Lumbar Muscle Fatty Infiltration and Atrophy in Patients with Low Back Pain and Degenerative Spinal Pathologies: A CT Imaging Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methodology of Scientific Research
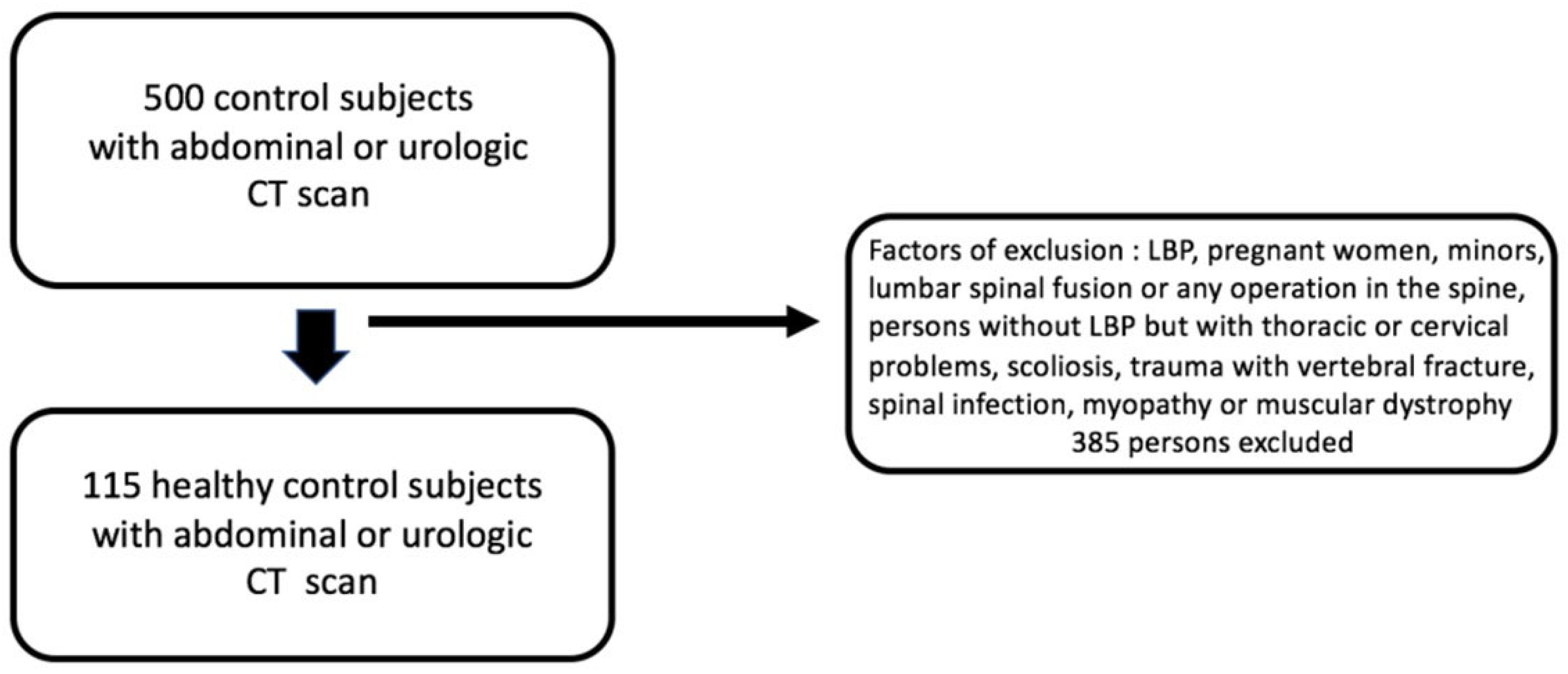
2.1.1. Selection of Patients
2.1.2. Statistical Analysis
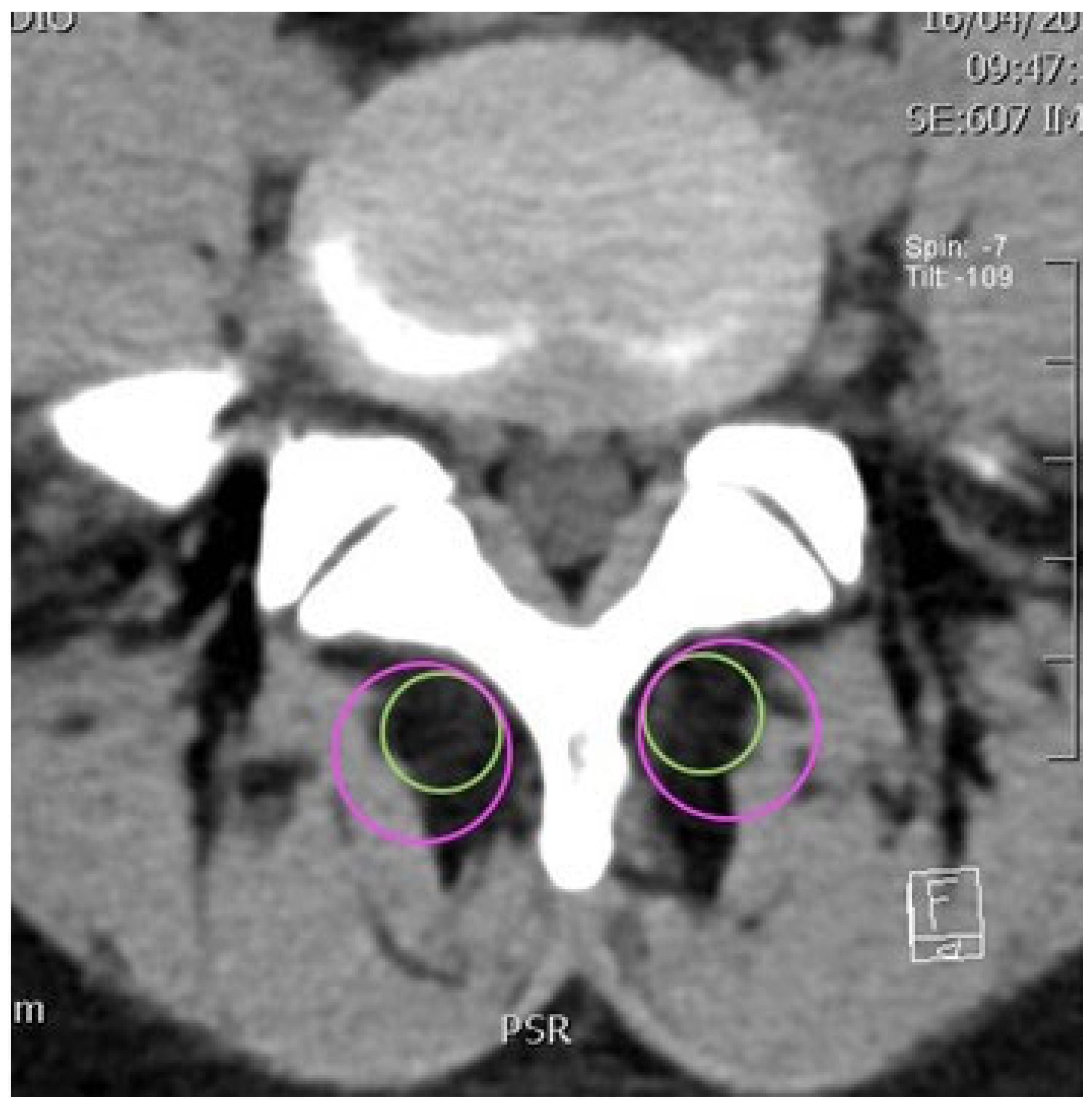
2.2. CT Imaging Research Methodology
3. Results
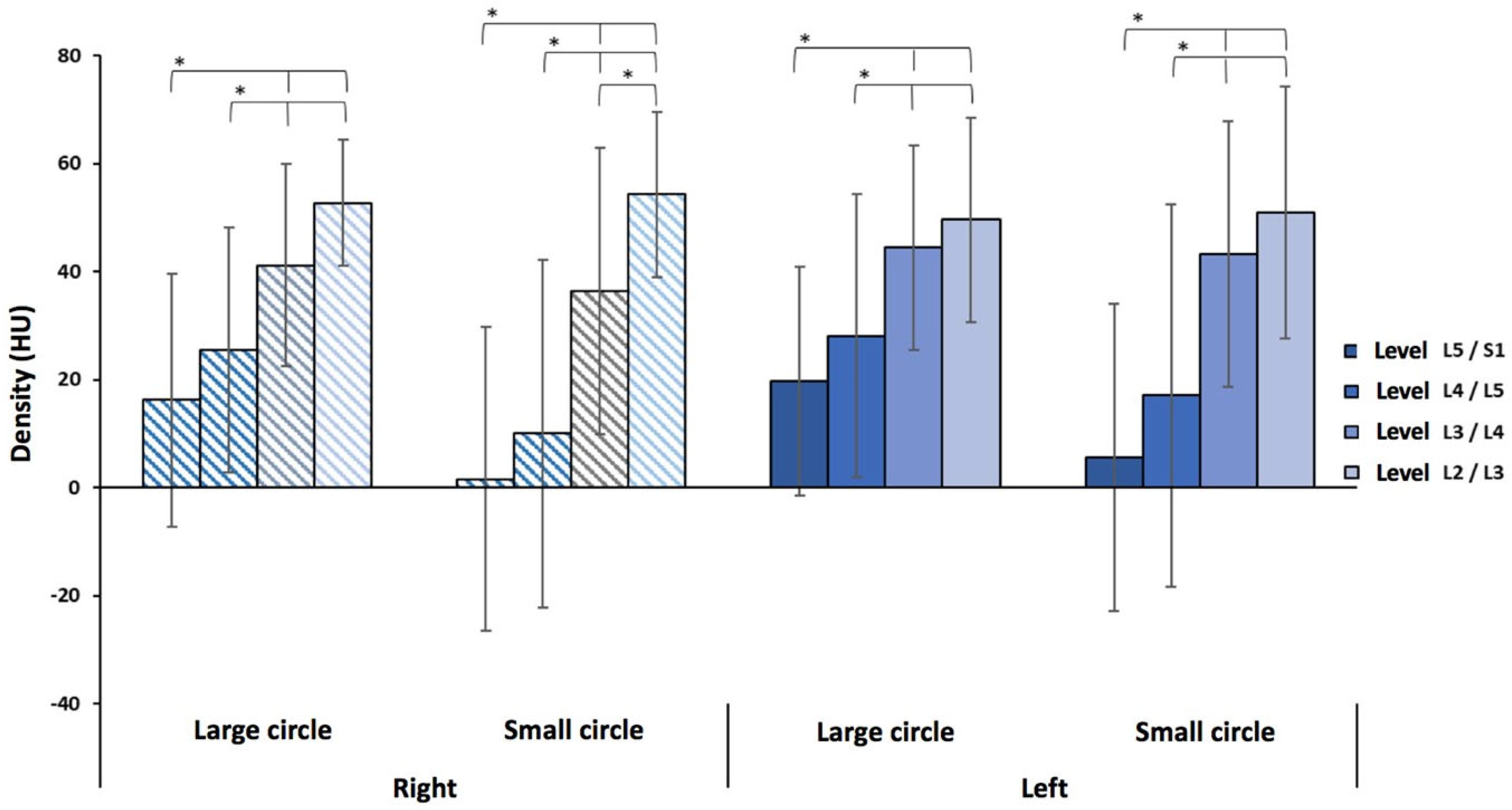
3.1. Low Back Pain Patients
3.2. Healthy Subjects
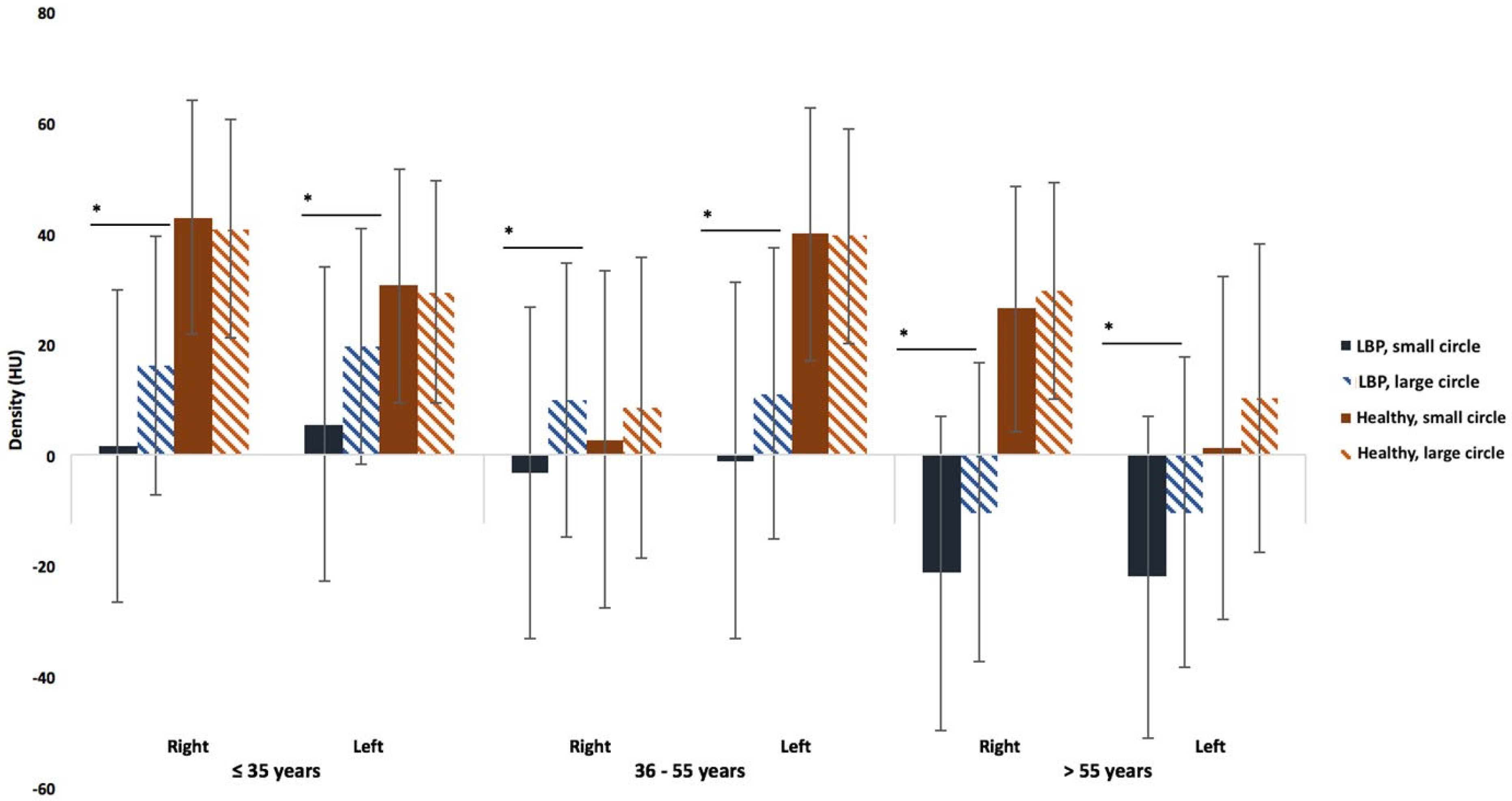
3.3. Comparisons Between the Healthy Subjects and the LBP Patients
3.4. Reliability of Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar]
- Steffens, D.; Maher, C.G.; Pereira, L.S.; Stevens, M.L.; Oliveira, V.C.; Chapple, M.; Teixeira-Salmela, L.F.; Hancock, M.J. Prevention of Low Back Pain: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2016, 176, 199–208. [Google Scholar] [PubMed]
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The Epidemiology of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar]
- Mengiardi, B.; Schmid, M.R.; Boos, N.; Pfirrmann, C.W.; Brunner, F.; Elfering, A.; Hodler, J. Fat content of lumbar paraspinal muscles in patients with chronic low back pain and in asymptomatic volunteers: Quantification with MR spectroscopy. Radiology 2006, 240, 786–792. [Google Scholar]
- Ferreira, M.L.; De Luca, K.; Haile, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Ferreira, P.H.; Blyth, F.M.; Buchbinder, R.; et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar]
