Comparison of Cryoballoon and Ablation Index-Guided Radiofrequency Ablation in Paroxysmal Atrial Fibrillation
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Procedural Workflow
2.3. Follow-Up Period
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAD | antiarrhythmic drug |
| AF | atrial fibrillation |
| AI-RF | ablation index-guided radiofrequency ablation |
| CB | cryoballoon |
| CT | computed tomography |
| CS | coronary sinus |
| ECG | electrocardiogram |
| HPSD | high-power short-duration radiofrequency ablation |
| ICE | intracardiac echocardiography |
| IQR | interquartile range |
| LPLD | low-power long-duration radiofrequency ablation |
| PV | pulmonary vein |
| PVI | pulmonary vein isolation |
| pAF | paroxysmal atrial fibrillation |
| SD | standard deviation |
| TIA | transient ischemic attack |
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar]
- Hindricks, G.; Potpara, T.; Dagres, N.; Bax, J.J.; Boriani, G.; Dan, G.A.; Dilaveris, P.E.; Arbelo, E.; Blomström-Lundqvist, C.; Castella, M.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Brugada, J.; Fürnkranz, A.; Metzner, A.; Ouyang, F.; Chun, J.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar]
- Ioannou, A.; Papageorgiou, N.; Lim, W.Y.; Wongwarawipat, T.; Hunter, R.J.; Dhillon, G.; Schilling, R.J.; Creta, A.; El Haddad, M.; Duytschaever, M.; et al. Efficacy and safety of ablation index-guided catheter ablation for atrial fibrillation: An updated meta-analysis. Europace 2020, 22, 1659–1671. [Google Scholar]
- Van Belle, Y.; Janse, P.; Rivero-Ayerza, M.J.; Thornton, A.S.; Jessurun, E.R.; Theuns, D.; Jordaens, L. Pulmonary vein isolation using an occluding cryoballoon for circumferential ablation: Feasibility, complications, and short-term outcome. Eur. Heart J. 2007, 28, 2231–2237. [Google Scholar]
- Luik, A.; Radzewitz, A.; Kieser, M.; Walter, M.; Bramlage, P.; Hörmann, P.; Schmidt, K.; Horn, N.; Brinkmeier-Theofanopoulou, M.; Kunzmann, K.; et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation. Circulation 2015, 132, 1311–1319. [Google Scholar]
- Straube, F.; Dorwarth, U.; Vogt, J.; Kuniss, M.; Kuck, K.H.; Tebbenjohanns, J.; Alberola, A.G.; Chun, K.R.J.; Souza, J.J.; Ouarrak, T.; et al. Differences of two cryoballoon generations: Insights from the prospective multicentre, multinational FREEZE Cohort Substudy. Europace 2014, 16, 1434–1442. [Google Scholar]
- Kuck, K.H.; Brugada, J.; Schlüter, M.; Braegelmann, K.M.; Kueffer, F.J.; Chun, K.R.J.; Albenque, J.-P.; Tondo, C.; Calkins, H.; FIRE AND ICE Trial Investigators. The FIRE AND ICE trial: What we know, what we can still learn, and what we need to address in the future. J. Am. Heart Assoc. 2018, 7, e010777. [Google Scholar] [PubMed]
- Lin, H.; Chen, Y.H.; Hou, J.W.; Lu, Z.Y.; Xiang, Y.; Li, Y.G. Role of contact force-guided radiofrequency catheter ablation for treatment of atrial fibrillation: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2017, 28, 994–1005. [Google Scholar] [PubMed]
