Axis I Psychiatric Disorders and Substance Abuse: A Systematic Review of Neuroimaging Findings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Selection Process
2.4. Data Collection Process and Data Items
2.5. Bias Assessment
2.6. Effect Measures and Synthesis Methods
3. Results
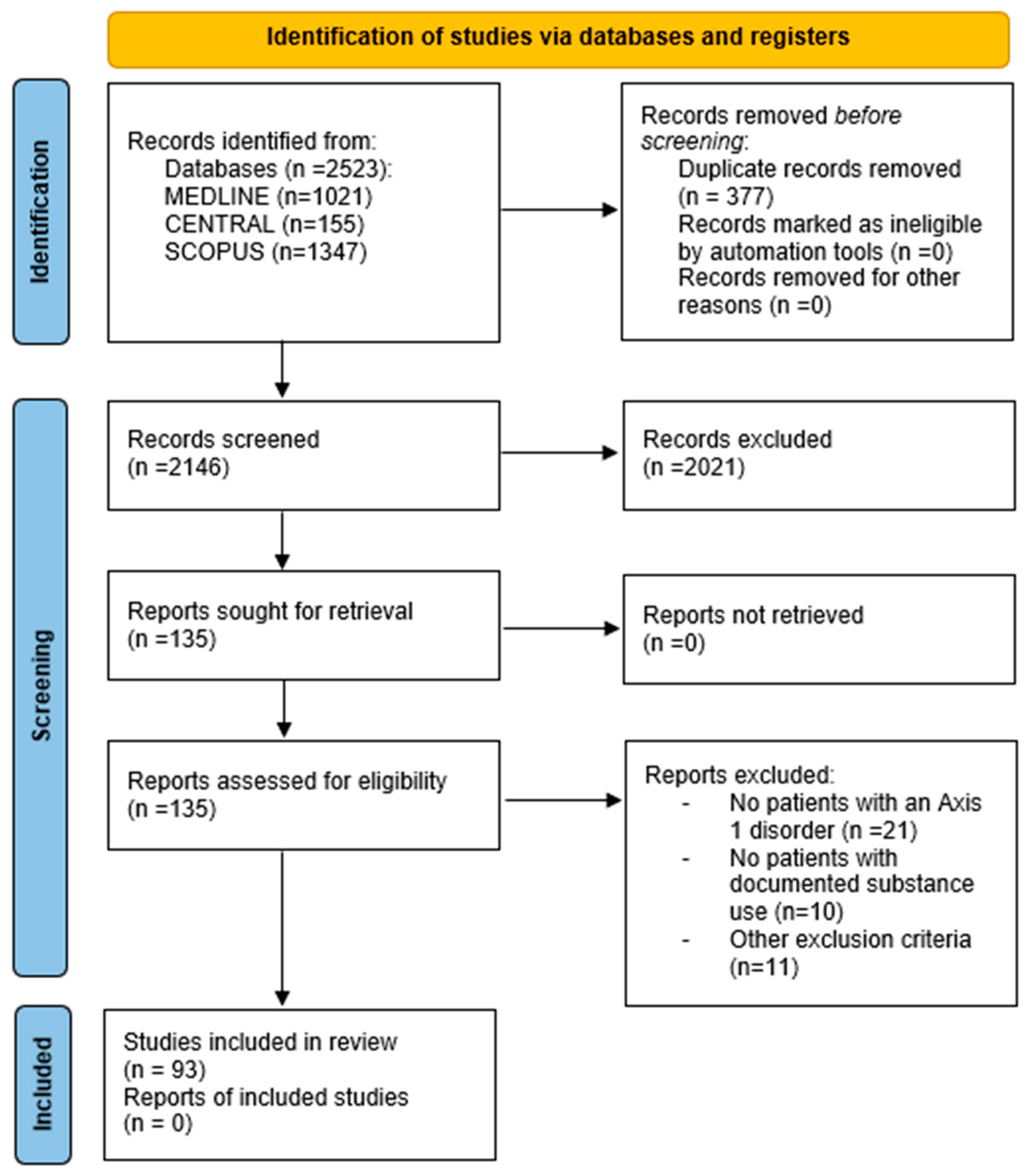
3.1. Study Selection
3.2. Risk of Bias
3.3. Schizophrenia and Substance Use Disorder
3.3.1. Cannabis and Volumetric/Morphometric MRI
3.3.2. Cannabis and Connectivity Imaging Studies (fMRI and DWI)
3.3.3. Smoking (Nicotine) and Volumetric/Morphometric MRI
3.3.4. Smoking (Nicotine) and Connectivity Imaging Studies (fMRI and DWI)
3.3.5. Alcohol and Volumetric/Morphometric MRI
3.3.6. Alcohol and Connectivity Imaging Studies (fMRI and DWI)
3.3.7. Stimulants (Cocaine/Amphetamines) and Structural/Functional MRI Studies
3.3.8. Cumulative Substance Abuse and Structural/Functional MRI Studies
3.4. Bipolar Disorder and Substance Use Disorder
3.4.1. Cannabis and Volumetric/Morphometric MRI
3.4.2. Cannabis and Magnetic Resonance Spectroscopy (MRS)
3.4.3. Smoking (Nicotine) and Volumetric/Morphometric MRI
3.4.4. Alcohol and Volumetric/Morphometric MRI
3.4.5. Alcohol and Magnetic Resonance Spectroscopy (MRS)
3.4.6. Unspecified Substance Abuse and Structural MRI and PET Scan
3.5. Depression and Substance Use Disorder
3.5.1. Cannabis and Volumetric/Morphometric MRI
3.5.2. Cannabis and Functional MRI
3.5.3. Smoking (Nicotine) and Volumetric/Functional MRI
3.5.4. Alcohol and Volumetric/Functional MRI
3.5.5. Opioids and Structural/Functional Connectivity in MRI
3.5.6. Ketamine and Functional MRI
3.5.7. Unspecified Substance Abuse and Functional MRI
3.6. Anxiety and Substance Use Disorder
3.6.1. Alcohol and Volumetric/Functional MRI
3.6.2. Heroin and Diffusion-Weighted MRI
3.7. Post-Traumatic Stress Disorder (PTSD) and Substance Use Disorder
Alcohol and Functional MRI
3.8. Key Findings
4. Discussion
4.1. Findings in Schizophrenia
4.2. Findings in Bipolar Disorder
4.3. Findings in Depressive Disorder
4.4. Findings in Anxiety Disorder
4.5. Findings in Post-Traumatic Stress Disorder
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greene, M.C.; Yangchen, T.; Lehner, T.; Sullivan, P.F.; Pato, C.N.; McIntosh, A.; Walters, J.; Gouveia, L.C.; Msefula, C.L.; Fumo, W.; et al. The epidemiology of psychiatric disorders in Africa: A scoping review. Lancet Psychiatry 2021, 8, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Robinson, Z.D.; Riggs, P.D. Cooccurring Psychiatric and Substance Use Disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2016, 25, 713–722. [Google Scholar] [CrossRef]
- Christie, G.; Merry, S.; Robinson, E. Do young people attending addiction services differ from those attending youth mental health services? Drug Alcohol Rev. 2010, 29, 406–412. [Google Scholar]
- Mueser, K.T.; Yarnold, P.R.; Levinson, D.F.; Singh, H.; Bellack, A.S.; Kee, K.; Morisson, R.L.; Yadalam, K.G. Prevalence of Substance Abuse in Schizophrenia: Demographic and Clinical Correlates. Schizophr. Bull. 1990, 16, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.E.; Leong, S.A.; Birnbaum, H.G. Assessing the economic impact of psychiatric disorders: Where to begin? Expert Opin Pharmacother. 2001, 2, 641–652. [Google Scholar] [PubMed]
- Regier, D.A.; Kuhl, E.A.; Kupfer, D.J. The DSM-5: Classification and criteria changes. World Psychiatry 2013, 12, 92–98. [Google Scholar] [CrossRef]
- Zanardi, R.; Prestifilippo, D.; Fabbri, C.; Colombo, C.; Maron, E.; Serretti, A. Precision psychiatry in clinical practice. Int. J. Psychiatry Clin. Pract. 2021, 25, 19–27. [Google Scholar] [CrossRef]
- Zametkin, A.J.; Ernst, M.; Silver, R. Laboratory and Diagnostic Testing in Child and Adolescent Psychiatry: A Review of the Past 10 Years. J. Am. Acad. Child Adolesc. Psychiatry 1998, 37, 464–472. [Google Scholar] [CrossRef]
- Anderson, A.N.; King, J.B.; Anderson, J.S. Neuroimaging in Psychiatry and Neurodevelopment: Why the emperor has no clothes. Br. J. Radiol. 2019, 92, 20180910. [Google Scholar] [CrossRef]
- Kapadia, M.; Desai, M.; Parikh, R. Fractures in the framework: Limitations of classification systems inpsychiatry. Dialog. Clin. Neurosci. 2020, 22, 17–26. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiology 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Abush, H.; Ghose, S.; Van Enkevort, E.A.; Clementz, B.A.; Pearlson, G.D.; Sweeney, J.A.; Keshavan, M.S.; Tamminga, C.A.; Ivleva, E.I. Associations between adolescent cannabis use and brain structure in psychosis. Psychiatry Res. Neuroimaging 2018, 276, 53–64. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Hough, M.; James, S.; Winmill, L.; Burge, L.; Nijhawan, S.; Matthews, P.; Zarei, M. Greater white and grey matter changes associated with early cannabis use in adolescent-onset schizophrenia (AOS). Schizophr. Res. 2011, 128, 91–97. [Google Scholar] [CrossRef]
- Schiffer, B.; Müller, B.W.; Scherbaum, N.; Forsting, M.; Wiltfang, J.; Leygraf, N.; Gizewski, E.R. Impulsivity-related brain volume deficits in schizophrenia-addiction comorbidity. Brain 2010, 133, 3093–3103. [Google Scholar] [CrossRef]
- Cohen, M.; Rasser, P.E.; Peck, G.; Carr, V.J.; Ward, P.B.; Thompson, P.M.; Johnston, P.; Baker, A.; Schall, U. Cerebellar grey-matter deficits, cannabis use and first-episode schizophrenia in adolescents and young adults. Int. J. Neuropsychopharmacol. 2012, 15, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Cookey, J.; Crocker, C.E.; Bernier, D.; Newman, A.J.; Stewart, S.; McAllindon, D.; Tibbo, P.G. Microstructural Findings in White Matter Associated with Cannabis and Alcohol Use in Early-Phase Psychosis: A Diffusion Tensor Imaging and Relaxometry Study. Brain Connect. 2018, 8, 567–576. [Google Scholar] [CrossRef]