- Deyo, R.A.; Weinstein, J.N. Low back pain. N. Engl. J. Med. 2001, 344, 363–370. [Google Scholar]
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.W.; Chen, L.E.; Deyo, R.A.; Halabi, S.; Turner, J.A.; Avins, A.L.; James, K.; et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef]
- Fortin, M.; Yuan, Y.; Battié, M.C. Factors associated with paraspinal muscle asymmetry in size and composition in a general population sample of men. Phys. Ther. 2013, 93, 1540–1550. [Google Scholar]
- Kalichman, L.; Hodges, P.; Li, L.; Guermazi, A.; Hunter, D.J. Changes in paraspinal muscles and their association with low back pain and spinal degeneration: CT study. Eur. Spine J. 2010, 19, 1136–1144. [Google Scholar]
- Chang, D.G.; Healey, R.M.; Snyder, A.J.; Sayson, J.V.; Macias, B.R.; Coughlin, D.G.; Bailey, J.F.; Parazynski, S.E.; Lotz, J.C.; Hargens, A.R. Lumbar Spine Paraspinal Muscle and Intervertebral Disc Height Changes in Astronauts After Long-Duration Spaceflight on the International Space Station. Spine 2016, 41, 1917–1924. [Google Scholar] [PubMed]
- Fortin, M.; Videman, T.; Gibbons, L.E.; Battié, M.C. Paraspinal muscle morphology and composition: A 15-yr longitudinal magnetic resonance imaging study. Med. Sci. Sports Exerc. 2014, 46, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.G.T.; Boas, F.V.; Clifton, T.; Piche, M.; Yoon, W.W.; Freeman, B.J.C. Does pre-operative magnetic resonance imaging of the lumbar multifidus muscle predict clinical outcomes following lumbar spinal decompression for symptomatic spinal stenosis? Eur. Spine J. 2017, 26, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- D’hooge, R.; Cagnie, B.; Crombez, G.; Vanderstraeten, G.; Dolphens, M.; Danneels, L. Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man. Ther. 2012, 17, 584–588. [Google Scholar]