- Ikeda, A.; Nakagawa, H.; Lambert, H.; Shah, D.C.; Fonck, E.; Yulzari, A.; Sharma, T.; Pitha, J.V.; Lazzara, R.; Jackman, W.M. Relationship between catheter contact force and radiofrequency lesion size and incidence of steam pop in the beating canine heart: Electrogram amplitude, impedance, and electrode temperature are poor predictors of electrode-tissue contact force and lesion size. Circ. Arrhythmia Electrophysiol. 2014, 7, 1174–1180. [Google Scholar]
- El Haddad, M.; Taghji, P.; Phlips, T.; Wolf, M.; Demolder, A.; Choudhury, R.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Nakagawa, H.; et al. Determinants of Acute and Late Pulmonary Vein Reconnection in Contact Force-Guided Pulmonary Vein Isolation: Identifying the Weakest Link in the Ablation Chain. Circ. Arrhythmia Electrophysiol. 2017, 10, e004867. [Google Scholar] [CrossRef]
- Taghji, P.; Haddad, M.; El Phlips, T.; Wolf, M.; Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Nakagawa, H.; Duytschaever, M. Evaluation of a Strategy Aiming to Enclose the Pulmonary Veins with Contiguous and Optimized Radiofrequency Lesions in Paroxysmal Atrial Fibrillation A Pilot Study. JACC Clin. Electrophysiol. 2018, 4, 99–108. [Google Scholar]
- Phlips, T.; Taghji, P.; Haddad, M.; El Wolf, M.; Bastien Knecht, S.; Vandekerckhove, Y.; Tavernier, R.; Duytschaever, M. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: The role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. EP Eur. 2018, 20, f419–f427. [Google Scholar] [CrossRef]
- Kumar, S.; Khatri, M.; Kumar, S.; Partab, F.N.U.; Kumar, F.N.U.M.; Neha, F.N.U.; Suman, F.N.U.; Rai, L.; Sangam, F.N.U.; Kumari, S. Comparative efficacy and safety profile of high-power short duration with low power long duration radiofrequency ablation in atrial fibrillation: An updated systematic review and meta-analysis. J. Innov. Card. Rhythm Manag. 2023, 15, 5514–5527. [Google Scholar] [CrossRef]
- Kotadia, I.D.; Williams, S.E.; O’Neill, M. High-power, short-duration radiofrequency ablation for the treatment of AF. Arrhythm Electrophysiol. Rev. 2019, 8, 265–272. [Google Scholar]
- La Fazia, V.M.; Pierucci, N.; Schiavone, M.; Compagnucci, P.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Horton, R.; Al-Ahmad, A.; Di Biase, L.; et al. Comparative effects of different power settings for achieving transmural isolation of the left atrial posterior wall with radiofrequency energy. Europace 2024, 26, euae265. [Google Scholar] [PubMed]
- Theis, C.; Kaiser, B.; Kaesemann, P.; Hui, F.; Pirozzolo, G.; Bekeredjian, R.; Huber, C. Pulmonary vein isolation using cryoballoon ablation versus RF ablation using ablation index following the CLOSE protocol: A prospective randomized trial. J. Cardiovasc. Electrophysiol. 2022, 33, 866–873. [Google Scholar]
