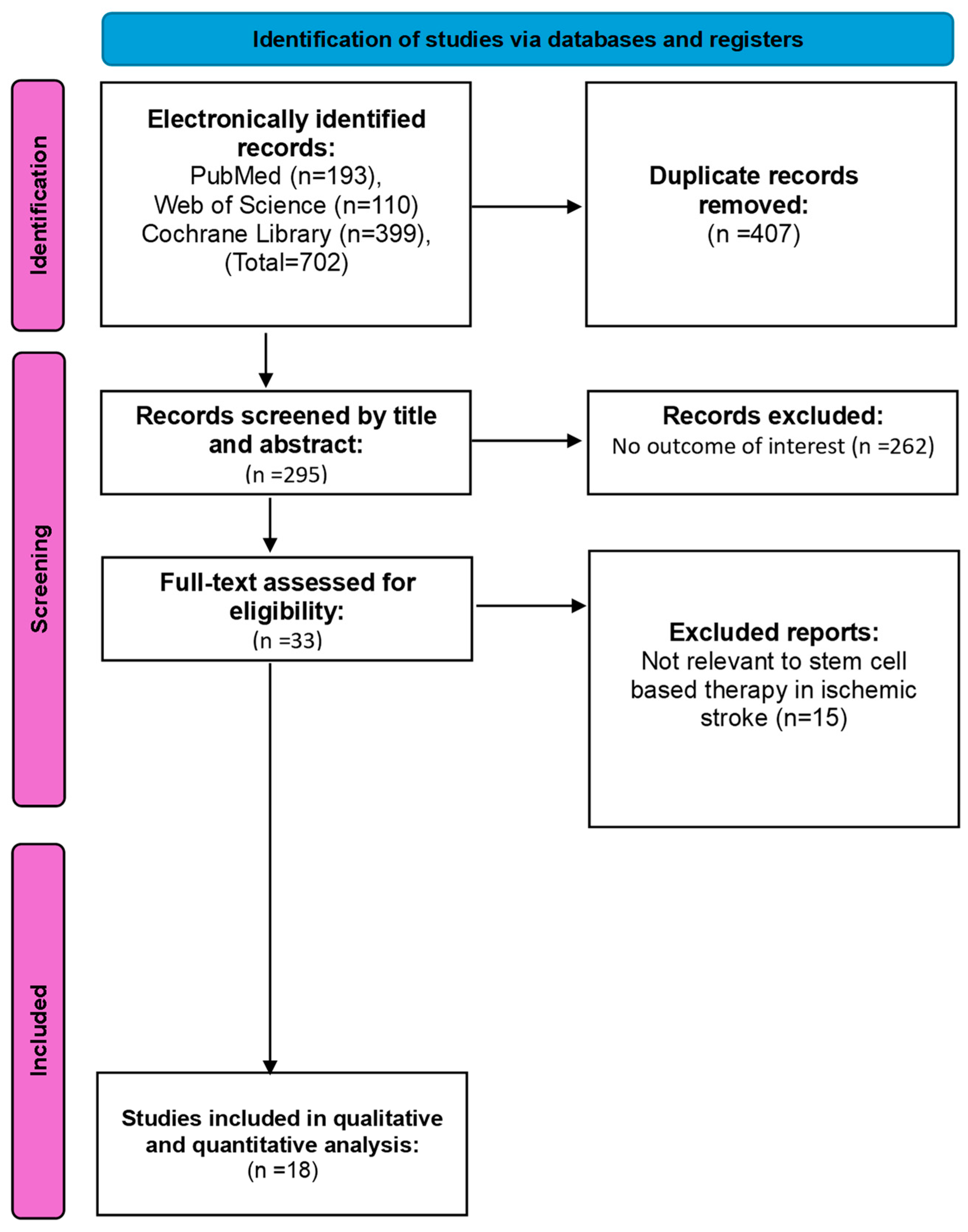
6.1. Search Results
6.2. Study Characteristics
| Study (Author) | Year of the Study | Country of the Study | Cell Type | Dosage | Administration | Administration Timing | Total Patients, N | Gender Distribution of SCT Group (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| SCT Group | Control Group | Males | Females | |||||||
| Bang et al. [13] | 2005 | South Korea | Mesenchymal stem cell | 5 × 107, twice | IV | 32–61 days | 5 | 25 | 80 | 20 |
| Lee et al. [29] | 2010 | South Korea | Mesenchymal stem cell | 5 × 107, twice | IV | 4 to 9 weeks | 16 | 36 | 50 | 50 |
| Chen et al. [30] | 2014 | China | Peripheral blood stem cell | 3–8 × 106 | Stereotaxic implantation | 6 months to 5 years | 15 | 15 | 80 | 20 |
| Prasad et al. [31] | 2014 | Indian | Mesenchymal stem cell | Mean of 2.8 × 108 | IV | Mean of 18.5 days | 60 | 60 | 68.3 | 60 |
| Bhasin et al. [25] | 2016 | India | Bone marrow-derived mononuclear cell | Mean of 6.28 × 107 | IV | 3 months to 2 years | 20 | 20 | 75 | 25 |
| Hess et al. [32] | 2017 | USA and UK | Multipotent adult progenitor cell | 1.2 × 109 | IV | 24 to 48 h | 71 | 63 | 56.3 | 43.7 |
| Bhatia et al. [28] | 2018 | India | Bone marrow-derived mononuclear cell | Mean of 6.1 × 108 | IA | Mean of 10 days | 10 | 10 | 80 | 20 |
| Fang et al. [23] | 2019 | China | Endothelial progenitor cell, (50%); mesenchymal stem cell (50%) | 2.5 × 106/kg, twice | IV | Mean of 33.5 days | 5 | 6 | 80 | 20 |
| Savitz et al. [33] | 2019 | America | Bone marrow-derived ALDHbr cells (ALD-401) | Mean of 3.08 × 106 | IA | 13 to 19 days | 29 | 19 | 69 | 31 |
| Wang et al. [26] | 2020 | China | Olfactory ensheathing cell | 10 × 106 | Intranasal | >12 months | 18 | 9 | 83.2 | 16.7 |
| Jaillard et al. [34] | 2020 | France | Mesenchymal stem cell | 10 × 107 (First cohort), 30 × 107 (Second cohort) | IV | <5–6 weeks | 16 | 15 | 68.8 | 31.2 |
| Law et al. [24] | 2021 | Malaysia | Bone marrow-derived mononuclear cell | 2 × 106/kg | IV | Median of 63 days | 9 | 8 | 88.9 | 11.1 |
| Chung et al. [27] | 2021 | South Korea | Mesenchymal stem cell | 1 × 106/kg | IV | >3 months | 39 | 15 | 43.6 | 56.4 |
| Celis-Ruiz et al. [21] | 2022 | Spain | Mesenchymal stem cell | 1 × 107 | IV | Mean of 13 days | 4 | 8 | 25 | 75 |
| Lee et al. [35] | 2022 | South Korea | Mesenchymal stem cell | 11 × 106 | IV | >3 months | 31 | 13 | 48.3 | 51.7 |
| Moniche et al. [36] | 2023 | Spain | Bone marrow-derived mononuclear cell | (2 × 106/kg or 5 × 106/kg) | IA | Median of 3 days | 39 | 38 | 54 | 56 |
| Houkin et al. [22] | 2024 | Japan | Multipotent adult progenitor cell | 1.2 × 109 | IV | 18 to 36 h | 104 | 102 | 53.8 | 46.2 |
| Laskowitz et al. [37] | 2024 | USA | Umbilical cord blood | 0.5–5 × 107 total nucleated cell count/kg | IV | 3–10 days | 47 | 26 | 61.7 | 38.3 |
| Study (Author) | Age Distribution, Mean (SD) | Duration After Stroke, Mean (SD) | Duration of Follow-Up, Mean (SD) | Baseline Infarct Volume, Mean (SD) | Baseline NIHSS Score, Mean (SD) | Baseline BI, Mean (SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SCT Group | Control Group | SCT Group | Control Group | SCT Group | Control Group | SCT Group | Control Group | SCT Group | Control Group | SCT Group | Control Group | |