- Danneels, L.A.; Vanderstraeten, G.G.; Cambier, D.C.; Witvrouw, E.E.; Cuyper, H.J.D.; Danneels, L. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur. Spine J. 2000, 9, 266–272. [Google Scholar]
- Lee, S.H.; Park, S.W.; Kim, Y.B.; Nam, T.K.; Lee, Y.S. The fatty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. Spine J. 2017, 17, 81–87. [Google Scholar]
- Kjaer, P.; Bendix, T.; Sorensen, J.S.; Korsholm, L.; Leboeuf-Yde, C. Are mri-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007, 5, 2. [Google Scholar]
- Hebert, J.J.; Kjaer, P.; Fritz, J.M.; Walker, B.F. The relationship of lumbar multifidus muscle morphology to previous, current, and future low back pain: A 9-year population-based prospective cohort study. Spine 2014, 39, 1417–1425. [Google Scholar]
- Crawford, R.J.; Filli, L.; Elliott, J.M.; Nanz, D.; Fischer, M.A.; Marcon, M.; Ulbrich, E.J. Age- and Level-Dependence of Fatty Infiltration in Lumbar Paravertebral Muscles of Healthy Volunteers. Am. J. Neuroradiol. 2016, 37, 742–748. [Google Scholar] [CrossRef]
- Gueniche, J.; Bierry, G. Rotator cuff muscles fatty infiltration increases with age: Retrospective review of 210 patients with intact cuff on computed tomography arthrography. J. Shoulder Elb. Surg. 2019, 28, 617–624. [Google Scholar] [CrossRef]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [PubMed]
