Biomarkers Affecting Treatment Outcomes of Febrile Neutropenia in Hematological Patients with Lymphomas: Is Presepsin the New Promising Diagnostic and Prognostic Biomarker?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Inclusion Criteria and Definitions
2.3. Clinical Assessments and Data Collection Procedures
- Patients with FN and confirmed infection (I group).
- Patients with FN without a confirmed infection (N group).
- Control group representing patients with neutropenic episodes (NEs) without fever (C group).
2.4. Biomarker Measurements
2.5. Statistical Analysis
3. Results
- Patients with FN with the proof of infection (“I” group; N = 23);
- Patients with FN without the proof of infection (“N” group; N = 14);
- Patients with NEs without the fever but with the established neutropenia diagnosis (“C” group; N = 18).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANC | Absolute neutrophil count |
| ALB | Albumin |
| APACHE II | Acute physiology and chronic health evaluation II |
| CBC | Complete blood count |
| CDI | Clostridioides difficile infection |
| CISNE | Clinical index of stable febrile neutropenia |
| CMIA | Chemiluminescent microparticle immunoassay |
| CVC | Central venous catheter |
| CRP | C-reactive protein |
| ECOG | Eastern Cooperative Oncology Group |
| ELISA | Enzyme-linked immunosorbent assay |
| FN | Febrile neutropenia |
| G-CSF | Granulocyte colony-stimulating factor |
| GNB | Gram-negative bacteria |
| GPB | Gram-positive bacteria |
| ICU | Intensive care unit |
| IgG | Immunoglobulin G |
| IQR | Interquartile range |
| MASCC | Multinational Association for Supportive Care in Cancer |
| MED | Median |
| mCD14 | Membrane-bound form of CD14 |
| NE | Neutropenic episode |
| NHL | Non-Hodgkin lymphoma |
| PCR | Polymerase chain reaction |
| PCT | Procalcitonin |
| PSP | Presepsin |
| qSOFA | Quick Sepsis-related organ failure assessment |
| SD | Standard deviation |
| sCD14-ST | Soluble subtype of CD14 |
| SOFA | Sequential organ failure assessment |
| UHM | University Hospital Merkur |
| WHO | World Health Organization |
References