| Bang et al. [13] | 63 (7.5) | 59.3 (11.5) | 7 days | 7 days | 12 months | 12 months | 127.4 (70.3) | 89.1 (77.4) | 10.6 (2.6) | 11.6 (4.9) | 9.0 (20.1) | 13.4 (22.2) |
| Lee et al. [29] | 64.0 (11.6) | 64.9 (14.5) | 7 days | 7 days | 129.6 weeks | 110.3 weeks | 115.7 (95.2) | 90.1 (86.8) | 10.63 (3.03) | 10.17 (3.67) | N/A | N/A |
| Chen et al. [30] | 50.1 (7.7) | 52.8 (9.0) | 2.7 years | 2.5 years | 12 months | 12 months | N/A | N/A | 9.3 (0.5) | 9.6 (1.3) | N/A | N/A |
| Prasad et al. [31] | 50.7 (11) | 52.5 (12) | 17 days | 17 days | 6 months | 6 months | 86.9 (57) | 111.7 (72.4) | 11 (3) | 13 (3) | 25 (12.7) | 27.5 (11) |
| Bhasin et al. [25] | 48.4 (8.16) | 49.6 (5.6) | 11.05 months | 10.5 months | 56 days | 56 days | N/A | N/A | N/A | N/A | 46.5 (5.9) | N/A |
| Hess et al. [32] | 61.8 (11.4) | 62.6 (11.4) | 37.2 h | 39.3 h | 12 months | 12 months | 43.7 (26.9) | 50.9 (41.3) | 13.4 (3.6) | 13.3 (3,7) | N/A | N/A |
| Bhatia et al. [28] | 57 (12.2) | 66 (7.3) | 10 days | 10 days | 6 months | 6 months | N/A | N/A | 10.6 | 10.5 | 76.3 | 78.1 |
| Fang et al. [23] | 50.1 (7.55) | 52.83 (14.95) | 7 days | 7 days | 4 years | 4 years | N/A | N/A | 12.20 (4.92) | 15.5 (3.02) | 39.00 (24.60) | 25 (20) |
| Savitz et al. [33] | 59.3 (10.03) | 62.9 (10.81) | 28 days | 16 days | 90 days | 90 days | N/A | N/A | 11 | 10 | N/A | N/A |
| Wang et al. [26] | 64.2 (5.7) | 66 (12) | 6 days | N/A | 6 months | 6 months | 69 (23.9) | 79 (33.5) | 12.5 (1.4) | 12.3 (4.5) | N/A | N/A |
| Jaillard et al. [34] | 55 (6) | 53 (9.12) | <14 days | <14 days | 2 years | 2 years | 92 (60.1) | 113 (48.2) | 17 (3.48) | 17 (3.33) | 48.75 (22.5) | 45 (20) |
| Law et al. [24] | 55 (6) | 53 (9.12) | <14 days | <14 days | 2 years | 2 years | 92 (60.1) | 113 (48.2) | 17 (3.48) | 17 (3.33) | 48.75 (22.5) | 45 (20) |
| Chung et al. [27] | 63.03 (14.36) | 64.27 (13.25) | 21 days | 18.4 days | 32.7 days | 30.6 days | 90.96 (79.57) | 96.46 (74.31) | 11.36 (5.2) | 14.5 (5.32) | 28.28 (26.63) | 19.8 (25.5) |
| Celis-Ruiz et al. [21] | 79.25 (3.83) | 77.13 (3.75) | 13.4 days | 12.5 days | 2 years | 2 years | 43.22 (41.84) | 88.16 (56.15) | 10.5 (3.19) | 11 (1.5) | N/A | N/A |
| Lee et al. [35] | 63.4 (14.0) | 61.5 (13.0) | 24.6 days | 20.9 days | 90 days | 90 days | 125 (115.7) | 127.3 (122.7) | N/A | N/A | N/A | N/A |
| Moniche et al. [36] | 64.2 (5.7) | 66 (12) | 6 days | N/A | 6 months | 6 months | 69 (23.9) | 79 (33.5) | 12.5 (1.4) | 12.3 (4.5) | N/A | N/A |
| Houkin et al. [22] | 76.7 (10.4) | 76.2 (10.6) | 18–36 h | 18–36 h | 12 months | 12 months | 42.0 (48.4) | 54.3 (57.0) | 13.7 (3.9) | 13.9 (3.9) | N/A | N/A |
| Laskowitz et al. [37] | 62.6 (12.1) | 64.4 (11.2) | 6 days | 6 days | 90 days | 90 days | N/A | N/A | 12.3 (3.6) | 12.2 (3.4) | 40 (9.4) | 45 |
6.3. Risk of Bias Assessment
6.4. Publication Bias
7. Evaluation of the Efficacy Outcomes
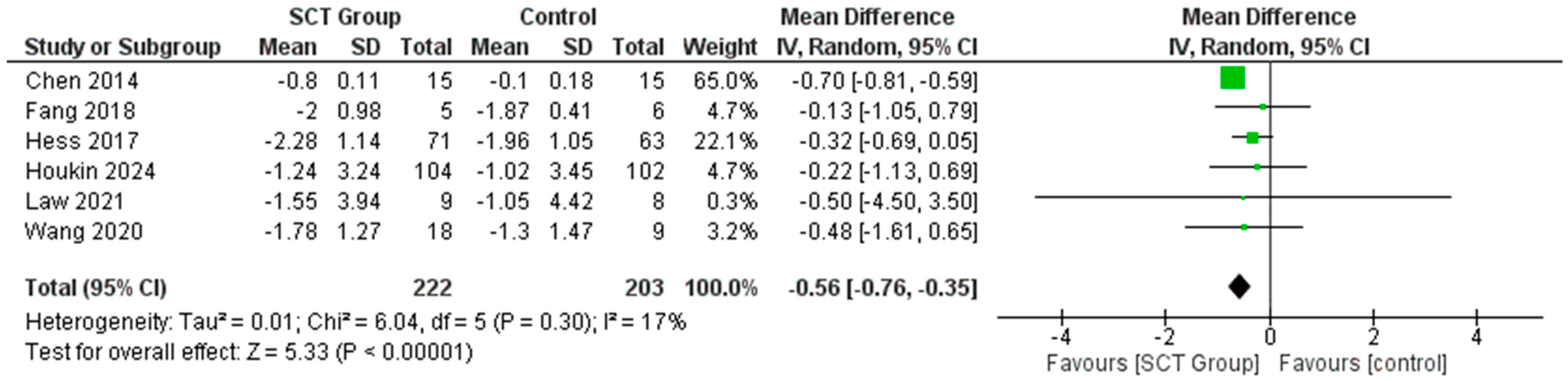
7.1. Difference in the Mean Change in NIHSS Score
7.2. Difference in the Mean Change in mRS Score
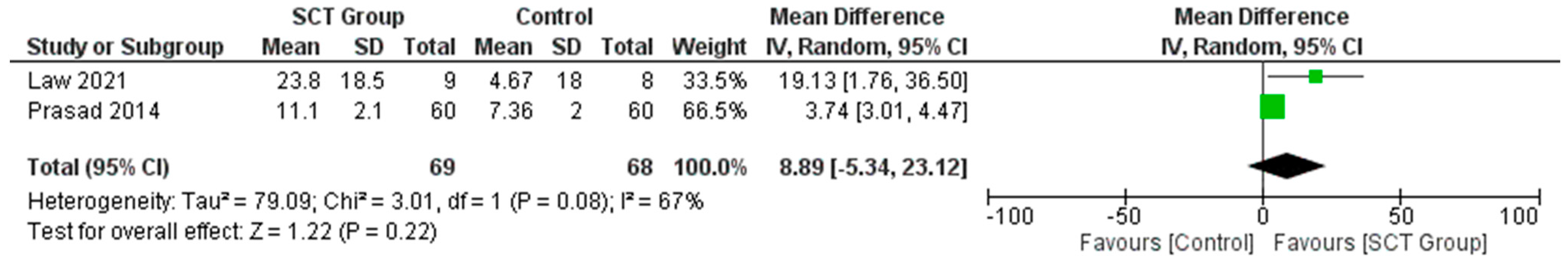
7.3. Difference in the Mean Change in BI
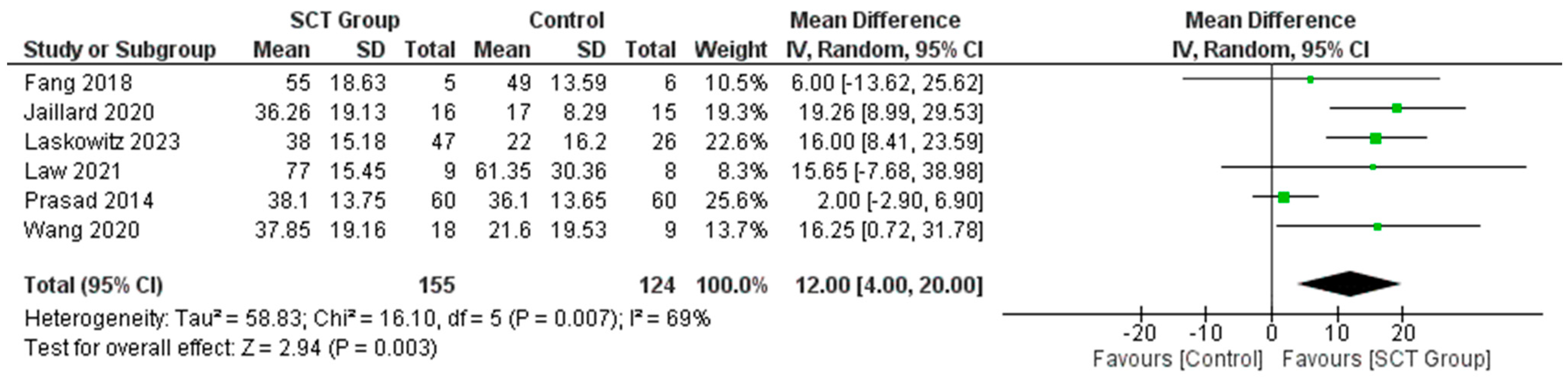