- Quinn, M.; McHugo, M.; Armstrong, K.; Woodward, N.; Blackford, J.; Heckers, S. Impact of substance use disorder on gray matter volume in schizophrenia. Psychiatry Res. Neuroimaging 2018, 280, 9–14. [Google Scholar] [CrossRef]
- Rapp, C.; Walter, A.; Studerus, E.; Bugra, H.; Tamagni, C.; Röthlisberger, M.; Borgwardt, S.; Aston, J.; Riecher-Rössler, A. Cannabis use and brain structural alterations of the cingulate cortex in early psychosis. Psychiatry Res. 2013, 214, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Solowij, N.; Yücel, M.; Respondek, C.; Whittle, S.; Lindsay, E.; Pantelis, C.; Lubman, D.I. Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychol. Med. 2011, 41, 2349–2359. [Google Scholar] [CrossRef]
- Solowij, N.; Walterfang, M.; Lubman, D.; Styner, M.; Yücel, M.; Velakoulis, D.; Whittle, S.; Pantelis, C. Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophr. Res. 2013, 143, 179–184. [Google Scholar] [CrossRef]
- Rais, M.; Cahn, W.; Van Haren, N.; Schnack, H.; Caspers, E.; Pol, H.H.; Kahn, R. Excessive Brain Volume Loss over Time in Cannabis-Using First-Episode Schizophrenia Patients. Am. J. Psychiatry 2008, 165, 490–496. [Google Scholar] [CrossRef]
- Dekker, N.; Schmitz, N.; Peters, B.D.; van Amelsvoort, T.A.; Linszen, D.H.; de Haan, L. Cannabis use and callosal white matter structure and integrity in recent-onset schizophrenia. Psychiatry Res. Neuroimaging 2010, 181, 51–56. [Google Scholar] [CrossRef]
- Domen, P.; Michielse, S.; Peeters, S.; Viechtbauer, W.; van Os, J.; Marcelis, M. Childhood trauma- and cannabis-associated microstructural white matter changes in patients with psychotic disorder: A longitudinal family-based diffusion imaging study. Psychol. Med. 2018, 49, 628–638. [Google Scholar] [CrossRef]
- Peters, B.D.; De Haan, L.; Vlieger, E.-J.; Majoie, C.B.; Heeten, G.J.D.; Linszen, D.H. Recent-onset schizophrenia and adolescent cannabis use: MRI evidence for structural hyperconnectivity? Psychopharmacol. Bull. 2009, 42, 75–88. [Google Scholar]
- Epstein, K.A.; Kumra, S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: A naturalistic diffusion tensor imaging study. Psychiatry Res. 2015, 232, 34–41. [Google Scholar] [CrossRef]
- Hartberg, C.B.; Lange, E.H.; Lagerberg, T.V.; Haukvik, U.K.; Andreassen, O.A.; Melle, I.; Agartz, I. Cortical thickness, cortical surface area and subcortical volumes in schizophrenia and bipolar disorder patients with cannabis use. Eur. Neuropsychopharmacol. 2018, 28, 37–47. [Google Scholar] [CrossRef]
- Rais, M.; van Haren, N.E.; Cahn, W.; Schnack, H.G.; Lepage, C.; Collins, L.; Evans, A.C.; Pol, H.E.H.; Kahn, R.S. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur. Neuropsychopharmacol. 2010, 20, 855–865. [Google Scholar] [CrossRef]
- Bangalore, S.S.; Prasad, K.M.R.; Montrose, D.M.; Goradia, D.D.; Diwadkar, V.A.; Keshavan, M.S. Cannabis use and brain structural alterations in first episode schizophrenia—A region of interest, voxel based morphometric study. Schizophr. Res. 2008, 99, 1–6. [Google Scholar]
- Ebdrup, B.H.; Glenthøj, B.; Rasmussen, H.; Aggernaes, B.; Langkilde, A.R.; Paulson, O.B.; Lublin, H.; Skimminge, A.; Baaré, W. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J. Psychiatry Neurosci. 2010, 35, 95–104. [Google Scholar] [CrossRef]
- Malchow, B.; Hasan, A.; Schneider-Axmann, T.; Jatzko, A.; Gruber, O.; Schmitt, A.; Falkai, P.; Wobrock, T. Effects of cannabis and familial loading on subcortical brain volumes in first-episode schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 155–168. [Google Scholar] [CrossRef]
- Smith, M.J.; Cobia, D.J.; Reilly, J.L.; Gilman, J.M.; Roberts, A.G.; Alpert, K.I.; Wang, L.; Breiter, H.C.; Csernansky, J.G. Cannabis-related episodic memory deficits and hippocampal morphological differences in healthy individuals and schizophrenia subjects: CANNABIS and EPISODIC MEMORY. Hippocampus 2015, 25, 1042–1051. [Google Scholar]
- Kumra, S.; Robinson, P.; Tambyraja, R.; Jensen, D.; Schimunek, C.; Houri, A.; Reis, T.; Lim, K. Parietal Lobe Volume Deficits in Adolescents with Schizophrenia and Adolescents with Cannabis Use Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 171–180. [Google Scholar] [CrossRef]
- Cunha, P.J.; Rosa, P.G.P.; de Mello Ayres, A.; Duran, F.L.; Santos, L.C.; Scazufca, M.; Menezes, P.R.; dos Santos, B.; Murray, R.M.; Crippa, J.A.S.; et al. Cannabis use, cognition and brain structure in first-episode psychosis. Schizophr. Res. 2013, 147, 209–215. [Google Scholar]
- Szeszko, P.R.; Robinson, D.G.; Sevy, S.; Kumra, S.; Rupp, C.I.; Betensky, J.D.; Lencz, T.; Ashtari, M.; Kane, J.M.; Malhotra, A.K.; et al. Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. Br. J. Psychiatry 2007, 190, 230–236. [Google Scholar] [CrossRef]
- Epstein, K.A.; Kumra, S. Altered cortical maturation in adolescent cannabis users with and without schizophrenia. Schizophr. Res. 2015, 162, 143–152. [Google Scholar] [CrossRef]
- Epstein, K.A.; Kumra, S. Executive attention impairment in adolescents with schizophrenia who have used cannabis. Schizophr. Res. 2014, 157, 48–54. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Zisook, S.; Heaton, R.K.; Moranville, J.T.; Hesselink, J.R.; Braff, D.L. Magnetic Resonance Imaging Abnormalities in Lenticular Nuclei and Cerebral Cortex in Schizophrenia. Arch. Gen. Psychiatry 1991, 48, 881–890. [Google Scholar] [CrossRef]