- Flowers, C.R.; Seidenfeld, J.; Bow, E.J.; Karten, C.; Gleason, C.; Hawley, D.K.; Kuderer, N.M.; Langston, A.A.; Marr, K.A.; Rolston, K.V.; et al. Antimicrobial Prophylaxis and Outpatient Management of Fever and Neutropenia in Adults Treated for Malignancy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 794–810. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.A.; Wingard, J.R. ; Infectious Diseases Society of America. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- Baluch, A.; Shewayish, S. Neutropenic Fever. In Infections in Neutropenic Cancer Patients; Springer: Berlin/Heidelberg, Germany, 2019; pp. 105–117. [Google Scholar] [CrossRef]
- Peters, R.P.; van Agtmael, M.A.; Danner, S.A.; Savelkoul, P.H.; Vandenbroucke-Grauls, C.M. New Developments in the Diagnosis of Bloodstream Infections. Lancet Infect. Dis. 2004, 4, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Suberviola, B.; Márquez-López, A.; Castellanos-Ortega, A.; Fernández-Mazarrasa, C.; Santibáñez, M.; Martínez, L.M. Microbiological Diagnosis of Sepsis: Polymerase Chain Reaction System Versus Blood Cultures. Am. J. Crit. Care 2016, 25, 68–75. [Google Scholar] [CrossRef]
- Vincent, J.L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.R.; Payen, D.; et al. Sepsis in European Intensive Care Units: Results of the SOAP Study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef]
- Kostić, I.; Gurrieri, C.; Piva, E.; Semenzato, G.; Plebani, M.; Caputo, I.; Vianello, F. Comparison of Presepsin, Procalcitonin, Interleukin-8 and C-Reactive Protein in Predicting Bacteraemia in Febrile Neutropenic Adult Patients with Haematological Malignancies. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019047. [Google Scholar] [CrossRef]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum Procalcitonin and C-Reactive Protein Levels as Markers of Bacterial Infection: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- Singer, M. Biomarkers for Sepsis–Past, Present and Future. Qatar Med. J. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Tang, B.M.; Eslick, G.D.; Craig, J.C.; McLean, A.S. Accuracy of Procalcitonin for Sepsis Diagnosis in Critically Ill Patients: Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2007, 7, 210–217. [Google Scholar] [CrossRef]
- Lee, S.H.; Chan, R.C.; Wu, J.Y.; Chen, H.W.; Chang, S.S.; Lee, C.C. Diagnostic Value of Procalcitonin for Bacterial Infection in Elderly Patients—A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2013, 67, 1350–1357. [Google Scholar] [CrossRef]