7.4. Difference in the Mean Change in FMA Score
7.5. Difference in the Mean Change in Infarct Volume
8. Safety Outcomes
8.1. Serious Adverse Effects
8.2. Immediate Adverse Effects
8.3. Delayed Adverse Effects
8.4. Subgroup Analysis
9. Discussion
9.1. Primary Findings and Clinical Significance
9.2. Subgroup Analyses and Considerations for Implementation
9.3. Stroke Burden and Present Therapeutic Landscape
9.4. Rationale and Mechanisms of Stem Cell Therapy
9.5. Patient-Reported Outcomes and Quality of Life
9.6. Study Strengths in Comparison to the Recent Literature
10. Study Limitations
11. Future Directions and Research Priorities
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barthels, D.; Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar]
- Xiong, Y.; Guo, X.; Gao, W.; Ke, C.; Huang, X.; Pan, Z.; Chen, C.; Zheng, H.; Hu, W.; Zheng, F.; et al. Efficacy and safety of stem cells in the treatment of ischemic stroke: A meta-analysis. Medicine 2024, 103, e37414. [Google Scholar]
- Chavez, L.M.; Huang, S.S.; MacDonald, I.; Lin, J.G.; Lee, Y.C.; Chen, Y.H. Mechanisms of Acupuncture Therapy in Ischemic Stroke Rehabilitation: A Literature Review of Basic Studies. Int. J. Mol. Sci. 2017, 18, 2270. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J. Long-Term Outcome after Ischaemic Stroke/Transient Ischaemic Attack. Cerebrovasc. Dis. 2003, 16 (Suppl. S1), 14–19. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Outes, A.; Alcubilla, P.; Calvo-Rojas, G.; Terleira-Fernández, A.I.; Suárez-Gea, M.L.; Lecumberri, R.; Vargas-Castrillón, E. Meta-Analysis of Reversal Agents for Severe Bleeding Associated with Direct Oral Anticoagulants. J. Am. Coll. Cardiol. 2021, 77, 2987–3001. [Google Scholar]
- Hovhannisyan, L.; Khachatryan, S.; Khamperyan, A.; Matinyan, S. A review and meta-analysis of stem cell therapies in stroke patients: Effectiveness and safety evaluation. Neurol. Sci. 2024, 45, 65–74. [Google Scholar]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 2013, 140, 2457–2461. [Google Scholar]
- Gautam, J.; Alaref, A.; Hassan, A.; Kandel, R.S.; Mishra, R.; Jahan, N. Safety and Efficacy of Stem Cell Therapy in Patients with Ischemic Stroke. Cureus 2020, 12, e9917. [Google Scholar]
- Zheng, H.; Zhang, B.; Chhatbar, P.Y.; Dong, Y.; Alawieh, A.; Lowe, F.; Hu, X.; Feng, W. Mesenchymal Stem Cell Therapy in Stroke: A Systematic Review of Literature in Pre-Clinical and Clinical Research. Cell Transplant. 2018, 27, 1723–1730. [Google Scholar]
- Bang, O.Y.; Lee, J.S.; Lee, P.H.; Lee, G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005, 57, 874–882. [Google Scholar] [PubMed]
- Li, Z.; Dong, X.; Tian, M.; Liu, C.; Wang, K.; Li, L.; Liu, Z.; Liu, J. Stem cell-based therapies for ischemic stroke: A systematic review and meta-analysis of clinical trials. Stem Cell Res. Ther. 2020, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Tang, X.; Zhang, Y.; Jia, Y.; Guo, X.; Guo, X.; Bao, J.; Xie, X.; Xing, Y.; Xing, J.; et al. Efficacy and safety of mesenchymal stem cell therapies for ischemic stroke: A systematic review and meta-analysis. Stem Cells Transl. Med. 2024, 13, 886–897. [Google Scholar]