- Fischer, A.S.; Whitfield-Gabrieli, S.; Roth, R.M.; Brunette, M.F.; Green, A.I. Impaired functional connectivity of brain reward circuitry in patients with schizophrenia and cannabis use disorder: Effects of cannabis and THC. Schizophr. Res. 2014, 158, 176–182. [Google Scholar] [CrossRef]
- Rigucci, S.; Xin, L.; Klauser, P.; Baumann, P.S.; Alameda, L.; Cleusix, M.; Jenni, R.; Ferrari, C.; Pompili, M.; Gruetter, R.; et al. Cannabis use in early psychosis is associated with reduced glutamate levels in the prefrontal cortex. Psychopharmacology 2018, 235, 13–22. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Fischer, A.S.; Henricks, A.M.; Khokhar, J.Y.; Roth, R.M.; Brunette, M.F.; Green, A.I. Understanding marijuana’s effects on functional connectivity of the default mode network in patients with schizophrenia and co-occurring cannabis use disorder: A pilot investigation. Schizophr. Res. 2018, 194, 70–77. [Google Scholar] [CrossRef]
- Peeters, S.; van Bronswijk, S.; van de Ven, V.; Gronenschild, E.; Goebel, R.; van Os, J.; Marcelis, M. Cognitive correlates of frontoparietal network connectivity ‘at rest’ in individuals with differential risk for psychotic disorder. Eur. Neuropsychopharmacol. 2015, 25, 1922–1932. [Google Scholar] [CrossRef]
- Bourque, J.; Mendrek, A.; Durand, M.; Lakis, N.; Lipp, O.; Stip, E.; Lalonde, P.; Grignon, S.; Potvin, S. Cannabis abuse is associated with better emotional memory in schizophrenia: A functional magnetic resonance imaging study. Psychiatry Res. Neuroimaging 2013, 214, 24–32. [Google Scholar] [CrossRef]
- Buchy, L.; Mathalon, D.H.; Cannon, T.D.; Cadenhead, K.S.; Cornblatt, B.A.; McGlashan, T.H.; Perkins, D.O.; Seidman, L.J.; Tsuang, M.T.; Walker, E.F.; et al. Relation between cannabis use and subcortical volumes in people at clinical high risk of psychosis. Psychiatry Res. Neuroimaging 2016, 254, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Koenders, L.; Machielsen, M.W.; van der Meer, F.J.; van Gasselt, A.C.; Meijer, C.J.; Brink, W.v.D.; Koeter, M.W.; Caan, M.W.; Cousijn, J.; Braber, A.D.; et al. Brain volume in male patients with recent onset schizophrenia with and without cannabis use disorders. J. Psychiatry Neurosci. 2015, 40, 197–206. [Google Scholar] [CrossRef]
- Machielsen, M.W.; Veltman, D.J.; Brink, W.v.D.; de Haan, L. Comparing the effect of clozapine and risperidone on cue reactivity in male patients with schizophrenia and a cannabis use disorder: A randomized fMRI study. Schizophr. Res. 2018, 194, 32–38. [Google Scholar] [CrossRef]
- Smith, M.J.; Cobia, D.J.; Wang, L.; Alpert, K.I.; Cronenwett, W.J.; Goldman, M.B.; Mamah, D.; Barch, D.M.; Breiter, H.C.; Csernansky, J.G. Cannabis-Related Working Memory Deficits and Associated Subcortical Morphological Differences in Healthy Individuals and Schizophrenia Subjects. Schizophr. Bull. 2013, 40, 287–299. [Google Scholar] [CrossRef]
- Haller, S.; Curtis, L.; Badan, M.; Bessero, S.; Albom, M.; Chantraine, F.; Alimenti, A.; Lovblad, K.-O.; Giannakopoulos, P.; Merlo, M. Combined Grey Matter VBM and White Matter TBSS Analysis in Young First Episode Psychosis Patients with and Without Cannabis Consumption. Brain Topogr. 2013, 26, 641–647. [Google Scholar] [CrossRef]
- Moser, D.A.; Doucet, G.E.; Lee, W.H.; Rasgon, A.; Krinsky, H.; Leibu, E.; Ing, A.; Schumann, G.; Rasgon, N.; Frangou, S. Multivariate Associations Among Behavioral, Clinical, and Multimodal Imaging Phenotypes in Patients with Psychosis. JAMA Psychiatry 2018, 75, 386–395. [Google Scholar] [CrossRef]
- Alexander, P.D.; Gicas, K.M.; Cheng, A.; Lang, D.J.; Procyshyn, R.M.; Vertinsky, A.T.; Panenka, W.J.; Thornton, A.E.; Rauscher, A.; Wong, J.Y.X.; et al. A comparison of regional brain volumes and white matter connectivity in subjects with stimulant induced psychosis versus schizophrenia. Psychopharmacology 2019, 236, 3385–3399. [Google Scholar] [CrossRef] [PubMed]
- Crocker, C.E.; Bernier, D.C.; Hanstock, C.C.; Lakusta, B.; Purdon, S.E.; Seres, P.; Tibbo, P.G. Prefrontal glutamate levels differentiate early phase schizophrenia and methamphetamine addiction: A 1H MRS study at 3Tesla. Schizophr. Res. 2014, 157, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bernier, D.; Bartha, R.; McAllindon, D.; Hanstock, C.C.; Marchand, Y.; Dillen, K.N.; Gallant, M.; Good, K.P.; Tibbo, P.G. Illness versus substance use effects on the frontal white matter in early phase schizophrenia: A 4 Tesla 1 H-MRS study. Schizophr. Res. 2016, 175, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Mancini-Marïe, A.; Fahim, C.; Mensour, B.; Lévesque, J.; Karama, S.; Beauregard, M.; Rompré, P.-P.; Stip, E. Increased striatal gray matter densities in patients with schizophrenia and substance use disorder: A voxel-based morphometry study. Psychiatry Res. Neuroimaging 2007, 154, 275–279. [Google Scholar] [CrossRef]
- Wobrock, T.; Sittinger, H.; Behrendt, B.; D’amelio, R.; Falkai, P. Comorbid substance abuse and brain morphology in recent-onset psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wojtalik, J.A.; Barch, D.M. An fMRI Study of the Influence of a History of Substance Abuse on Working Memory-Related Brain Activation in Schizophrenia. Front. Psychiatry 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.R.; Wallace, S.; Magnotta, V.A.; Bockholt, J.; Ehrlich, S.; Gollub, R.L.; Manoach, D.S.; Ho, B.C.; Clark, V.P.; Lauriello, J.; et al. Cigarette smoking and white matter microstructure in schizophrenia. Psychiatry Res. Neuroimaging 2012, 201, 152–158. [Google Scholar] [CrossRef]