- Vincent, J.L.; Beumier, M. Diagnostic and Prognostic Markers in Sepsis. Expert Rev. Anti-Infect. Ther. 2013, 11, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.T.; Jenyon, T.; van Hamel Parsons, V.; King, A.J. Neutropenic Sepsis: Management and Complications. Clin. Med. 2013, 13, 185–187. [Google Scholar] [CrossRef]
- Zanoni, I.; Granucci, F. Role of CD14 in Host Protection Against Infections and in Metabolism Regulation. Front. Cell. Infect. Microbiol. 2013, 3, 32. [Google Scholar] [CrossRef]
- Meuleman, P.; Steyaert, S.; Libbrecht, L.; Couvent, S.; Van Houtte, F.; Clinckspoor, F.; de Hemptinne, B.; Roskams, T.; Vanlandschoot, P.; Leroux-Roels, G. Human Hepatocytes Secrete Soluble CD14, a Process Not Directly Influenced by HBV and HCV Infection. Clin. Chim. Acta Int. J. Clin. Chem. 2006, 366, 156–162. [Google Scholar] [CrossRef]
- Memar, M.Y.; Baghi, H.B. Presepsin: A Promising Biomarker for the Detection of Bacterial Infections. Biomed. Pharmacother. 2019, 111, 649–656. [Google Scholar] [CrossRef]
- Chenevier-Gobeaux, C.; Bardet, V.; Poupet, H.; Poyart, C.; Borderie, D.; Claessens, Y.E. Presepsin (sCD14-ST) Secretion and Kinetics by Peripheral Blood Mononuclear Cells and Monocytic THP-1 Cell Line. Ann. De Biol. Clin. 2016, 74, 93–97. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Hinedi, K.; Khairallah, H.; Saadeh, B.; Abbasi, S.; Noureen, M.; Raza, S.; Alkhatti, A. Epidemiology and Source of Infection in Patients with Febrile Neutropenia: A Ten-Year Longitudinal Study. J. Infect. Public Health 2019, 12, 364–366. [Google Scholar] [CrossRef]
- Parodi, R.L.; Lagrutta, M.; Tortolo, M.; Navall, E.; Rodríguez, M.S.; Sasia, G.F.; De Candia, L.F.; Gruvman, M.A.; Bottasso, O.; Greca, A. A Multicenter Prospective Study of 515 Febrile Neutropenia Episodes in Argentina during a 5-Year Period. PLoS ONE 2019, 14, e0224299. [Google Scholar] [CrossRef]
- Shmuely, H.; Monely, L.; Shvidel, L. All-Cause Mortality and Its Predictors in Haemato-Oncology Patients with Febrile Neutropenia. J. Clin. Med. 2023, 12, 5635. [Google Scholar] [CrossRef]
- Schmidt, K.; Gensichen, J.; Fleischmann-Struzek, C.; Bahr, V.; Pausch, C.; Sakr, Y.; Reinhart, K.; Vollmar, H.C.; Thiel, P.; Scherag, A.; et al. Long-Term Survival Following Sepsis. Dtsch. Arztebl. Int. 2020, 117, 775–782. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Curr. Estim. Limit. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; Paesmans, M.; Rubenstein, E.B.; Boyer, M.; Elting, L.; Feld, R.; Gallagher, J.; Herrstedt, J.; Rapoport, B.; Rolston, K.; et al. The Multinational Association for Supportive Care in Cancer Risk Index: A Multinational Scoring System for Identifying Low-Risk Febrile Neutropenic Cancer Patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 3038–3051. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.V.; Emmich, M.; Knee, A.; Ali, F.; Walia, R.; Roychowdhury, P.; Clark, J.; Sridhar, A.; Lagu, T.; Loh, K.P. Use of MASCC Score in the Inpatient Management of Febrile Neutropenia: A Single-Center Retrospective Study. Support Care Cancer 2021, 29, 5905–5914. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Ramazani, J. Evaluation of Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment Scoring Systems for Prognostication of Outcomes among Intensive Care Unit Patients. Saudi J. Anaesth. 2016, 10, 168–173. [Google Scholar] [CrossRef]
- Deepak, C.L.; Bhat, S.S. Prediction of Outcome in Patients with Sepsis Using C-Reactive Protein & APACHE II Scoring System. Iosr J. Dent. Med. Sci. 2014, 13, 17–20. [Google Scholar] [CrossRef]
- Baldirà, J.; Ruiz-Rodríguez, J.C.; Ruiz-Sanmartin, A.; Chiscano, L.; Cortes, A.; Sistac, D.Á.; Ferrer-Costa, R.; Comas, I.; Villena, Y.; Larrosa, M.N.; et al. Use of Biomarkers to Improve 28-Day Mortality Stratification in Patients with Sepsis and SOFA ≤ 6. Biomedicines 2023, 11, 2149. [Google Scholar] [CrossRef]
- Aliu-Bejta, A.; Kurshumliu, M.; Namani, S.; Dreshaj, S.; Baršić, B. Ability of Presepsin Concentrations to Predict Mortality in Adult Patients with Sepsis. J. Clin. Transl. Sci. 2023, 7, e121. [Google Scholar] [CrossRef]
- Yang, H.S.; Hur, M.; Yi, A.; Kim, H.; Lee, S.; Kim, S.N. Prognostic Value of Presepsin in Adult Patients with Sepsis: Systematic Review and Meta-Analysis. PLoS ONE 2018, 13, e0191486. [Google Scholar] [CrossRef]
- Paraskevas, T.; Chourpiliadi, C.; Demiri, S.; Micahilides, C.; Karanikolas, E.; Lagadinou, M.; Velissaris, D. Presepsin in the Diagnosis of Sepsis. Clin. Chim. Acta 2023, 550, 117588. [Google Scholar] [CrossRef]