- PRISMA Group. PRISMA Statement [Internet]. 2020. Available online: https://www.prisma-statement.org/ (accessed on 24 December 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- RevMan Group. Cochrane. RevMan5.4_Statment. 2020. Available online: https://training.cochrane.org/online-learning/core-software (accessed on 20 June 2024).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [PubMed]
- Cumpston, M.; Li, T.; Page, M.; Chandler, J.; Welch, V.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar]
- de Celis-Ruiz, E.; Fuentes, B.; Alonso de Leciñana, M.; Gutiérrez-Fernández, M.; Borobia, A.M.; Gutiérrez-Zúñiga, R.; Ruiz-Ares, G.; Otero-Ortega, L.; Laso-García, F.; Gómez-de Frutos, M.C.; et al. Final Results of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial. Cell Transplant. 2022, 31, 9636897221083864. [Google Scholar]
- Houkin, K.; Houkin, K.; Osanai, T.; Osanai, T.; Uchiyama, S.; Uchiyama, S.; Minematsu, K.; Minematsu, K.; Taguchi, A.; Taguchi, A.; et al. Allogeneic Stem Cell Therapy for Acute Ischemic Stroke: The Phase 2/3 TREASURE Randomized Clinical Trial. JAMA Neurol. 2024, 81, 154–162. [Google Scholar] [CrossRef]
- Fang, J.; Guo, Y.; Tan, S.; Li, Z.; Xie, H.; Chen, P.; Wang, K.; He, Z.; He, P.; Ke, Y.; et al. Autologous Endothelial Progenitor Cells Transplantation for Acute Ischemic Stroke: A 4-Year Follow-Up Study. Stem Cells Transl. Med. 2019, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Law, Z.K.; Tan, H.J.; Chin, S.P.; Wong, C.Y.; Yahya, W.N.N.W.; Muda, A.S.; Zakaria, R.; Ariff, M.I.; Ismail, N.A.; Cheong, S.K.; et al. The effects of intravenous infusion of autologous mesenchymal stromal cells in patients with subacute middle cerebral artery infarct: A phase 2 randomized controlled trial on safety, tolerability and efficacy. Cytotherapy 2021, 23, 833–840. [Google Scholar] [CrossRef]
- Bhasin, A.; Srivastava, M.P.; Mohanty, S.; Vivekanandhan, S.; Sharma, S.; Kumaran, S.; Bhatia, R. Paracrine Mechanisms of Intravenous Bone Marrow-Derived Mononuclear Stem Cells in Chronic Ischemic Stroke. Cerebrovasc. Dis. Extra 2016, 6, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, X.; Liu, J.; Zheng, Z.; Liu, Y.; Gao, W.; Xiao, J.; Liu, Y.; Li, Y.; Tang, M.; et al. Olfactory ensheathing cells in chronic ischemic stroke: A phase 2, double-blind, randomized, controlled trial. J. Neurorestoratol. 2020, 8, 182–193. [Google Scholar] [CrossRef]
- Chung, J.-W.; Chang, W.H.; Bang, O.Y.; Moon, G.J.; Kim, S.J.; Kim, S.-K.; Lee, J.S.; Sohn, S.-I.; Kim, Y.-H. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology 2021, 96, e1012–e1023. [Google Scholar] [CrossRef]
- Bhatia, V.; Gupta, V.; Khurana, D.; Sharma, R.; Khandelwal, N. Randomized Assessment of the Safety and Efficacy of Intra-Arterial Infusion of Autologous Stem Cells in Subacute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2018, 39, 899–904. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, J.M.; Moon, G.J.; Lee, P.H.; Ahn, Y.H.; Bang, O.Y. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010, 28, 1099–1106. [Google Scholar] [CrossRef]
- Chen, D.C.; Lin, S.Z.; Fan, J.R.; Lin, C.H.; Lee, W.; Lin, C.C.; Liu, Y.J.; Tsai, C.H.; Chen, J.C.; Cho, D.Y.; et al. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: A randomized phase II study. Cell Transplant. 2014, 23, 1599–1612. [Google Scholar] [CrossRef]