- Zhang, X.; Stein, E.A.; Hong, L.E. Smoking and Schizophrenia Independently and Additively Reduce White Matter Integrity Between Striatum and Frontal Cortex. Biol. Psychiatry 2010, 68, 674–677. [Google Scholar] [CrossRef]
- Jørgensen, K.N.; Psychol, C.; Skjærvø, I.; Mørch-Johnsen, L.; Haukvik, U.K.; Lange, E.H.; Melle, I.; Andreassen, O.A.; Agartz, I. Cigarette smoking is associated with thinner cingulate and insular cortices in patients with severe mental illness. J. Psychiatry Neurosci. 2015, 40, 241–249. [Google Scholar] [CrossRef]
- Moran, L.V.; Betts, J.M.; Ongur, D.; Janes, A.C. Neural Responses to Smoking Cues in Schizophrenia. Schizophr. Bull. 2018, 44, 525–534. [Google Scholar] [CrossRef]
- Moran, L.V.; Sampath, H.; Kochunov, P.; Hong, L.E. Brain Circuits That Link Schizophrenia to High Risk of Cigarette Smoking. Schizophr. Bull. 2013, 39, 1373–1381. [Google Scholar] [CrossRef]
- Liu, H.; Luo, Q.; Du, W.; Li, X.; Zhang, Z.; Yu, R.; Chen, X.; Meng, H.; Du, L. Cigarette smoking and schizophrenia independently and reversibly altered intrinsic brain activity. Brain Imaging Behav. 2018, 12, 1457–1465. [Google Scholar] [CrossRef]
- Postma, P.; Gray, J.A.; Sharma, T.; Geyer, M.; Mehrotra, R.; Das, M.; Zachariah, E.; Hines, M.; Williams, S.C.R.; Kumari, V. A behavioural and functional neuroimaging investigation into the effects of nicotine on sensorimotor gating in healthy subjects and persons with schizophrenia. Psychopharmacology 2006, 184, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Lungu, O.; Lipp, O.; Lalonde, P.; Zaharieva, V.; Stip, E.; Melun, J.-P.; Mendrek, A. Increased ventro-medial prefrontal activations in schizophrenia smokers during cigarette cravings. Schizophr. Res. 2016, 173, 30–36. [Google Scholar] [CrossRef]
- Potvin, S.; Dugré, J.R.; Fahim, C.; Dumais, A. Increased Connectivity Between the Nucleus Accumbens and the Default Mode Network in Patients with Schizophrenia During Cigarette Cravings. J. Dual Diagn. 2019, 15, 8–15. [Google Scholar] [PubMed]
- Nesvag, R.; Frigessi, A.; Jonsson, E.; Agartz, I. Effects of alcohol consumption and antipsychotic medication on brain morphology in schizophrenia. Schizophr. Res. 2007, 90, 52–61. [Google Scholar] [CrossRef]
- Mathalon, D.H.; Pfefferbaum, A.; Lim, K.O.; Rosenbloom, M.J.; Sullivan, E.V. Compounded Brain Volume Deficits in Schizophrenia-Alcoholism Comorbidity. Arch. Gen. Psychiatry 2003, 60, 245–252. [Google Scholar] [CrossRef]
- Gizewski, E.R.; Müller, B.W.; Scherbaum, N.; Lieb, B.; Forsting, M.; Wiltfang, J.; Leygraf, N.; Schiffer, B. The impact of alcohol dependence on social brain function. Addict. Biol. 2013, 18, 109–120. [Google Scholar] [CrossRef]
- Lange, E.H.; Nerland, S.; Jørgensen, K.N.; Mørch-Johnsen, L.; Nesvåg, R.; Hartberg, C.B.; Haukvik, U.K.; Osnes, K.; Melle, I.; Andreassen, O.A.; et al. Alcohol use is associated with thinner cerebral cortex and larger ventricles in schizophrenia, bipolar disorder and healthy controls. Psychol. Med. 2017, 47, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.; Rosenbloom, M.; Derosa, E.; Sullivan, E.; Pfefferbaum, A. Regional striatal volume abnormalities in schizophrenia: Effects of comorbidity for alcoholism, recency of alcoholic drinking, and antipsychotic medication type. Schizophr. Res. 2005, 79, 189–200. [Google Scholar] [CrossRef]
- Smith, M.J.; Wang, L.; Cronenwett, W.; Goldman, M.B.; Mamah, D.; Barch, D.M.; Csernansky, J.G. Alcohol use disorders contribute to hippocampal and subcortical shape differences in schizophrenia. Schizophr. Res. 2011, 131, 174–183. [Google Scholar] [CrossRef]
- Joyal, C.C.; Pennanen, C.; Tiihonen, E.; Laakso, M.P.; Tiihonen, J.; Aronen, H.J. MRI volumetry of the vermis and the cerebellar hemispheres in men with schizophrenia. Psychiatry Res. Neuroimaging 2004, 131, 115–124. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Deshmukh, A.; Desmond, J.E.; Mathalon, D.H.; Rosenbloom, M.J.; Lim, K.O.; Pfefferbaum, A. Contribution of Alcohol Abuse to Cerebellar Volume Deficits in Men with Schizophrenia. Arch. Gen. Psychiatry 2000, 57, 894–902. [Google Scholar] [CrossRef]
- Joyal, C.; Putkonen, A.; Mancini-Marïe, A.; Hodgins, S.; Kononen, M.; Boulay, L.; Pihlajamaki, M.; Soininen, H.; Stip, E.; Tiihonen, J.; et al. Violent persons with schizophrenia and comorbid disorders: A functional magnetic resonance imaging study. Schizophr. Res. 2007, 91, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Scheller-Gilkey, G.; Lewine, R.R.J.; Caudle, J.; Brown, F.W. Schizophrenia, substance use, and brain morphology. Schizophr. Res. 1999, 35, 113–120. [Google Scholar] [PubMed]
- Bitter, S.M.; Weber, W.A.; Chu, W.-J.; Adler, C.M.; Eliassen, J.C.; Strakowski, S.M.; DelBello, M.P. N-acetyl Aspartate Levels in Adolescents with Bipolar and/or Cannabis Use Disorders. J. Dual Diagn. 2014, 10, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, K.; DelBello, M.P.; Mills, N.; Elman, I.; Strakowski, S.M.; Adler, C.M. Neuroanatomic Comparison of Bipolar Adolescents with and Without Cannabis Use Disorders. J. Child Adolesc. Psychopharmacol. 2008, 18, 557–563. [Google Scholar] [CrossRef]
- De Bellis, M.D.; Narasimhan, A.; Thatcher, D.L.; Keshavan, M.S.; Soloff, P.; Clark, D.B. Prefrontal Cortex, Thalamus, and Cerebellar Volumes in Adolescents and Young Adults with Adolescent-Onset Alcohol Use Disorders and Comorbid Mental Disorders. Alcohol. Clin. Exp. Res. 2005, 29, 1590–1600. [Google Scholar] [CrossRef]
- Nery, F.G.; Matsuo, K.; Nicoletti, M.A.; Monkul, E.S.; Zunta-Soares, G.B.; Hatch, J.P.; Lafer, B.; Soares, J.C. Association between prior alcohol use disorders and decreased prefrontal gray matter volumes in bipolar I disorder patients. Neurosci. Lett. 2011, 503, 136–140. [Google Scholar] [CrossRef]