- Minuk, L.A.; Monkman, K.; Chin-Yee, I.H.; Lazo-Langner, A.; Bhagirath, V.; Chin-Yee, B.H.; Mangel, J.E. Treatment of Hodgkin Lymphoma with Adriamycin, Bleomycin, Vinblastine and Dacarbazine without Routine Granulocyte-Colony Stimulating Factor Support Does Not Increase the Risk of Febrile Neutropenia: A Prospective Cohort Study. Leuk. Lymphoma 2012, 53, 57–63. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Dale, D.C.; Crawford, J.; Cosler, L.E.; Lyman, G.H. Mortality, Morbidity, and Cost Associated with Febrile Neutropenia in Adult Cancer Patients. Cancer 2006, 106, 2258–2266. [Google Scholar] [CrossRef] [PubMed]
- Arıkan, K.; Karadag-Oncel, E.; Aytac, S.; Cetin, M.; Cengiz, A.B.; Gümrük, F.; Kara, A.; Ceyhan, M. Usage of Plasma Presepsin, C-Reactive Protein, Procalcitonin and Proadrenomedullin to Predict Bacteremia in Febril Neutropenia of Pediatric Hematological Malignancy Patients. Lab. Med. 2021, 52, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Chenevier-Gobeaux, C.; Borderie, D.; Weiss, N.; Mallet-Coste, T.; Claessens, Y.E. Presepsin (sCD14-ST), an Innate Immune Response Marker in Sepsis. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 450, 97–103. [Google Scholar] [CrossRef]
- Moustafa, R.; Albouni, T.; Aziz, G. The Role of Procalcitonin and Presepsin in the Septic Febrile Neutropenia in Acute Leukemia Patients. PLoS ONE 2021, 16, e0253842. [Google Scholar] [CrossRef]
- Behnes, M.; Bertsch, T.; Lepiorz, D.; Lang, S.; Trinkmann, F.; Brueckmann, M.; Borggrefe, M.; Hoffmann, U. Diagnostic and Prognostic Utility of Soluble CD14 Subtype (Presepsin) for Severe Sepsis and Septic Shock During the First Week of Intensive Care Treatment. Crit. Care 2014, 18, 507. [Google Scholar] [CrossRef]
- Masson, S.; Caironi, P.; Spanuth, E.; Thomae, R.; Panigada, M.; Sangiorgi, G.; Fumagalli, R.; Mauri, T.; Isgrò, S.; Fanizza, C.; et al. ALBIOS Study Investigators. Presepsin (Soluble CD14 Subtype) and Procalcitonin Levels for Mortality Prediction in Sepsis: Data from the Albumin Italian Outcome Sepsis Trial. Crit. Care 2014, 18, R6. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, Y.; Liang, X.; Gui, S.; Ren, D.; Liu, Y.; She, J.; Zhang, X.; Song, F.; Yu, L.; et al. Elevations in Presepsin, PCT, hs-CRP, and IL-6 Levels Predict Mortality Among Septic Patients in the ICU. J. Leukoc. Biol. 2024, 116, 890–900. [Google Scholar] [CrossRef]
- Velissaris, D.; Zareifopoulos, N.; Karamouzos, V.; Karanikolas, E.; Pierrakos, C.; Koniari, I.; Karanikolas, M. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus 2021, 13, e15019. [Google Scholar] [CrossRef]