- Marcus, R.L.; Addison, O.; Kidde, J.P.; Dibble, L.E.; Lastayo, P.C. Skeletal muscle fat infiltration: Impact of age, inactivity, and exercise. J. Nutr. Health Aging 2010, 14, 362–366. [Google Scholar] [PubMed]
- Mannion, A.F.; Käser, L.; Weber, E.; Rhyner, A.; Dvorak, J.; Müntener, M. Influence of age and duration of symptoms on fibre type distribution and size of the back muscles in chronic low back pain patients. Eur. Spine J. 2000, 9, 273–281. [Google Scholar] [PubMed]
- Fortin, M.; Gibbons, L.E.; Videman, T.; Battié, M.C. Do variations in paraspinal muscle morphology and composition predict low back pain in men? Scand. J. Med. Sci. Sports 2015, 25, 880–887. [Google Scholar]
- Hides, J.A.; Williamson, E.; Richardson, C.A. Multifidus muscle recovery and function in patients with low back pain: A review. Clin. Biomech. 1994, 9, 121–128. [Google Scholar]
- MacDonald, D.; Moseley, G.L.; Hodges, P.W. The lumbar multifidus: Does it really matter? A review of its role in lumbar stability and low back pain. J. Orthop. Sports Phys. Ther. 2006, 36, 664–669. [Google Scholar]
- Goodpaster, B.H.; Kelley, D.E.; Thaete, F.L.; He, J.; Ross, R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J. Appl. Physiol. 2000, 89, 104–110. [Google Scholar]
- Niemeläinen, R.; Briand, M.-M.; Battié, M.C. Substantial asymmetry in paraspinal muscle cross-sectional area in healthy adults questions its value as a marker of low back pain and pathology. Spine 2011, 36, 2152–2157. [Google Scholar]
- Engelke, K.; Museyko, O.; Wang, L.; Laredo, J.D. Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J. Orthop. Transl. 2018, 15, 91–103. [Google Scholar]