- Kirsch, D.E.; Tretyak, V.; Radpour, S.; Weber, W.A.; Nemeroff, C.B.; Fromme, K.; Strakowski, S.M.; Lippard, E.T.C. Childhood maltreatment, prefrontal-paralimbic gray matter volume, and substance use in young adults and interactions with risk for bipolar disorder. Sci. Rep. 2021, 11, 123. [Google Scholar] [CrossRef]
- Nery, F.G.; Stanley, J.A.; Chen, H.-H.; Hatch, J.P.; Nicoletti, M.A.; Monkul, E.S.; Lafer, B.; Soares, J.C. Bipolar disorder comorbid with alcoholism: A 1H magnetic resonance spectroscopy study. J. Psychiatr. Res. 2010, 44, 278–285. [Google Scholar] [CrossRef]
- Prisciandaro, J.J.; Tolliver, B.K.; Prescot, A.P.; Brenner, H.M.; Renshaw, P.F.; Brown, T.R.; Anton, R.F. Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Transl. Psychiatry 2017, 7, e1163. [Google Scholar] [CrossRef]
- Chitty, K.M.; Lagopoulos, J.; Hickie, I.B.; Hermens, D.F. The impact of alcohol and tobacco use on in vivo glutathione in youth with bipolar disorder: An exploratory study. J. Psychiatr. Res. 2014, 55, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Lippard, E.T.; Mazure, C.M.; Johnston, J.A.; Spencer, L.; Weathers, J.; Pittman, B.; Wang, F.; Blumberg, H.P. Brain circuitry associated with the development of substance use in bipolar disorder and preliminary evidence for sexual dimorphism in adolescents. J. Neurosci. Res. 2017, 95, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Shad, M.U.; Prasad, K.; Forman, S.D.; Haas, G.L.; Walker, J.D.; Pisarov, L.A.; Goldstein, G. Insight and neurocognitive functioning in bipolar subjects. Compr. Psychiatry 2015, 56, 112–120. [Google Scholar] [CrossRef]
- Strakowski, S.M.; Woods, B.T.; Tohen, M.; Wilson, D.R.; Douglas, A.W.; Stoll, A.L. MRI subcortical signal hyperintensities in Mania at first hospitalization. Biol. Psychiatry 1993, 33, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Altamura, A.; Delvecchio, G.; Marotta, G.; Oldani, L.; Pigoni, A.; Ciappolino, V.; Caletti, E.; Rovera, C.; Dobrea, C.; Arici, C.; et al. Structural and metabolic differentiation between bipolar disorder with psychosis and substance-induced psychosis: An integrated MRI/PET study. Eur. Psychiatry 2017, 41, 85–94. [Google Scholar] [CrossRef]
- Chye, Y.; Solowij, N.; Ganella, E.P.; Suo, C.; Yücel, M.; Batalla, A.; Cousijn, J.; Goudriaan, A.E.; Martin-Santos, R.; Whittle, S.; et al. Role of orbitofrontal sulcogyral pattern on lifetime cannabis use and depressive symptoms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 392–400. [Google Scholar] [CrossRef]
- Radoman, M.; Hoeppner, S.S.; Schuster, R.M.; Evins, A.E.; Gilman, J.M. Marijuana use and major depressive disorder are additively associated with reduced verbal learning and altered cortical thickness. Cogn. Affect. Behav. Neurosci. 2019, 19, 1047–1058. [Google Scholar] [CrossRef]
- Osuch, E.A.; Manning, K.; Hegele, R.A.; Théberge, J.; Neufeld, R.; Mitchell, D.; Williamson, P.; Gardner, R.C. Depression, marijuana use and early-onset marijuana use conferred unique effects on neural connectivity and cognition. Acta Psychiatr. Scand. 2016, 134, 399–409. [Google Scholar] [CrossRef]
- Thayer, R.E.; YorkWilliams, S.L.; Hutchison, K.E.; Bryan, A.D. Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Res. Neuroimaging 2019, 285, 58–63. [Google Scholar] [CrossRef]
- Nichols, E.S.; Penner, J.; Ford, K.A.; Wammes, M.; Neufeld, R.W.; Mitchell, D.G.; Greening, S.G.; Théberge, J.; Williamson, P.C.; Osuch, E.A. Emotion regulation in emerging adults with major depressive disorder and frequent cannabis use. NeuroImage Clin. 2021, 30, 102575. [Google Scholar] [CrossRef]
- Cornelius, J.R.; Aizenstein, H.J.; Hariri, A.R. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict. Behav. 2010, 35, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, A.; Bandelow, B.; Stein, D.J.; Bloch, S.; Engel, K.R.; Havemann-Reinecke, U.; Wedekind, D. Grey matter structural differences in alcohol-dependent individuals with and without comorbid depression/anxiety—An MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 285–294. [Google Scholar] [CrossRef]
- Sjoerds, Z.; Brink, W.v.D.; Beekman, A.T.F.; Penninx, B.W.J.H.; Veltman, D.J. Response inhibition in alcohol-dependent patients and patients with depression/anxiety: A functional magnetic resonance imaging study. Psychol. Med. 2014, 44, 1713–1725. [Google Scholar] [CrossRef]
- Zorlu, N.; Cropley, V.L.; Zorlu, P.K.; Delibas, D.H.; Adibelli, Z.H.; Baskin, E.P.; Esen, Ö.S.; Bora, E.; Pantelis, C. Effects of cigarette smoking on cortical thickness in major depressive disorder. J. Psychiatr. Res. 2017, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, V.; Menon, M.; Balducci, X.L.; Selby, P.; Busto, U.; Zawertailo, L. Enhanced smoking cue salience associated with depression severity in nicotine-dependent individuals: A preliminary fMRI study. Int. J. Neuropsychopharmacol. 2013, 16, 997–1008. [Google Scholar] [CrossRef]
- Li, C.-S.R.; Zhang, S.; Hung, C.-C.; Chen, C.-M.; Duann, J.-R.; Lin, C.-P.; Lee, T.S.-H. Depression in chronic ketamine users: Sex differences and neural bases. Psychiatry Res. Neuroimaging 2017, 269, 1–8. [Google Scholar] [CrossRef]
- Wong, N.M.L.; Cheung, S.-H.; Chan, C.C.H.; Zeng, H.; Liu, Y.-P.; So, K.-F.; Lee, T.M.C. Diffusivity of the uncinate fasciculus in heroin users relates to their levels of anxiety. Transl. Psychiatry 2015, 5, e554. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.Y.; Dichter, G.S.; Reese, E.D.; Bell, R.P.; Bartuska, A.D.; Stein, J.R.; Daughters, S.B. Neural reward response to substance-free activity images in opiate use disorder patients with depressive symptoms. Drug Alcohol Depend. 2019, 198, 180–189. [Google Scholar] [CrossRef]