- Enguix-Armada, A.; Escobar-Conesa, R.; García-De La Torre, A.; De La Torre-Prados, M.V. Usefulness of Several Biomarkers in the Management of Septic Patients: C-Reactive Protein, Procalcitonin, Presepsin and Mid-Regional Pro-Adrenomedullin. Clin. Chem. Lab. Med. 2016, 54, 163–168. [Google Scholar] [CrossRef]
- Kim, H.; Hur, M.; Moon, H.W.; Yun, Y.M.; Di Somma, S.; GREAT Network. Multi-Marker Approach Using Procalcitonin, Presepsin, Galectin-3, and Soluble Suppression of Tumorigenicity 2 for the Prediction of Mortality in Sepsis. Ann. Intensive Care 2017, 7, 27. [Google Scholar] [CrossRef]
- Molano-Franco, D.; Arevalo-Rodriguez, I.; Muriel, A.; del Campo-Albendea, L.; Fernandez-Garcia, S.; Alvarez-Méndez, A.; Simancas-Racines, D.; Viteri, A.; Sanchez, G.; Fernandez-Felix, B.; et al. Basal Procalcitonin, C-Reactive Protein, Interleukin-6, and Presepsin for Prediction of Mortality in Critically Ill Septic Patients: A Systematic Review and Meta-Analysis. Diagn. Progn. Res. 2023, 7, 15. [Google Scholar] [CrossRef]
- Abdelshafey, E.E.; Nasa, P.; Elgohary, A.E.; Khalil, M.F.; Rashwan, M.A.; Ghezala, H.B.; Tayar, A.A. Role of Presepsin for the Diagnosis of Sepsis and ICU Mortality: A Prospective Controlled Study. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2021, 25, 153–157. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, K.H.; Jeong, N.R.; Kim, S.C.; Oh, E.J. The Association between Dynamic Changes in Serum Presepsin Levels and Mortality in Immunocompromised Patients with Sepsis: A Prospective Cohort Study. Diagnostics 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Klouche, K.; Cristol, J.P.; Devin, J.; Gilles, V.; Kuster, N.; Larcher, R.; Amigues, L.; Corne, P.; Jonquet, O.; Dupuy, A.M. Diagnostic and Prognostic Value of Soluble CD14 Subtype (Presepsin) for Sepsis and Community-Acquired Pneumonia in ICU Patients. Ann. Intensive Care 2016, 6, 59. [Google Scholar] [CrossRef]
- Babel, J.; Košuta, I.; Vujaklija Brajković, A.; Lončar Vrančić, A.; Premužić, V.; Rogić, D.; Duraković, N. Early Fever in Allogeneic Stem Cell Transplantation: Are Presepsin and YKL-40 Valuable Diagnostic Tools? J. Clin. Med. 2024, 13, 5991. [Google Scholar] [CrossRef]
- Handke, J.; Piazza, O.; Larmann, J.; Tesoro, S.; De Robertis, E. Presepsin as a Biomarker in Perioperative Medicine. Minerva Anestesiol. 2020, 86, 768–776. [Google Scholar] [CrossRef]

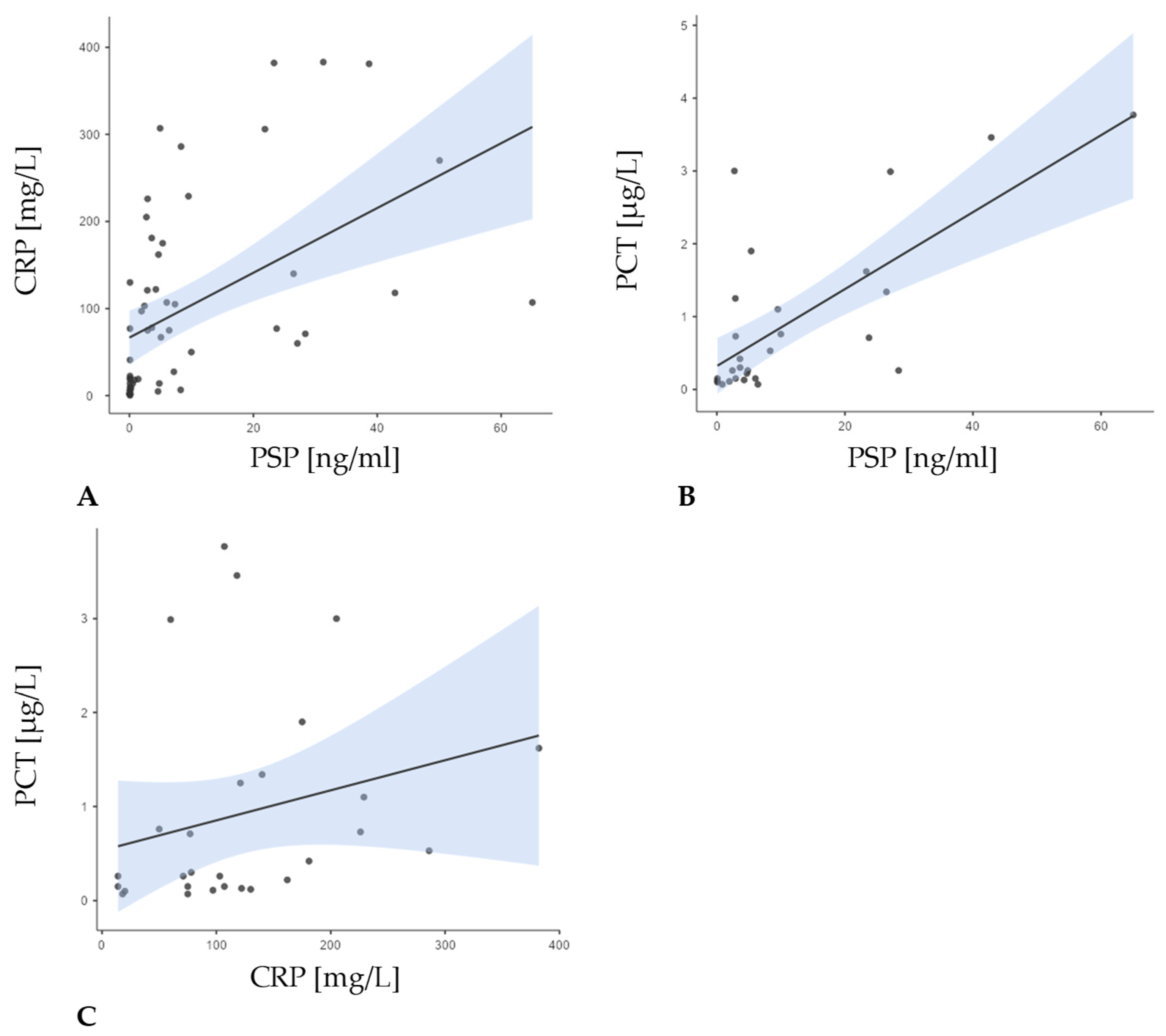