- Huang, C.B.; Lin, D.D.; Huang, J.Q.; Hu, W. Based on CT at the third lumbar spine level, the skeletal muscle index and psoas muscle index can predict osteoporosis. BMC Musculoskelet. Disord. 2022, 23, 933. [Google Scholar]
- Fortin, M.; Macedo, L.G. Multifidus and paraspinal muscle group cross-sectional areas of patients with low back pain and control patients: A systematic review with a focus on blinding. Phys. Ther. 2013, 93, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.; Gilmore, C.; Stanton, W.; Bohlscheid, E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man. Ther. 2008, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Elliott, J.M.; Volken, T. Change in fatty infiltration of lumbar multifidus, erector spinae, and psoas muscles in asymptomatic adults of Asian or Caucasian ethnicities. Eur. Spine J. 2017, 26, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, E.; Castiglione, J.A.; Brady, S.L.; Trout, A.T. Defining Normal Ranges of Skeletal Muscle Area and Skeletal Muscle Index in Children on CT Using an Automated Deep Learning Pipeline: Implications for Sarcopenia Diagnosis. AJR Am. J. Roentgenol. 2022, 219, 326–336. [Google Scholar] [CrossRef]
- van der Werf, A.; Dekker, I.M.; Meijerink, M.R.; Wierdsma, N.J.; de van der Schueren, M.A.E.; Langius, J.A.E. Skeletal muscle analyses: Agreement between non-contrast and contrast CT scan measurements of skeletal muscle area and mean muscle attenuation. Clin. Physiol. Funct. Imaging 2018, 38, 366–372. [Google Scholar] [CrossRef]