- Prasad, K.; Sharma, A.; Garg, A.; Mohanty, S.; Bhatnagar, S.; Johri, S.; Singh, K.K.; Nair, V.; Sarkar, R.S.; Gorthi, S.P.; et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014, 45, 3618–3624. [Google Scholar] [CrossRef]
- Hess, D.C.; Wechsler, L.R.; Clark, W.M.; Savitz, S.I.; Ford, G.A.; Chiu, D.; Yavagal, D.R.; Uchino, K.; Liebeskind, D.S.; Auchus, A.P.; et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017, 16, 360–368. [Google Scholar] [CrossRef]
- Savitz, S.I.; Yavagal, D.; Rappard, G.; Likosky, W.; Rutledge, N.; Graffagnino, C.; Alderazi, Y.; Elder, J.A.; Chen, P.R.; Budzik, R.F., Jr.; et al. A Phase 2 Randomized, Sham-Controlled Trial of Internal Carotid Artery Infusion of Autologous Bone Marrow-Derived ALD-401 Cells in Patients with Recent Stable Ischemic Stroke (RECOVER-Stroke). Circulation 2019, 139, 192–205. [Google Scholar]
- Jaillard, A.; Hommel, M.; Moisan, A.; Zeffiro, T.A.; Favre-Wiki, I.M.; Barbieux-Guillot, M.; Vadot, W.; Marcel, S.; Lamalle, L.; Grand, S.; et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: A Randomized Clinical Trial. Transl. Stroke Res. 2020, 11, 910–923. [Google Scholar] [PubMed]
- Lee, J.; Chang, W.H.; Chung, J.W.; Kim, S.J.; Kim, S.K.; Lee, J.S.; Sohn, S.I.; Kim, Y.H.; Bang, O.Y. Efficacy of Intravenous Mesenchymal Stem Cells for Motor Recovery After Ischemic Stroke: A Neuroimaging Study. Stroke 2022, 53, 20–28. [Google Scholar] [PubMed]
- Moniche, F.; Cabezas-Rodriguez, J.A.; Valverde, R.; Escudero-Martinez, I.; Lebrato-Hernandez, L.; Pardo-Galiana, B.; Ainz, L.; Medina-Rodriguez, M.; de la Torre, J.; Escamilla-Gomez, V.; et al. Safety and efficacy of intra-arterial bone marrow mononuclear cell transplantation in patients with acute ischaemic stroke in Spain (IBIS trial): A phase 2, randomised, open-label, standard-of-care controlled, multicentre trial. Lancet Neurol. 2023, 22, 137–146. [Google Scholar] [PubMed]
- Laskowitz, D.T.; Troy, J.; Poehlein, E.; Bennett, E.R.; Shpall, E.J.; Wingard, J.R.; Freed, B.; Belagaje, S.R.; Khanna, A.; Jones, W.; et al. A Randomized, Placebo-Controlled, Phase II Trial of Intravenous Allogeneic Non-HLA Matched, Unrelated Donor, Cord Blood Infusion for Ischemic Stroke. Stem Cells Transl. Med. 2024, 13, 125–136. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar]
- Trounson, A.; Thakar, R.G.; Lomax, G.; Gibbons, D. Clinical trials for stem cell therapies. BMC Med. 2011, 9, 52. [Google Scholar] [CrossRef]
- eClinicalMedicine. The rising global burden of stroke. eClinicalMedicine 2023, 59, 102028. [Google Scholar] [CrossRef]
- Pu, L.; Wang, L.; Zhang, R.; Zhao, T.; Jiang, Y.; Han, L. Projected Global Trends in Ischemic Stroke Incidence, Deaths and Disability-Adjusted Life Years From 2020 to 2030. Stroke 2023, 54, 1330–1339. [Google Scholar] [CrossRef]
- Marín-Medina, D.S.; Arenas-Vargas, P.A.; Arias-Botero, J.C.; Gómez-Vásquez, M.; Jaramillo-López, M.F.; Gaspar-Toro, J.M. New approaches to recovery after stroke. Neurol. Sci. 2024, 45, 55–63. [Google Scholar]