- Zhang, W.; Wang, Y.; Wang, H.; Shao, Y.; Dong, Q.; Li, S.; Gu, Y. Left Atrial Low Voltage Areas Predicts Recurrence of Atrial Fibrillation after Catheter Ablation: A Meta-Analysis. Hear. Surg. Forum 2024, 27, E58–E67. [Google Scholar]
- Karakasis, P.; Theofilis, P.; Vlachakis, P.K.; Korantzopoulos, P.; Patoulias, D.; Antoniadis, A.P.; Fragakis, N. Atrial Fibrosis in Atrial Fibrillation: Mechanistic Insights, Diagnostic Challenges, and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 26, 209. [Google Scholar] [CrossRef]
- Akerström, F.; Bastani, H.; Insulander, P.; Schwieler, J.; Arias, M.A.; Jensen-Urstad, M. Comparison of regular atrial tachycardia incidence after circumferential radiofrequency versus cryoballoon pulmonary vein isolation in real-life practice. J. Cardiovasc. Electrophysiol. 2014, 25, 948–952. [Google Scholar] [PubMed]
- Liu, X.H.; Chen, C.F.; Gao, X.F.; Xu, Y.Z. Safety and Efficacy of Different Catheter Ablations for Atrial Fibrillation: A Systematic Review and Meta-Analysis. Pacing Clin. Electrophysiol. 2016, 39, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Mekhael, M.; Feng, H.; Younes, H.; Chouman, N.; Assaf, A.; Lim, C.; Huang, C.; Donnellan, E.; Rao, S.; Marrouche, N.; et al. Lesion monitoring parameters as predictors of atrial arrhythmia recurrence after catheter ablation in persistent AF: A DECAAF II sub-analysis. J. Cardiovasc. Electrophysiol. 2024, 35, 2414–2422. [Google Scholar]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2024, 26, euae043. [Google Scholar]
- Fitzpatrick, N.; Mittal, A.; Galvin, J.; Jauvert, G.; Keaney, J.; Keelan, E.; O’brien, J.; Széplaki, G. The impact of steerable sheath visualization during catheter ablation for atrial fibrillation. Europace 2023, 25, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Janosi, K.; Debreceni, D.; Janosa, B.; Bocz, B.; Simor, T.; Kupo, P. Visualizable vs. standard, non-visualizable steerable sheath for pulmonary vein isolation procedures: Randomized, single-centre trial. Front. Cardiovasc. Med. 2022, 9, 1033755. [Google Scholar] [CrossRef]
- Debreceni, D.; Janosi, K.; Bocz, B.; Turcsan, M.; Lukacs, R.; Simor, T.; Antolič, B.; Vamos, M.; Komocsi, A.; Kupo, P. Zero fluoroscopy catheter ablation for atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1178783. [Google Scholar]
- Torma, D.; Janosi, K.; Debreceni, D.; Bocz, B.; Keseru, M.; Simor, T.; Kupo, P. Initial experience with zero-fluoroscopy pulmonary vein isolation in patients with atrial fibrillation: Single-center observational trial. Sci. Rep. 2024, 14, 16332. [Google Scholar] [CrossRef]
- Ahn, J.; Shin, D.G.; Han, S.J.; Lim, H.E. Safety and efficacy of intracardiac echocardiography–guided zero-fluoroscopic cryoballoon ablation for atrial fibrillation: A prospective randomized controlled trial. Europace 2023, 25, euad086. [Google Scholar] [CrossRef]