- Ekşi, M.Ş.; Özcan-Ekşi, E.E. Fatty infiltration of the erector spinae at the upper lumbar spine could be a land-mark for low back pain. Pain Pract. 2024, 24, 278–287. [Google Scholar] [CrossRef]
- Wesselink, E.O.; Pool-Goudzwaard, A.; De Leener, B.; Law, C.S.W.; Fenyo, M.B.; Ello, G.M.; Coppieters, M.W.; Elliott, J.M.; Mackey, S.; Weber, K.A., II. Investigating the associations between lumbar paraspinal muscle health and age, BMI, sex, physical activity, and back pain using an automated computer-vision model: A UK Biobank study. Spine J. 2024, 24, 1253–1266. [Google Scholar] [CrossRef]
- Wesselink, E.O.; Pool, J.J.M.; Mollema, J.; Weber, K.A.; Elliott, J.M.; Coppieters, M.W.; Pool-Goudzwaard, A.L. Is fatty infiltration in paraspinal muscles reversible with exercise in people with low back pain? A systematic review. Eur. Spine J. 2023, 32, 787–796. [Google Scholar] [CrossRef]
- Hoppe, S.; Maurer, D.; Valenzuela, W.; Benneker, L.M.; Bigdon, S.F.; Häckel, S.; Wangler, S.; Albers, C.E. 3D analysis of fatty infiltration of the paravertebral lumbar muscles using T2 images-a new approach. Eur. Spine J. 2021, 30, 2570–2576. [Google Scholar] [CrossRef]
- Zaed, I.; Bossi, B.; Ganau, M.; Tinterri, B.; Giordano, M.; Chibbaro, S. Current state of benefits of Enhanced Recovery After Surgery (ERAS) in spinal surgeries: A systematic review of the literature. Neurochirurgie 2022, 68, 61–68. [Google Scholar] [CrossRef]


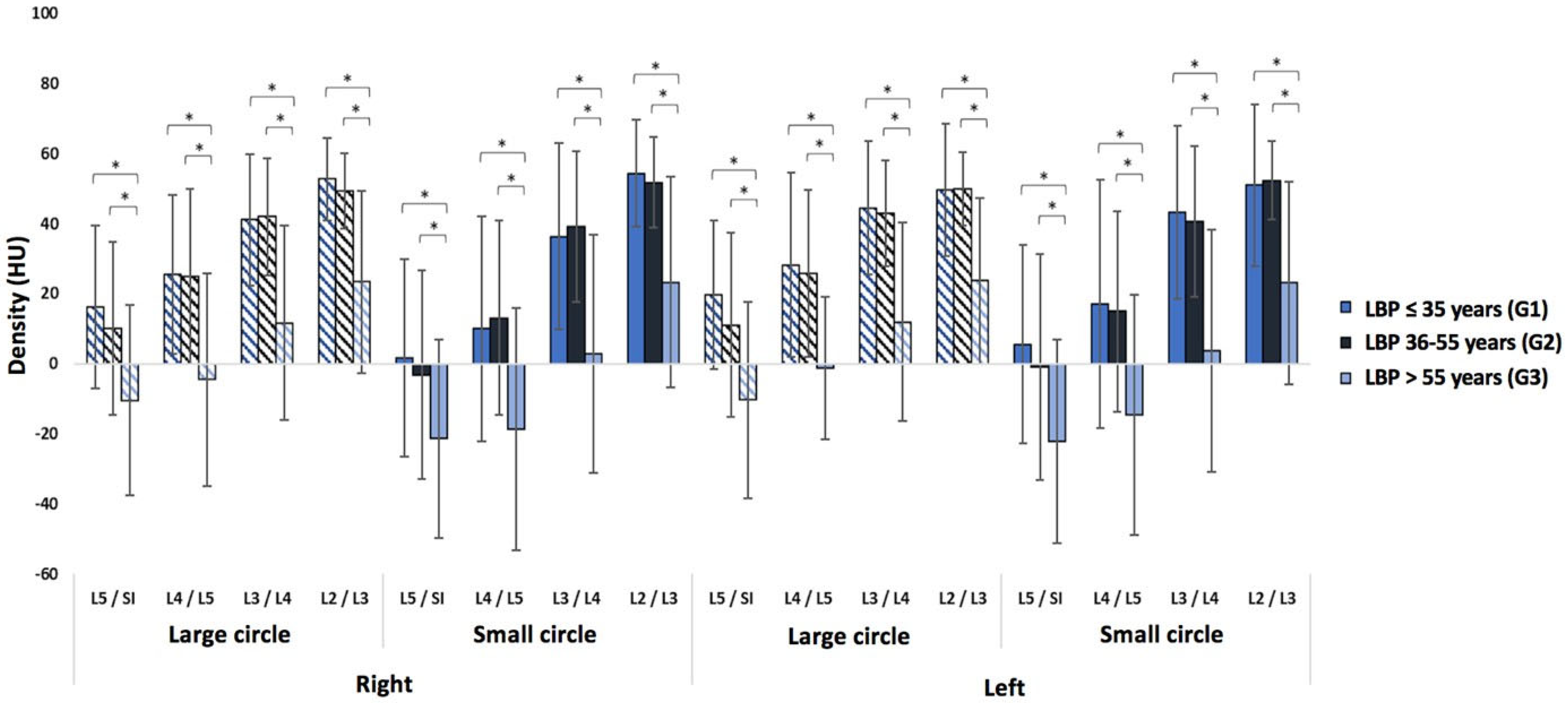
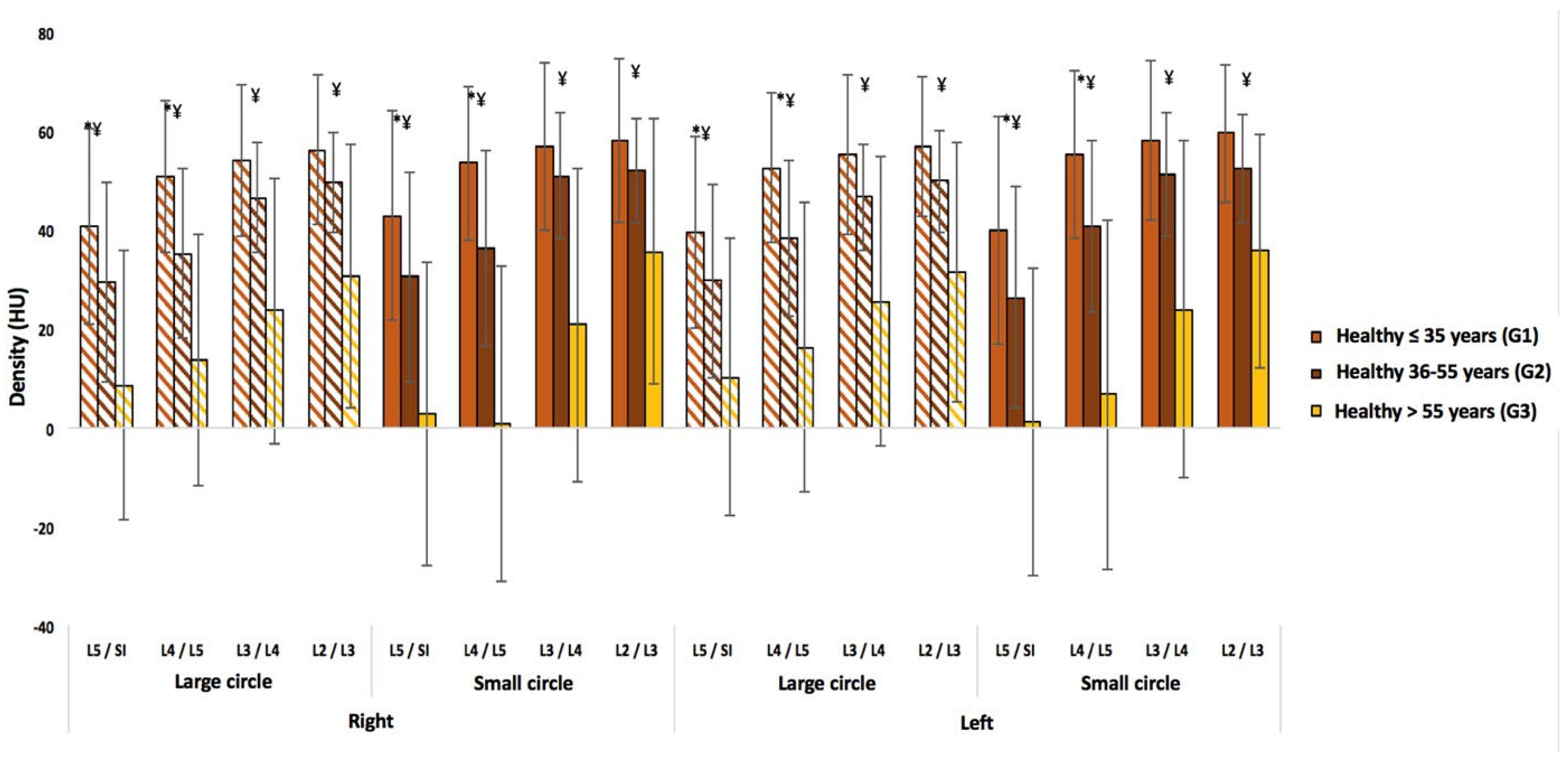
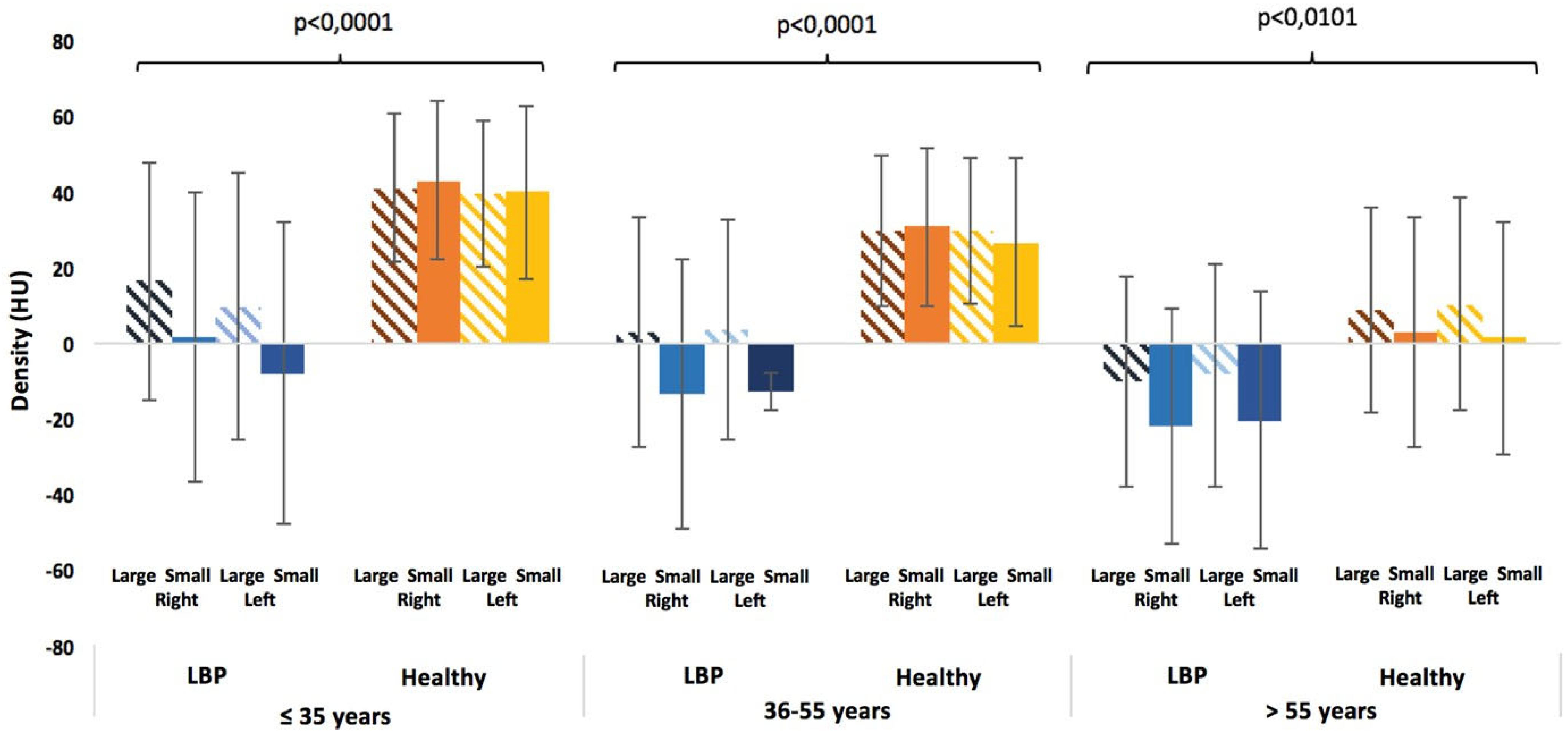
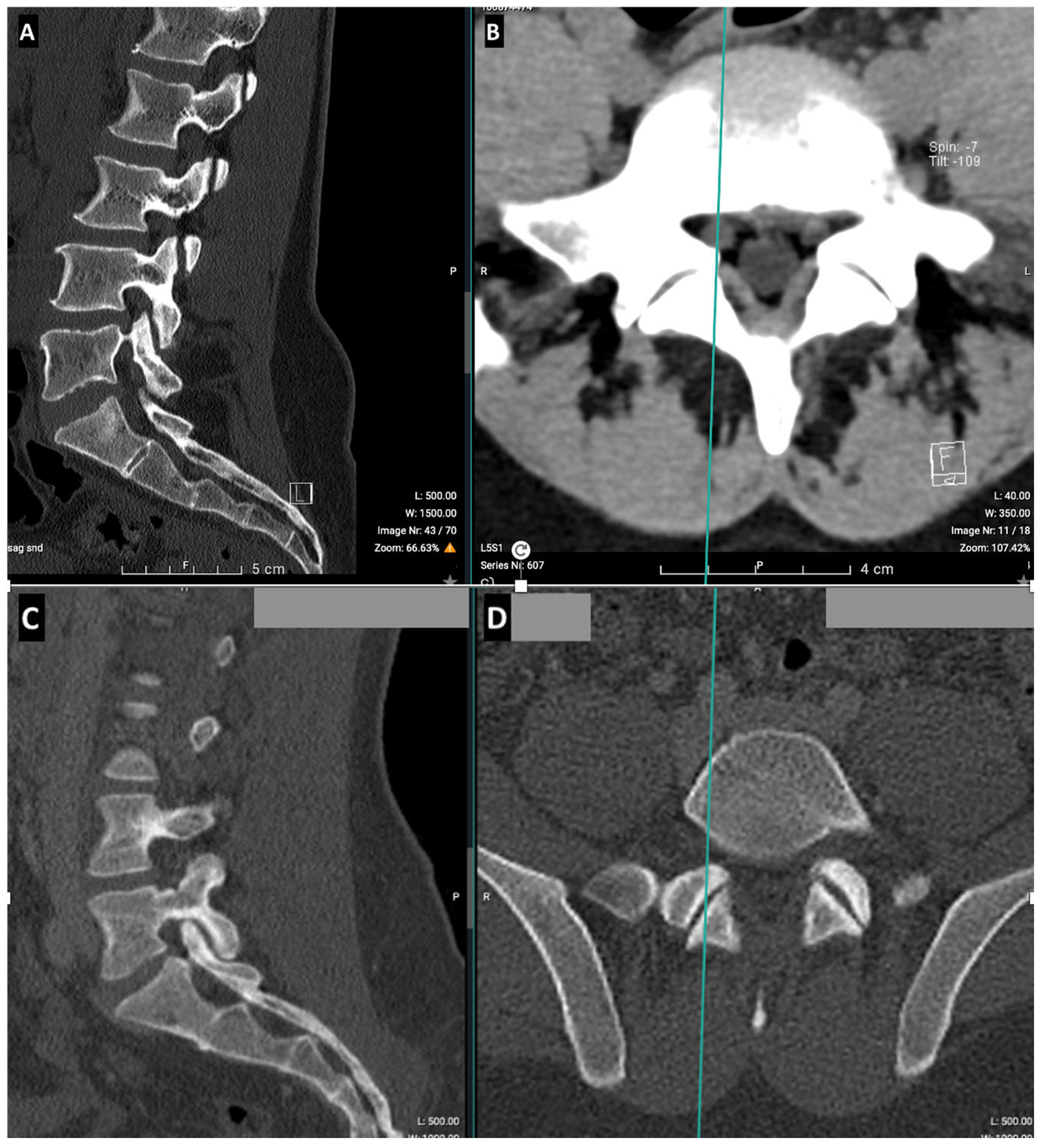