- Martins, B.S.; Cáceda, R.; Cisler, J.M.; Kilts, C.D.; James, G.A. The neural representation of the association between comorbid drug use disorders and childhood maltreatment. Drug Alcohol Depend. 2018, 192, 215–222. [Google Scholar] [CrossRef]
- Karch, S.; Jäger, L.; Karamatskos, E.; Graz, C.; Stammel, A.; Flatz, W.; Lutz, J.; Holtschmidt-Täschner, B.; Genius, J.; Leicht, G.; et al. Influence of trait anxiety on inhibitory control in alcohol-dependent patients: Simultaneous acquisition of ERPs and BOLD responses. J. Psychiatr. Res. 2008, 42, 734–745. [Google Scholar] [CrossRef]
- Viswanath, H.; Velasquez, K.M.; Savjani, R.; Molfese, D.L.; Curtis, K.; Molfese, P.J.; Eagleman, D.M.; Baldwin, P.R.; Frueh, B.C.; Fowler, J.C.; et al. Interhemispheric insular and inferior frontal connectivity are associated with substance abuse in a psychiatric population. Neuropharmacology 2015, 92, 63–68. [Google Scholar] [CrossRef]
- Kim, B.; Shin, W.S.; Kim, M.K.; Lee, S.H. White matter microstructural changes are associated with alcohol use in patients with panic disorder. J. Affect. Disord. 2016, 199, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Harlé, K.M.; Simmons, A.N.; Norman, S.B.; Spadoni, A.D. Neural affective mechanisms associated with treatment responsiveness in veterans with PTSD and comorbid alcohol use disorder. Psychiatry Res. Neuroimaging 2020, 305, 111172. [Google Scholar] [CrossRef] [PubMed]
- Schuff, N.; Neylan, T.C.; Fox-Bosetti, S.; Lenoci, M.; Samuelson, K.W.; Studholme, C.; Kornak, J.; Marmar, C.R.; Weiner, M.W. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. Neuroimaging 2008, 162, 147–157. [Google Scholar] [CrossRef]
- Fischer, A.G.; Jocham, G. The role of serotonin in performance monitoring and cognitive control. In Handbook of Behavioral Neuroscience [Internet]; Elsevier: Amsterdam, The Netherlands, 2020; pp. 571–588. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, C.; Li, X.; Duan, M.; He, H.; Chen, X.; Yang, H.; Gong, J.; Chang, X.; Woelfer, M.; et al. Progressive Reduction in Gray Matter in Patients with Schizophrenia Assessed with MR Imaging by Using Causal Network Analysis. Radiology 2018, 287, 729. [Google Scholar] [CrossRef]
- Iversen, L. Cannabis and the brain. Brain 2003, 126, 1252–1270. [Google Scholar] [CrossRef]
- Jacobus, J.; Tapert, S.F. Effects of Cannabis on the Adolescent Brain. Curr. Pharm. Des. 2014, 20, 2186–2193. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, R.C.K.; Shum, D.H.K. Schizophrenia and prospective memory impairments: A review. Clin. Neuropsychol. 2018, 32, 836–857. [Google Scholar] [CrossRef]
- Chambers, R.; Krystal, J.H.; Self, D.W. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol. Psychiatry 2001, 50, 71–83. [Google Scholar] [CrossRef]
- De La Monte, S.M.; Kril, J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014, 127, 71–90. [Google Scholar] [CrossRef]
- Abrahao, K.P.; Salinas, A.G.; Lovinger, D.M. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 2017, 96, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Maric, N.P.; Jovicic, M.J.; Mihaljevic, M.; Miljevic, C. Improving Current Treatments for Schizophrenia. Drug Dev. Res. 2016, 77, 357–367. [Google Scholar] [CrossRef]
- Swan, G.E.; Lessov-Schlaggar, C.N. The Effects of Tobacco Smoke and Nicotine on Cognition and the Brain. Neuropsychol. Rev. 2007, 17, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Shearman, E.; Fallon, S.; Sershen, H.; Lajtha, A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Res. Bull. 2008, 76, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.; McCutcheon, R.; Stone, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015, 29, 97–115. [Google Scholar] [CrossRef]
- Juckel, G.; Schlagenhauf, F.; Koslowski, M.; Wüstenberg, T.; Villringer, A.; Knutson, B.; Wrase, J.; Heinz, A. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage 2006, 29, 409–416. [Google Scholar] [CrossRef]
- Khokhar, J.Y.; Dwiel, L.L.; Henricks, A.M.; Doucette, W.T.; Green, A.I. The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophr. Res. 2018, 194, 78–85. [Google Scholar] [CrossRef]
- Messer, T.; Lammers, G.; Müller-Siecheneder, F.; Schmidt, R.-F.; Latifi, S. Substance abuse in patients with bipolar disorder: A systematic review and meta-analysis. Psychiatry Res. 2017, 253, 338–350. [Google Scholar] [CrossRef]
- Levin, F.R.; Hennessy, G. Bipolar disorder and substance abuse. Biol. Psychiatry 2004, 56, 738–748. [Google Scholar] [CrossRef]
- Frazier, J.A.; Ahn, M.S.; DeJong, S.; Bent, E.K.; Breeze, J.L.; Giuliano, A.J. Magnetic Resonance Imaging Studies in Early-Onset Bipolar Disorder: A Critical Review. Harv. Rev. Psychiatry 2005, 13, 125–140. [Google Scholar] [CrossRef]
- Garrett, A.; Chang, K. The role of the amygdala in bipolar disorder development. Dev. Psychopathol. 2008, 20, 1285–1296. [Google Scholar] [CrossRef]
- Preuss, U.W.; Schaefer, M.; Born, C.; Grunze, H. Bipolar Disorder and Comorbid Use of Illicit Substances. Medicina 2021, 57, 1256. [Google Scholar] [CrossRef]
- Gardner, E.L. Addiction and Brain Reward and Antireward Pathways. In Advances in Psychosomatic Medicine [Internet]; Clark, M.R., Treisman, G.J., Eds.; Karger: Basel, Switzerland, 2011; pp. 22–60. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC4549070/.324065 (accessed on 15 September 2024).