- Rubesch-Kütemeyer, V.; Fischbach, T.; Guckel, D.; Körber, B.; Horstkotte, D.; Gutleben, K.J.; Nölker, G. Long-term development of radiation exposure, fluoroscopy time and contrast media use in daily routine in cryoballoon ablations after implementation of intracardiac echocardiography and other radioprotective measures: Experiences from a large single-centre cohort. J. Interv. Card. Electrophysiol. 2019, 58, 169–175. [Google Scholar] [CrossRef]

| Group AI-RF (n = 103) | Group CB (n = 51) | p-Value | |
|---|---|---|---|
| Male, n (%) | 67 (65.0) | 32 (62.7) | 0.77 |
| Age, y | 62.2 (49.6; 70.0) | 61.1 (51.6; 69.0) | 0.67 |
| Hypertension, n (%) | 77 (74.8) | 41 (80.4) | 0.35 |
| Diabetes mellitus, n (%) | 18 (17.4) | 9 (16.0) | 0.92 |
| Prior stroke/TIA, n (%) | 6 (5.8) | 5 (11.8) | 0.12 |
| Heart failure, n (%) | 12 (11.7) | 2 (3.9) | 0.11 |
| Coronary artery disease, n (%) | 17 (16.5) | 5 (7.7) | 0.26 |
| Chronic kidney disease, n (%) | 15 (15.0) | 4 (7.8) | 0.21 |
| No antiarrhythmic drug during the blanking period, n (%) | 44 (42.7) | 25 (49.0) | 0.45 |
| Propafenone during the blanking period, n (%) | 34 (33.0) | 14 (27.5) | 0.12 |
| Sotalol during the blanking period, n (%) | 3 (2.9) | 3 (5.9) | 0.29 |
| Amiodarone during the blanking period, n (%) | 19 (18.4) | 8 (15.6) | 0.86 |
| CB (n = 51) | AI-RF (n = 103) | p-Value | |
|---|---|---|---|
| Total procedure time, min | 64 (57;74.8) | 92 (76; 119) | <0.001 |
| Total fluoroscopy time, s | 559 (395; 868) | 167 (126; 224) | <0.001 |
| Total fluoroscopy dose, mGy | 21.8 (11.7; 40.1) | 7.65 (5.21; 14.5) | <0.001 |
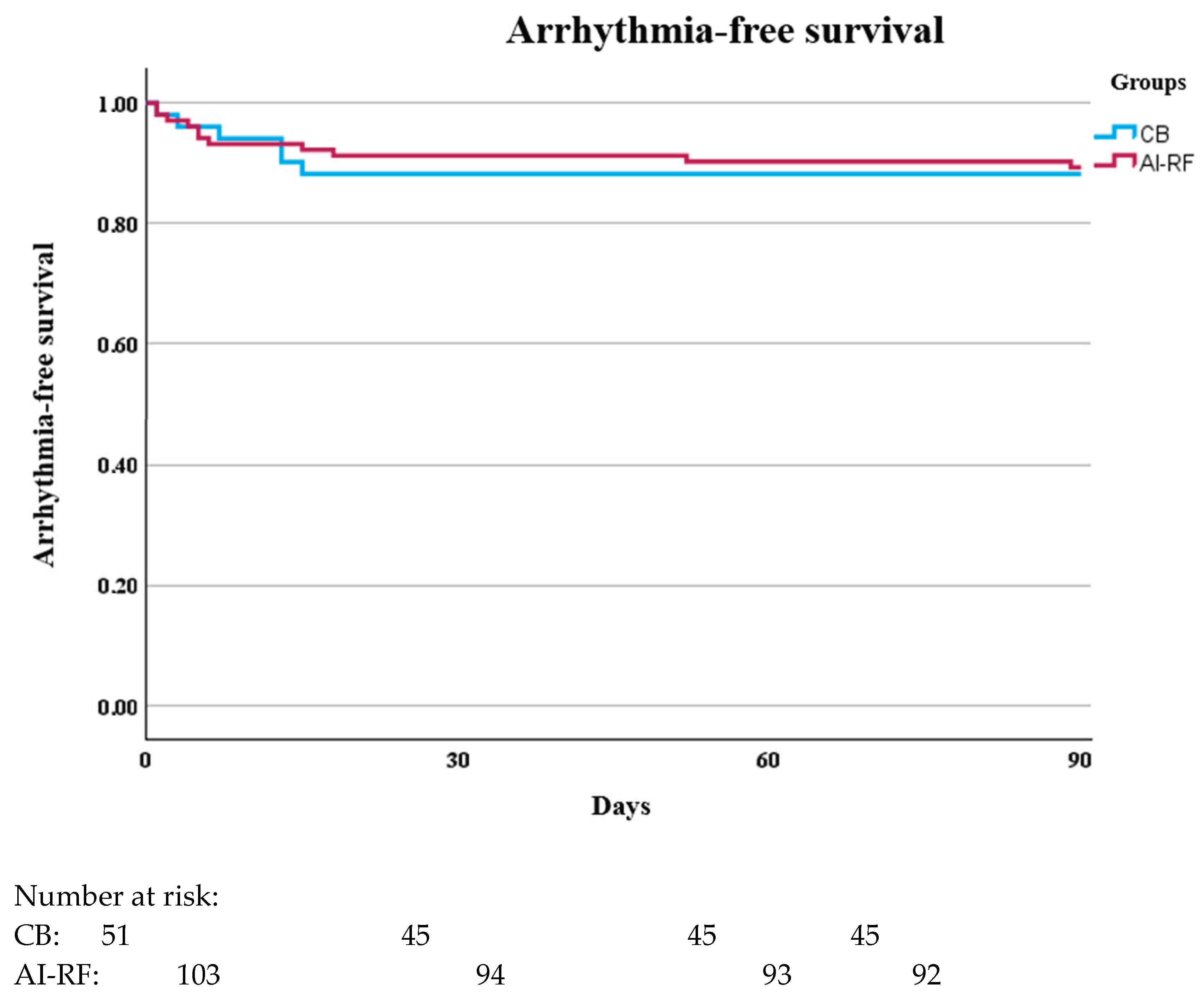
| Recurrence rate during the blanking period, % | 11.7 | 10.7 | 0.84 |
| Recurrence rate outside the blanking period, % | 21.6 | 13.4 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocz, B.; Debreceni, D.; Jánosi, K.-F.; Torma, D.; Kupo, P. Comparison of Cryoballoon and Ablation Index-Guided Radiofrequency Ablation in Paroxysmal Atrial Fibrillation. J. Clin. Med. 2025, 14, 2119. https://doi.org/10.3390/jcm14062119
Bocz B, Debreceni D, Jánosi K-F, Torma D, Kupo P. Comparison of Cryoballoon and Ablation Index-Guided Radiofrequency Ablation in Paroxysmal Atrial Fibrillation. Journal of Clinical Medicine. 2025; 14(6):2119. https://doi.org/10.3390/jcm14062119
Chicago/Turabian StyleBocz, Botond, Dorottya Debreceni, Kristof-Ferenc Jánosi, Dalma Torma, and Peter Kupo. 2025. "Comparison of Cryoballoon and Ablation Index-Guided Radiofrequency Ablation in Paroxysmal Atrial Fibrillation" Journal of Clinical Medicine 14, no. 6: 2119. https://doi.org/10.3390/jcm14062119
APA StyleBocz, B., Debreceni, D., Jánosi, K.-F., Torma, D., & Kupo, P. (2025). Comparison of Cryoballoon and Ablation Index-Guided Radiofrequency Ablation in Paroxysmal Atrial Fibrillation. Journal of Clinical Medicine, 14(6), 2119. https://doi.org/10.3390/jcm14062119