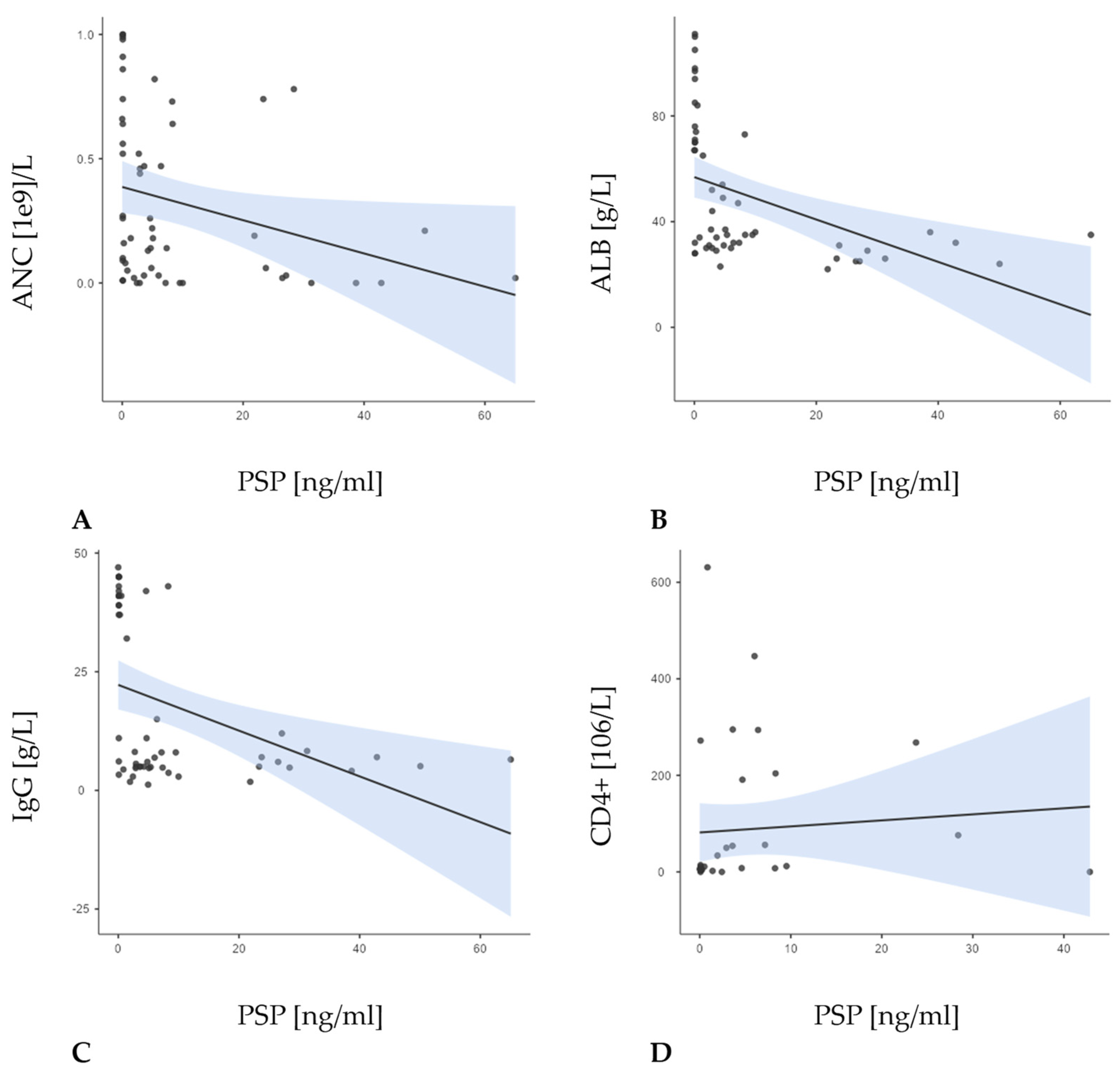


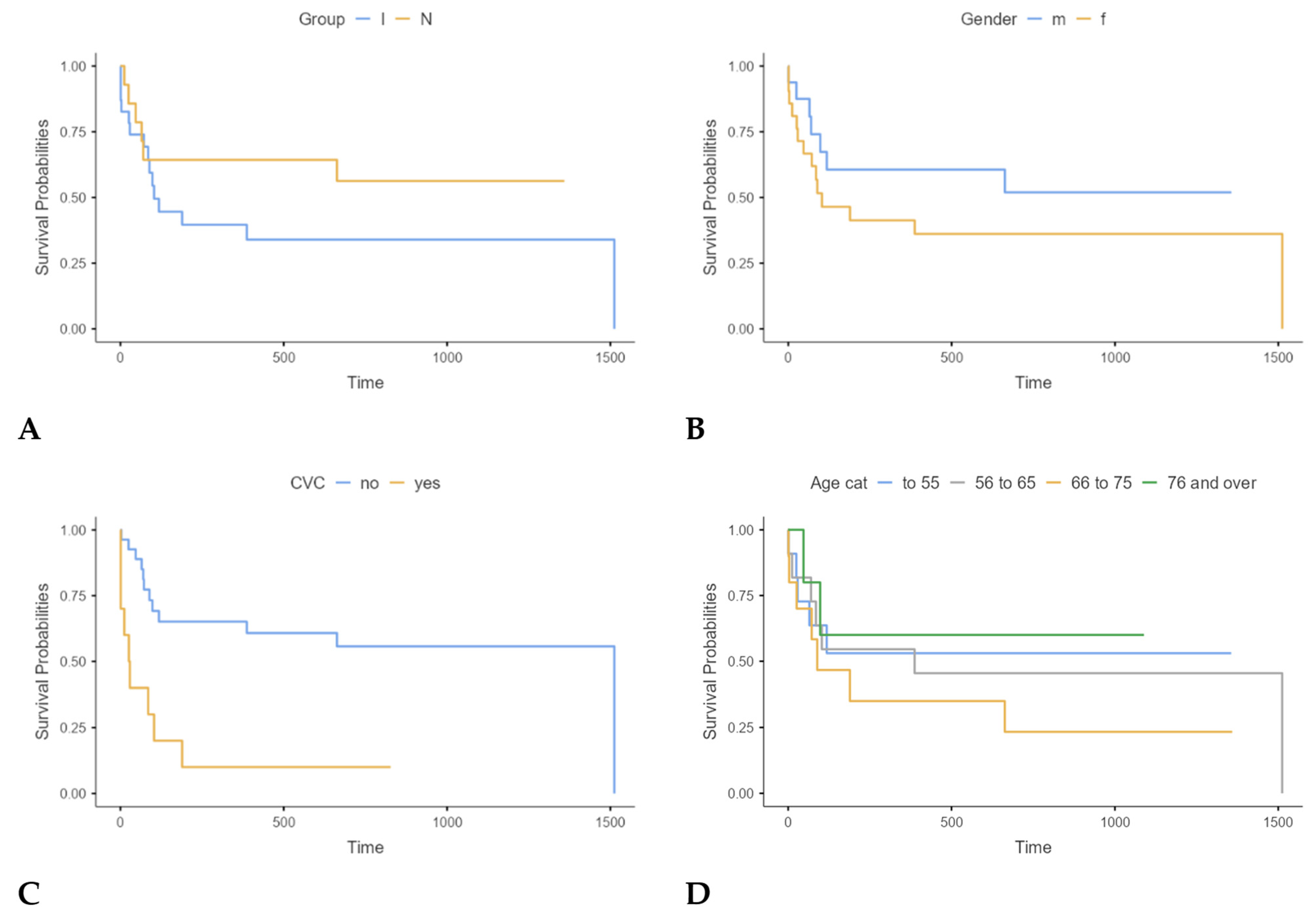
| Patients with FN and Proven Infection (Group I) (n = 23; 41.8%) | Patients with FN Without Proven Infection (Group N) (n = 14; 25.5%) | Patients with NEs (Group C) (n = 18; 32.7%) | Total (n = 55; 100.0%) | p-Value | |
|---|---|---|---|---|---|
| Male Female | 8 (34.8%) 15 (65.2%) | 8 (57.1%) 6 (42.9%) | 10 (55.6%) 8 (44.4%) | 26 (47.3%) 29 (52.7%) | 0.289 |
| ECOG 0 ECOG ≥ 1 | 8 (34.8%) 15 (65.2%) | 10 (71.4%) 4 (28.6%) | 12 (66.7%) 6 (33.3%) | 30 (54.5%) 25 (45.5%) | 0.043 |
| IgG < 4 g/L | 5 (21.7%) | 2 (14.3%) | 1 (5.6%) | 8 (14.5%) | 0.345 |
| CVC | 0.139 | ||||
| 8 (34.8%) | 2 (14.3%) | 2 (11.1%) | 12 (21.8%) | |
| 15 (65.2%) | 12 (85.7%) | 16 (88.9%) | 43 (78.2%) | |
| MASCC score ≥ 21 | 1 (4.3%) | 3 (21.4%) | 0 (0.0%) | 4 (10.8%) | - |
| qSOFA ≥ 2 | 14 (60.9%) | 7 (50%) | 0 (0.0%) | 21 (56.8%) | - |
| Use of G-CSF following the protocol | 0.127 | ||||
| 17 (73.9%) | 7 (50.0%) | 8 (44.4%) | 32 (58.2%) | |
| 6 (26.1%) | 7 (50.0%) | 10 (55.6%) | 23 (41.8%) | |
| Use of antimicrobial prophylaxis | 0.786 | ||||
| 22 (95.7%) | 11 (78.6%) | 18 (100.0%) | 51 (92.7%) | |
| 12 (52.2%) | 6 (42.9%) | 14 (77.8%) | 32 (58.2%) | |
| 11 (47.8%) | 4 (28.6%) | 5 (27.8%) | 20 (36.4%) | |
| 4 (17.4%) | 1 (7.1%) | 0 (0.0%) | 5 (9.1%) | |
| Subtype of lymphoproliferative disease: | 0.819 | ||||
| 21 (91.3%) | 13 (92.9%) | 18 (100.0%) | 52 (94.5%) | |
| 2 (8.7%) | 1 (7.1%) | 0 (0.0%) | 3 (5.5%) | |
| Line of therapy | 0.109 | ||||
| 14 (60.9%) | 5 (35.7%) | 13 (72.2%) | 32 (58.2%) | |
| 9 (39.1%) | 9 (64.3%) | 5 (27.8%) | 23 (41.8%) | |
| No comorbidities | 13 (56.5%) | 12 (85.7%) | 4 (22.2%) | 29 (52.7%) | 0.178 |
| Line of Therapy | Patients with FN N (%) | Patients with Nes N (%) |
|---|---|---|
| 1 | 19 (51.4) | 13 (72.2) |
| 2 | 5 (13.5) | 3 (16.7) |
| 3 | 6 (16.2) | 2 (11.1) |
| 4 | 2 (5.4) | 0 (0.0) |
| 5 | 5 (13.5) | 0 (0.0) |
| FN with Proven Infection | (Group I, N = 23) |
|---|---|
| Only microbiologically documented infection | (N = 11; 47.9%) |
| 4 (17.4%) |
| 1 (4.3%) |
| 1 (4.3%) |
| 1 (4.3%) |
| 1 (4.3%) |
| 2 (8.7%) |
| 1 (4.3%) |
| 1 (4.3%) |
| 2 (8.7%) |
| 1 (4.3%) |
| 1 (4.3%) |
| 3 (13.0%) |
| 1 (4.3%) |
| 2 (8.7%) |
| Only radiologically documented infection | (N = 7; 30.4%) |
| 6 (26.1%) |
| 1 (4.3%) |