- Höflich, A.; Michenthaler, P.; Kasper, S.; Lanzenberger, R. Circuit Mechanisms of Reward, Anhedonia, and Depression. Int. J. Neuropsychopharmacol. 2019, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Hasler, B.P.; Smith, L.J.; Cousins, J.C.; Bootzin, R.R. Circadian rhythms, sleep, and substance abuse. Sleep Med. Rev. 2012, 16, 67–81. [Google Scholar]
- Daut, R.A.; Fonken, L.K. Circadian regulation of depression: A role for serotonin. Front. Neuroendocr. 2019, 54, 100746. [Google Scholar] [CrossRef]
- Arunogiri, S.; I Lubman, D. Anxiety and substance use disorders: A worrying combination. Australas. Psychiatry 2015, 23, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Brumback, T.; Thompson, W.; Cummins, K.; Brown, S.; Tapert, S. Psychosocial predictors of substance use in adolescents and young adults: Longitudinal risk and protective factors. Addict. Behav. 2021, 121, 106985. [Google Scholar] [CrossRef]
- Ng, E.; Browne, C.J.; Samsom, J.N.; Wong, A.H.C. Depression and substance use comorbidity: What we have learned from animal studies. Am. J. Drug Alcohol Abus. 2017, 43, 456–474. [Google Scholar] [CrossRef]
- Howe, L.K.; Fisher, L.R.; Atkinson, E.A.; Finn, P.R. Symptoms of anxiety, depression, and borderline personality in alcohol use disorder with and without comorbid substance use disorder. Alcohol 2021, 90, 19–25. [Google Scholar] [CrossRef]
- Williams, G.C.; Patte, K.A.; Ferro, M.A.; Leatherdale, S.T. Substance use classes and symptoms of anxiety and depression among Canadian secondary school students. Health Promot. Chronic Dis. Prev. Can. 2021, 41, 153–164. [Google Scholar] [CrossRef]
- Babaev, O.; Chatain, C.P.; Krueger-Burg, D. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. A role for GABA mechanisms in the motivational effects of alcohol. Biochem. Pharmacol. 2004, 68, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.B.; Jaffee, M.S.; Jorge, R.E. Posttraumatic Stress Disorder and Anxiety-Related Conditions. Contin. Lifelong Learn. Neurol. 2021, 27, 1738–1763. [Google Scholar] [CrossRef]
- Harnett, N.G.; Goodman, A.M.; Knight, D.C. PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Exp. Neurol. 2020, 330, 113331. [Google Scholar] [CrossRef]
| Reference | Author | Year | Selection | Comparability | Exposure/Outcome |
|---|---|---|---|---|---|
| [12] | Abush | 2018 | **** | ** | *** |
| [13] | James | 2011 | **** | ** | *** |
| [14] | Schiffer | 2010 | **** | ** | *** |
| [15] | Cohen | 2012 | **** | ** | *** |
| [16] | Cookey | 2018 | *** | ** | ** |
| [17] | Quinn | 2018 | **** | * | *** |
| [18] | Rapp | 2013 | **** | ** | *** |
| [19] | Solowij | 2011 | **** | ** | *** |
| [20] | Solowij | 2013 | **** | ** | *** |
| [21] | Rais | 2008 | **** | ** | *** |
| [22] | Dekker | 2010 | **** | ** | *** |
| [23] | Domen | 2019 | **** | ** | *** |
| [24] | Peters | 2009 | *** | ** | *** |
| [25] | Epstein | 2015 | **** | ** | *** |
| [26] | Hartberg | 2018 | **** | ** | *** |
| [27] | Rais | 2010 | **** | ** | *** |
| [28] | Bangalore | 2008 | **** | ** | *** |
| [29] | Ebdrup | 2010 | **** | ** | *** |
| [30] | Malchow | 2013 | **** | ** | *** |
| [31] | Smith | 2015 | **** | ** | *** |
| [32] | Kumra | 2012 | **** | ** | *** |
| [33] | Cunha | 2013 | **** | ** | *** |
| [34] | Szeszko | 2007 | **** | ** | *** |
| [35] | Epstein | 2015 | **** | ** | *** |
| [36] | Epstein | 2014 | **** | ** | *** |
| [37] | Jernigan | 1991 | **** | ** | *** |
| [38] | Fischer | 2014 | **** | ** | *** |
| [39] | Rigucci | 2018 | **** | ** | *** |
| [40] | Whitfield-Gabrieli | 2018 | **** | ** | *** |
| [41] | Peeters | 2015 | **** | ** | *** |
| [42] | Bourque | 2013 | **** | ** | *** |
| [43] | Buchy | 2016 | **** | ** | *** |
| [44] | Koenders | 2015 | **** | ** | *** |
| [45] | Machielsen | 2018 | **** | ** | *** |
| [46] | Smith | 2014 | **** | ** | *** |
| [47] | Haller | 2013 | **** | ** | *** |
| [48] | Moser | 2018 | **** | ** | *** |
| [49] | Alexander | 2019 | **** | ** | *** |
| [50] | Crocker | 2014 | **** | ** | *** |
| [51] | Bernier | 2016 | **** | ** | *** |
| [52] | Potvin | 2007 | **** | ** | *** |
| [53] | Wobrock | 2009 | ** | ** | *** |
| [54] | Wojtalik | 2014 | **** | ** | *** |
| [55] | Cullen | 2012 | **** | ** | *** |
| [56] | Zhang | 2010 | **** | * | *** |
| [57] | Jørgensen | 2015 | **** | ** | *** |
| [58] | Moran | 2018 | **** | ** | *** |
| [59] | Moran | 2013 | **** | ** | *** |
| [60] | Liu | 2018 | **** | ** | *** |
| [61] | Postma | 2006 | **** | ** | *** |
| [62] | Potvin | 2016 | **** | ** | *** |
| [63] | Potvin | 2019 | **** | ** | *** |
| [64] | Nesvag | 2007 | **** | ** | *** |
| [65] | Mathalon | 2003 | **** | ** | *** |
| [66] | Gizewski | 2018 | **** | ** | *** |
| [67] | Lange | 2017 | **** | ** | *** |
| [68] | Deshmukh | 2005 | **** | ** | *** |
| [69] | Smith | 2011 | **** | ** | *** |
| [70] | Joyal | 2004 | **** | ** | *** |
| [71] | Sullivan | 2000 | **** | ** | *** |
| [72] | Joyal | 2007 | *** | * | ** |
| [73] | Scheller-Gilkey | 1999 | **** | ** | *** |
| [74] | Bitter | 2014 | **** | ** | *** |
| [75] | Jarvis | 2008 | **** | ** | *** |
| [76] | De Bellis | 2005 | **** | ** | *** |
| [77] | Nery | 2011 | **** | ** | *** |
| [78] | Kirsch | 2021 | **** | ** | *** |
| [79] | Nery | 2010 | **** | ** | *** |
| [80] | Prisciandaro | 2017 | **** | ** | *** |
| [81] | Chitty | 2014 | **** | ** | *** |
| [82] | Lippard | 2017 | **** | * | *** |
| [83] | Shad | 2015 | **** | ** | *** |
| [84] | Strakowski | 1993 | **** | ** | *** |
| [85] | Altamura | 2016 | **** | ** | *** |
| [86] | Chye | 2017 | **** | ** | *** |
| [87] | Radoman | 2019 | **** | ** | *** |
| [88] | Osuch | 2016 | **** | ** | *** |
| [89] | Thayer | 2019 | **** | ** | *** |
| [90] | Nichols | 2021 | **** | ** | *** |
| [91] | Cornelius | 2010 | **** | ** | *** |
| [92] | Uhlmann | 2019 | **** | * | *** |
| [93] | Sjoerds | 2014 | **** | ** | *** |
| [94] | Zorlu | 2017 | **** | ** | *** |
| [95] | Kushnir | 2013 | **** | ** | *** |
| [96] | Li | 2017 | **** | ** | *** |
| [97] | Wong | 2015 | **** | ** | *** |
| [98] | Yi | 2019 | **** | ** | *** |
| [99] | Martins | 2018 | **** | ** | *** |
| [100] | Karch | 2008 | **** | ** | *** |
| [101] | Viswanath | 2015 | ** | ** | * |
| [102] | Kim | 2016 | **** | ** | *** |
| [103] | Harlé | 2020 | **** | * | ** |
| [104] | Schuff | 2008 | **** | ** | *** |
| Disorder Type | Substance | MRI Study Type | Regions of Interest | Findings |
|---|---|---|---|---|
| Schizophrenia | Cannabis | Volumetry | TBV * | Reduction in TB *, GM *, and WM * volumes |