| LBP Patients by Age (n = 254) | LBP Patients by Gender (n = 254) | ||||
|---|---|---|---|---|---|
| ≤35 Years (n = 23) | 36–55 Years (n = 115) | >55 Years (n = 116) | Men (n = 108) | Women (n = 146) | |
| Age | 28.8 ± 6.0 | 45.9 ± 5.2 | 66.3 ± 8.5 | 50.1 ± 13.0 | 56.3 ± 14.7 |
| Disc Herniation | 39.1% | 22.8% | 6.1% | 22.4% | 12.5% |
| Discopathy | 13.0% | 44.7% | 29.8% | 31.8% | 37.5% |
| Simple LBP (no pathology) | 21.7% | 16.7% | 7.9% | 15.9% | 11.1% |
| Spondylolysis | 8.7% | 4.4% | 2.6% | 4.7% | 3.5% |
| Spondylolisthesis | 8.7% | 3.5% | 7.0% | 4.7% | 6.3% |
| Narrow Canal | 8.7% | 7.9% | 46.5% | 20.6% | 29.2% |
| Narrow Canal (n = 64) | Discopathy (n = 88) | Disc Herniation (n = 42) | Simple LBP (n = 33) | Spondylolysis/Listhesis | |||
|---|---|---|---|---|---|---|---|
| RIGHT | Large circle | L5/SI | −8.66 ± 29.23 | 2.22 ± 27.00 | 6.55 ± 26.03 | 9.02 ± 27.94 | 5.57 ± 25.57 |
| L4/L5 | 17.11 ± 33.79 | 14.20 ± 29.09 | 22.40 ± 21.93 | 20.91 ± 27.42 | 5.96 ± 27.54 | ||
| L3/L4 | 14.86 ± 31.99 | 30.87 ± 25.72 | 38.81 ± 16.11 | 48.70 ± 13.15 | 39.82 ± 27.18 | ||
| L2/L3 | 22.49 ± 29.20 | 39.52 ± 20.15 | 35.25 ± 16.57 | 48.75 ± 13.15 | 39.82 ± 27.18 | ||
| Small circle | L5/SI | −18.36 ± 30.10 | 2.58 ± 33.19 | 8.70 ± 34.21 | 9.30 ± 23.90 | 37.41 ± 32.59 | |
| L4/L5 | 17.87 ± 37.87 | 20.64 ± 32.73 | 33.25 ± 16.75 | 35.29 ± 21.16 | 46.40 ± 43.63 | ||
| L2/L3 | 23.36 ± 32.01 | 3.15 ± 28.11 | 7.37 ± 30.20 | 9.16 ± 29.19 | 37.41 ± 32.59 | ||
| LEFT | Large circle | L5/SI | −8.51 ± 29.16 | 3.15 ± 28.11 | 7.37 ± 30.20 | 9.16 ± 29.19 | 39.82 ± 27.18 |