| Microbiologically and radiologically documented infection | (N = 5; 21.7%) |
| 1 (4.3%) |
| 2 (8.7%) |
| 2 (8.7%) |
| PSP Levels [ng/mL] on Day 1 | |||||||
|---|---|---|---|---|---|---|---|
| Mortality Outcome | Group | N | Mean | Median | SD | Minimum | Maximum |
| yes | I | 15 | 23.622 | 26.4900 | 19.824 | 0.8500 | 65.04 |
| N | 6 | 4.612 | 4.1450 | 3.670 | 0.0900 | 9.99 | |
| C | 3 | 0.587 | 0.2600 | 0.718 | 0.0900 | 1.41 | |
| no | I | 8 | 7.164 | 5.6150 | 7.029 | 0.0900 | 23.34 |
| N | 8 | 5.981 | 3.2800 | 7.785 | 0.0900 | 23.76 | |
| C | 15 | 0.959 | 0.0900 | 2.333 | 0.0000 | 8.27 | |
| Mortality Days | N | % |
|---|---|---|
| up to 30 | 8 | 21.6% |
| 31–90 | 8 | 21.6% |
| 91–180 | 3 | 8.1% |
| 181–365 | 2 | 5.4% |
| 366 and more | 16 | 43.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mišura Jakobac, K.; Milunović, V.; Kušec, V.; Hrabač, P.; Martinović, M.; Radić-Krišto, D.; Ostojić Kolonić, S.; Pavliša, G. Biomarkers Affecting Treatment Outcomes of Febrile Neutropenia in Hematological Patients with Lymphomas: Is Presepsin the New Promising Diagnostic and Prognostic Biomarker? J. Clin. Med. 2025, 14, 2238. https://doi.org/10.3390/jcm14072238
Mišura Jakobac K, Milunović V, Kušec V, Hrabač P, Martinović M, Radić-Krišto D, Ostojić Kolonić S, Pavliša G. Biomarkers Affecting Treatment Outcomes of Febrile Neutropenia in Hematological Patients with Lymphomas: Is Presepsin the New Promising Diagnostic and Prognostic Biomarker? Journal of Clinical Medicine. 2025; 14(7):2238. https://doi.org/10.3390/jcm14072238
Chicago/Turabian StyleMišura Jakobac, Karla, Vibor Milunović, Vesna Kušec, Pero Hrabač, Marko Martinović, Delfa Radić-Krišto, Slobodanka Ostojić Kolonić, and Gordana Pavliša. 2025. "Biomarkers Affecting Treatment Outcomes of Febrile Neutropenia in Hematological Patients with Lymphomas: Is Presepsin the New Promising Diagnostic and Prognostic Biomarker?" Journal of Clinical Medicine 14, no. 7: 2238. https://doi.org/10.3390/jcm14072238
APA StyleMišura Jakobac, K., Milunović, V., Kušec, V., Hrabač, P., Martinović, M., Radić-Krišto, D., Ostojić Kolonić, S., & Pavliša, G. (2025). Biomarkers Affecting Treatment Outcomes of Febrile Neutropenia in Hematological Patients with Lymphomas: Is Presepsin the New Promising Diagnostic and Prognostic Biomarker? Journal of Clinical Medicine, 14(7), 2238. https://doi.org/10.3390/jcm14072238