| Cortical areas (left prefrontal, middle frontal, right fusiform, left superior gyrus, right supplementary motor cortex, inferior frontal, superior temporal, angular cortex, and parietal) | Reduction in total cortex volume | |||
| Amygdala, hippocampus, cerebellum | Reduction in TB * and GM * volume | |||
| Thalamus | Increased volume | |||
| Connectivity | Left thalamic radiation, left parahippocampal radiation, brainstem, internal capsule, corona radiata, superior and inferior longitudinal fasciculus; connectivity between nucleus accumbens and structures involved in reward circuits | Reduction in FA * | ||
| Left posterior corpus callosum, anterior internal capsule, fasciculus uncinatus, and frontal white matter; connectivity between prefrontal cortex and the precuneus | Increase in FA * | |||
| Nicotine | Volumetry | Cingular and insulate cortices | Total volume reduction | |
| Connectivity | TB-WM * | Reduction in FA * | ||
| Bilateral midline frontal cortices | Decreased activation | |||
| Right insula, cerebellum, right caudate nucleus, ventromedial PFC *. | Increased activation | |||
| Alcohol | Volumetry | TBV * | TBV * reduction | |
| Insula, medial and dorsolateral frontal cortex, ventrolateral prefrontal cortex, and parieto-occipital cortex; putamen, nucleus accumbens, and caudate nucleus; hippocampus, thalamus, striatum and globus pallidus; cerebellar vermis | Volume reduction | |||
| Connectivity | Broca’s area, Wernicke’s area, primary motor cortex, temporal fusiform gyrus, and anterior cingulate area | Increased activation | ||
| Stimulants | Volumetry | Left planum temporale | Reduced volume | |
| Connectivity | Subcortical structures and cerebral peduncles | Reduction in FA * | ||
| Spectroscopy | Medial PFC * | Reduction in NAA * and glutamate levels | ||
| Bipolar disorder | Cannabis | Volumetry | Left and right fusiform gyri, right middle frontal gyrus | GM * decrease |
| Right caudate nucleus and precentral gyrus | GM * increase | |||
| Spectroscopy | Ventrolateral PFC | NAA * increase | ||
| Nicotine | Volumetry | Left ACC | Decreased cortical thickness | |
| Alcohol | Volumetry | Prefrontal area; left superior frontal gyrus, right superior frontal gyrus, right insula, bilateral parieto-occipital cortices; cingulate cortex; hippocampus | Decreased GM * and WM * | |
| CSF * volumes | Decreased | |||
| Ventricle volumes | Increased | |||
| Spectroscopy | Left dorsolateral prefrontal cortex | Increased glutamate/glutamine | ||
| ACC * | Decreased glutamate; decreased GABA | |||
| Depression | Cannabis | Volumetry | Medial orbitofrontal cortex and superior frontal cortex | Decreased cortical thickness |
| Connectivity | Right caudate/temporal gyrus/parahippocampal gyrus circuit, right medial frontal gyrus, left culmen/fusiform gyrus area; right and left temporal, occipital, and fusiform cortices, right precuneus and culmen, right ACC *, and left superior frontal gyrus | Decreased connectivity | ||
| Left supramarginal gyrus | Increased connectivity | |||
| Amygdala | Increased activation with less cannabis consumption | |||
| Nicotine | Volumetry | Left hemisphere cortical thickness | Decreased volume in depressive smokers | |
| Connectivity | Frontal gyrus, superior temporal gyrus, hippocampus, and anterior cingulate | Increased activation after smoking | ||
| Alcohol | Volumetry | Overall cortical volume as well as in the lateral occipital cortex, medial orbitofrontal cortex, middle temporal cortex, and isthmus of the cingulate cortex | Increased GM * volume | |
| Connectivity | Thalamus and putamen | Increased activation | ||
| Opioids | Connectivity | Right superior frontal gyrus, supplementary motor area, paracingulate gyrus, frontal pole, precuneus, caudate, thalamus, posterior cingulate gyrus, left precentral gyrus, anterior cingulate cortex, middle frontal gyrus, and inferior frontal gyrus | Decreased activation | |
| Ketamine | Connectivity | Subgenual anterior cingulate cortex and the orbitofrontal cortex | Decreased connectivity | |
| Subgenual anterior cingulate cortex and bilateral superior temporal gyrus or dorsomedial prefrontal cortex | Increased connectivity | |||
| Anxiety | Alcohol | Volumetry | Lateral occipital cortex, medial orbitofrontal cortex, middle temporal cortex, isthmus of the cingulate cortex | Increased volume |
| Prefrontal cortical grey and white matter and cerebrospinal fluid volumes | Decreased volume in depressive smokers | |||
| Connectivity | Corpus callosum, internal capsule, corona radiata, superior parietal, lateral occipital, and posterior cingulate | Increased FA * | ||
| Right superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, precentral gyrus, thalamus, putamen, and insula | Increased activation | |||
| Heroin | Connectivity | Left uncinate fasciculus | Increased diffusivity | |
| PTSD | Alcohol | Connectivity | Parahippocampal gyrus | Increased activation |
| Hippocampus, anterior cingulate cortex | Decreased activation |
| Imaging Study 1 | Articles Included | % |
|---|---|---|
| Structural MRI | 55 | 52 |
| HMRS | 9 | 9 |
| FMRI | 28 | 26 |
| DWI | 14 | 13 |
| TOTAL | 106 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa-Moscoso, B.; Rivadeneira-Limongi, A.; Moncayo, F.; Loor-Vera, E.; Álvarez, D.; Vasquez Mena, L.G.; Rodas, J.A.; Leon-Rojas, J.E. Axis I Psychiatric Disorders and Substance Abuse: A Systematic Review of Neuroimaging Findings. J. Clin. Med. 2025, 14, 2156. https://doi.org/10.3390/jcm14072156
Sosa-Moscoso B, Rivadeneira-Limongi A, Moncayo F, Loor-Vera E, Álvarez D, Vasquez Mena LG, Rodas JA, Leon-Rojas JE. Axis I Psychiatric Disorders and Substance Abuse: A Systematic Review of Neuroimaging Findings. Journal of Clinical Medicine. 2025; 14(7):2156. https://doi.org/10.3390/jcm14072156
Chicago/Turabian StyleSosa-Moscoso, Bernardo, Alina Rivadeneira-Limongi, Filip Moncayo, Enrique Loor-Vera, Diana Álvarez, Lucia Geannett Vasquez Mena, Jose A. Rodas, and Jose E. Leon-Rojas. 2025. "Axis I Psychiatric Disorders and Substance Abuse: A Systematic Review of Neuroimaging Findings" Journal of Clinical Medicine 14, no. 7: 2156. https://doi.org/10.3390/jcm14072156
APA StyleSosa-Moscoso, B., Rivadeneira-Limongi, A., Moncayo, F., Loor-Vera, E., Álvarez, D., Vasquez Mena, L. G., Rodas, J. A., & Leon-Rojas, J. E. (2025). Axis I Psychiatric Disorders and Substance Abuse: A Systematic Review of Neuroimaging Findings. Journal of Clinical Medicine, 14(7), 2156. https://doi.org/10.3390/jcm14072156