- AAderinto, N.; Olatunji, G.; Kokori, E.; Babalola, A.E.; Yusuf, I.A.; Apampa, O.O.; Ukoaka, B.M.; Aboje, J.E.; Adefusi, T.; Moradeyo, A.; et al. Stem cell therapies in stroke rehabilitation: A narrative review of current strategies and future prospects. Egypt J. Neurol. Psychiatr. Neurosurg. 2024, 60, 79. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, N.; Hong, H.; Qi, J.; Zhang, S.; Wang, J. Mesenchymal Stem Cells: Therapeutic Mechanisms for Stroke. Int. J. Mol. Sci. 2022, 23, 2550. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shi, L.; Hu, B.; Hong, Y.; Zhang, H.; Li, X.; Zhang, Y. Mesenchymal Stem Cell-Based Therapy for Stroke: Current Understanding and Challenges. Front. Cell. Neurosci. 2021, 15, 628940. [Google Scholar] [CrossRef]
- Kaneko, N.; Kako, E.; Sawamoto, K. Prospects and Limitations of Using Endogenous Neural Stem Cells for Brain Regeneration. Genes 2011, 2, 107. [Google Scholar] [CrossRef]
- Scheiner, Z.S.; Talib, S.; Feigal, E.G. The Potential for Immunogenicity of Autologous Induced Pluripotent Stem Cell-derived Therapies. J. Biol. Chem. 2013, 289, 4571–4577. [Google Scholar] [CrossRef]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O.; et al. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients with Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Ikonomou, L.; Cuende, N.; Forte, M.; Grilley, B.J.; Levine, A.D.; Munsie, M.; Rasko, J.E.; Turner, L.; Bidkhori, H.R.; Ciccocioppo, R.; et al. International Society for Cell & Gene Therapy Position Paper: Key considerations to support evidence-based cell and gene therapies and oppose marketing of unproven products. Cytotherapy 2023, 25, 920–929. [Google Scholar]
- Renesme, L.; Pierro, M.; Cobey, K.D.; Mital, R.; Nangle, K.; Shorr, R.; Lalu, M.M.; Thébaud, B. Definition and Characteristics of Mesenchymal Stromal Cells in Preclinical and Clinical Studies: A Scoping Review. Stem Cells Transl. Med. 2022, 11, 44–54. [Google Scholar]
- Kawabori, M.; Shichinohe, H.; Kuroda, S.; Houkin, K. Clinical Trials of Stem Cell Therapy for Cerebral Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 7380. [Google Scholar] [CrossRef]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 2016, 47, 1817–1824. [Google Scholar]
- Donega, V.; van Velthoven, C.T.J.; Nijboer, C.H.; van Bel, F.; Kas, M.J.H.; Kavelaars, A.; Heijnen, C.J. Intranasal mesenchymal stem cell treatment for neonatal brain damage: Long-term cognitive and sensorimotor improvement. PLoS ONE 2013, 8, e51253. [Google Scholar]
- Nagpal, A.; Choy, F.C.; Howell, S.; Hillier, S.; Chan, F.; Hamilton-Bruce, M.A.; Koblar, S.A. Safety and effectiveness of stem cell therapies in early-phase clinical trials in stroke: A systematic review and meta-analysis. Stem Cell Res. Ther. 2017, 8, 191. [Google Scholar] [PubMed]
- Ouyang, Q.; Li, F.; Xie, Y.; Han, J.; Zhang, Z.; Feng, Z.; Su, D.; Zou, X.; Cai, Y.; Zou, Y.; et al. Meta-Analysis of the Safety and Efficacy of Stem Cell Therapies for Ischemic Stroke in Preclinical and Clinical Studies. Stem Cells Dev. 2019, 28, 497–514. [Google Scholar]
- Kumar, A.; Rawat, D.; Prasad, K. Stem Cell Therapy in Ischemic Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Indian Acad. Neurol. 2021, 24, 164–172. [Google Scholar] [PubMed]