| L4/L5 | 14.44 ± 32.87 | 31.47 ± 23.87 | 19.11 ± 18.92 | 9.18 ± 11.89 | 37.41 ± 32.59 | ||
| Small circle | L5/SI | −19.38 ± 36.10 | 5.11 ± 33.29 | 37.06 ± 34.15 | 37.06 ± 34.15 | 16.43 ± 33.90 | |
| L4/L5 | 17.87 ± 39.61 | 26.71 ± 32.16 | 37.06 ± 34.15 | 47.54 ± 20.48 | 39.07 ± 25.99 | ||
| L2/L3 | 23.94 ± 32.74 | 41.33 ± 23.05 | 50.61 ± 12.78 | 47.54 ± 20.48 | 39.07 ± 25.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardulyn, T.; Delafontaine, A.; Jissendi, P.; Fabeck, L. Lumbar Muscle Fatty Infiltration and Atrophy in Patients with Low Back Pain and Degenerative Spinal Pathologies: A CT Imaging Study. J. Clin. Med. 2025, 14, 2125. https://doi.org/10.3390/jcm14062125
Mardulyn T, Delafontaine A, Jissendi P, Fabeck L. Lumbar Muscle Fatty Infiltration and Atrophy in Patients with Low Back Pain and Degenerative Spinal Pathologies: A CT Imaging Study. Journal of Clinical Medicine. 2025; 14(6):2125. https://doi.org/10.3390/jcm14062125
Chicago/Turabian StyleMardulyn, Tess, Arnaud Delafontaine, Patrice Jissendi, and Laurent Fabeck. 2025. "Lumbar Muscle Fatty Infiltration and Atrophy in Patients with Low Back Pain and Degenerative Spinal Pathologies: A CT Imaging Study" Journal of Clinical Medicine 14, no. 6: 2125. https://doi.org/10.3390/jcm14062125
APA StyleMardulyn, T., Delafontaine, A., Jissendi, P., & Fabeck, L. (2025). Lumbar Muscle Fatty Infiltration and Atrophy in Patients with Low Back Pain and Degenerative Spinal Pathologies: A CT Imaging Study. Journal of Clinical Medicine, 14(6), 2125. https://doi.org/10.3390/jcm14062125