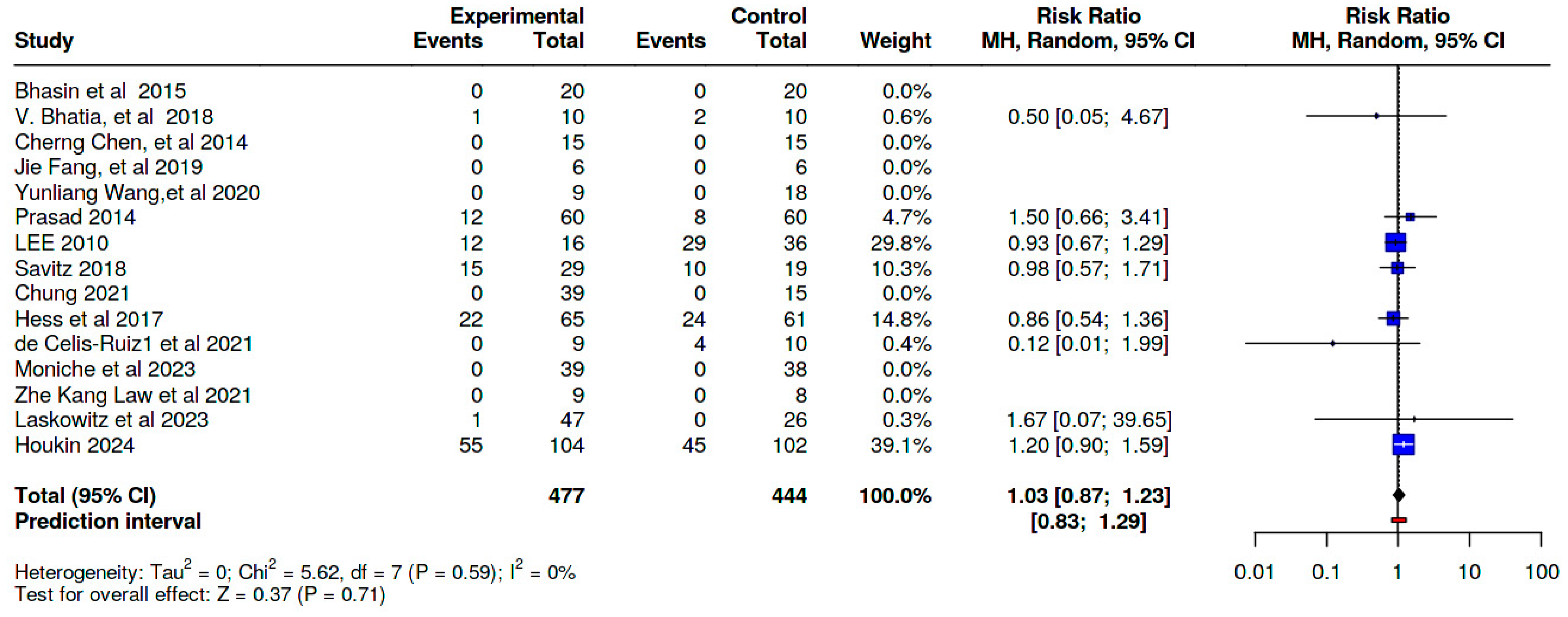

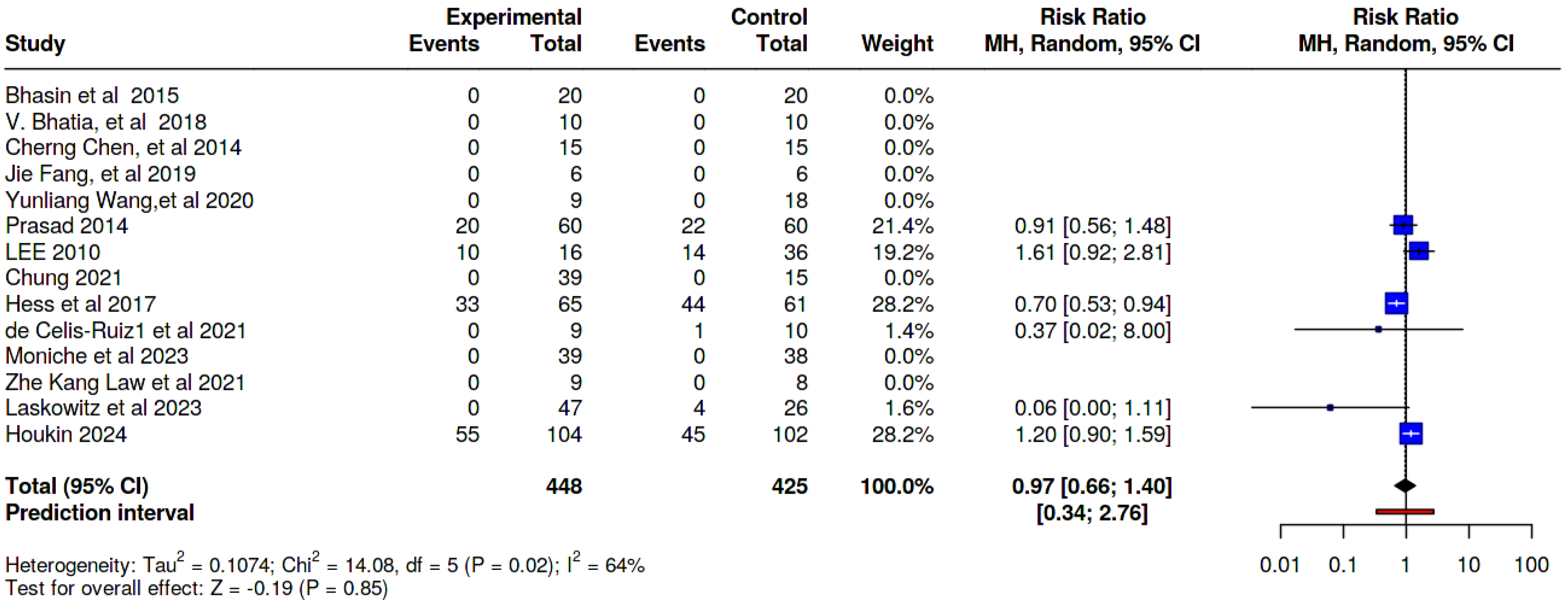
| Study (Author) | Route of Administration | Serious Adverse Effects, N (%) | Immediate Adverse Effects, N (%) | Delayed Adverse Effects, N (%) | |||
|---|---|---|---|---|---|---|---|
| SCT Group | Control Group | SCT Group | Control Group | SCT Group | Control Group | ||
| Bang et al. [13] | Intravenous | 0% | N/A | 0% | N/A | 1 (20%) (Cellulitis = 1) | N/A |
| Bhasin et al. [25] | Intravenous | 0% | 0% | 0% | 0% | 0% | 0% |
| Lee et al. 2022 [35] | Intravenous | N/A | N/A | N/A | N/A | N/A | N/A |
| Jaillard et al. [34] | Intravenous | 16 (Depression = 2, Urinary tract infection = 3, Humeral fracture = 1, Epileptic seizures = 6, Deep lower limb venous thrombosis = 1, Pneumonia = 2, Rotator cuff tear = 1), | 23 (Recurrent Ischemic stroke = 2, TIA = 1, Urinary tract infection = 2, Crytpogenic fever = 1, Algodystrophia = 2,Humeral fracture = 2, Foot skin infection = 1, Epileptic seizures = 5, Pneumonia = 3, Gastrostomy = 1, Ankle sprain = 1, Atrial flutter = 1, Kidney pain = 1) | 0% | 0% | 16 (Depression = 2, Urinary tract infection = 3, Humeral fracture = 1, Epileptic seizures = 6, Deep lower limb venous thrombosis = 1, Pneumonia = 2, Rotator cuff tear = 1) | 23 (Recurrent ischemic stroke = 2, TIA = 1, Urinary tract infection = 2, Crytpogenic fever = 1, Algodystrophia = 2, Humeral fracture = 2, Foot skin infection = 1, Epileptic seizures = 5, Pneumonia = 3, Gastrostomy = 1, Ankle sprain = 1, Atrial flutter = 1, Kidney pain = 1) |
| Bhatia et al. [28] | Intra-arterial | 2 (20%) (Death = 1, New Infarct = 1) | 2 (20%) (Death = 2) | 0% | 0% | 0% | 0% |
| Chen et al. [30] | Subcutaneously | 0% | 0% | 0% | 0% | 0% | 0% |
| Fang et al. [23] | Intravenous | 0% | 0% | 0% | 0% | 0% | 0% |
| Wang et al. [26] | Olfactory sub-mucosa Injection | 0% | 0% | 0% | 0% | 0% | 0% |
| Prasad et al. [31] | Intravenous | 12 (20%) (Pneumonitis = 1, Fracture in lower limb = 2, Death = 8, Bilateral lower limb ischemia = 1) | 8 (13.3%) (Hypertension = 1, Fracture in lower limb = 1, Septicaemia with shock = 1, Death = 5) | 0% | 0% | 61 (33%) (Rise in urea > 2.77 mmol/l = 2, Hematological = 10, Hepatic = 22, Serious deterioration in sensorium = 1, Pneumonitis = 1, Fever = 1, Hyperglycaemia = 1, Bilateral lower limb ischemia = 1, Frozen shoulder = 2, Traumatic injury = 1, Fracture in lower limb = 2, Death = 8, CNS = 6, GI = 3) | 60 (36%) (Rise in urea > 2.77 mmol/l = 1, Hematological = 20, Hepatic = 13, Hypotension = 1, Edoema = 1, Hyperglycaemia = 3, Hypertension = 1, Septicaemia with shock = 1, Traumatic injury = 1, Fracture in lower limb = 1, Death = 5, CNS = 7, GI = 4, Increase in standardized uptake value of breast = 1, Uterine lesion on PET scan = 1) |
| Lee et al. 2010 [29] | Intravenous | 12 (75%) (Death = 4, Small mass at lateral malleolus of the left ankle = 1, Seizure = 3, Recurrent vascular episode = 4) | 29 (80.5%) (Death = 21, Seizure = 5, Recurrent vascular episode = 3) | 8 (50%) (Recurrent stroke = 2, Myocardial infarction or angina = 1, Peripheral artery occlusive disease = 1, Infection = 3, Liver enzyme elevation = 1) | 15 (36.1%) (Recurrent stroke = 1, Myocardial infarction or angina = 2, Infection = 9, Acute renal failure = 1, Liver enzyme elevation = 2) | 10 (62.5%) (Benign mass = 1, Seizure = 3, Neuropyschological illness = 6) | 14 (38.9%) (Systemic cancer = 1, Benign mass = 1, Seizure = 5, Neuropyschological illness = 7) |
| Savitz et al. [33] | Intravenous | 16 (51.76%) (Convulsion = 2, Cerebral hemorrhage = 1, Deep vein thrombosis = 2, Hypertension = 2, Hypotension = 1, Angina = 1, Sick sinus syndrome = 1, Pulmonary embolism = 2, Urinary tract infection = 1, Chest pain = 1, Anxiety = 1, Craniectomy = 1) | 10 (52.6%) (Cerebrovascular accident = 1, Hemorrhagic transformation = 1, Syncope = 1, Tachycardia = 1, Ventricular tachycardia = 1, Dyspnea = 1, Pneumonia = 1, Thrombocytopenia = 1, Retinal artery embolism = 1, Astrocytoma = 1) | 0% | 0% | N/A | N/A |
| Chung et al. [27] | Intravenous | 0% | 0% | 0% | 0% | 0% | 0% |
| Hess et al. [32] | Intravenous | 34% | 39% | 99% | 97% | 33 (21.5%) (Life-threatening adverse events or death = 8, Infections = 25) | 44 (72.1%) (Life-threatening adverse events or death = 15, Infections = 29) |
| Celis-Ruiz et al. [21] | intravenous | 0% | 4 (44.4%) (Deaths = 1, other = 3) | 0% | 0% | 0% | 1 (10%) (Death = 1) |
| Moniche et al. [36] | Intra-arterial | 0% | 0% | 0% | 0% | 0% | 0% |
| Law et al. [24] | Intravenous | 0% | 0% | 0% | 0% | 0% | 0% |
| Laskowitz et al. [37] | Intravenous | 1 (2.1%) (1 Thromboembolic Event) | 0.00% | 0% | 0% | 0% | 4 (15.4%) (Hypertension = 3, Seizure = 1) |
| Houkin et al. [22] | Intravenous | 55 (52.8%) (Death = 7, infections = 48) | 45 (44.1%) (Death = 6, infections = 38, SAE occurring within 7 days after treatment related to the investigational product = 1) | N/A | N/A | 55 (52.8%) (Death = 7, Infections = 48) | 45 (44.1%) (Death = 6, Infections = 38, SAEs occurring within 7 days after treatment related to the investigational product = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrasheed, A.S.; Aljahdali, T.A.; Alghafli, I.A.; Alghafli, G.A.; Almuslim, M.F.; AlMohish, N.M.; Alabdali, M.M. Safety and Efficacy of Stem Cell Therapy in Ischemic Stroke: A Comprehensive Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2118. https://doi.org/10.3390/jcm14062118
Alrasheed AS, Aljahdali TA, Alghafli IA, Alghafli GA, Almuslim MF, AlMohish NM, Alabdali MM. Safety and Efficacy of Stem Cell Therapy in Ischemic Stroke: A Comprehensive Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(6):2118. https://doi.org/10.3390/jcm14062118
Chicago/Turabian StyleAlrasheed, Abdulrahim Saleh, Tala Abdullah Aljahdali, Israa Aqeel Alghafli, Ghadeer Aqeel Alghafli, Majd Fouad Almuslim, Noor Mohammad AlMohish, and Majed Mohammad Alabdali. 2025. "Safety and Efficacy of Stem Cell Therapy in Ischemic Stroke: A Comprehensive Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 6: 2118. https://doi.org/10.3390/jcm14062118
APA StyleAlrasheed, A. S., Aljahdali, T. A., Alghafli, I. A., Alghafli, G. A., Almuslim, M. F., AlMohish, N. M., & Alabdali, M. M. (2025). Safety and Efficacy of Stem Cell Therapy in Ischemic Stroke: A Comprehensive Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(6), 2118. https://doi.org/10.3390/jcm14062118






