Dual-Energy Computed Tomography, a New Metal Artifact Reduction Technique for Total Hip Arthroplasty: Is There a Light in the Darkness?
Abstract
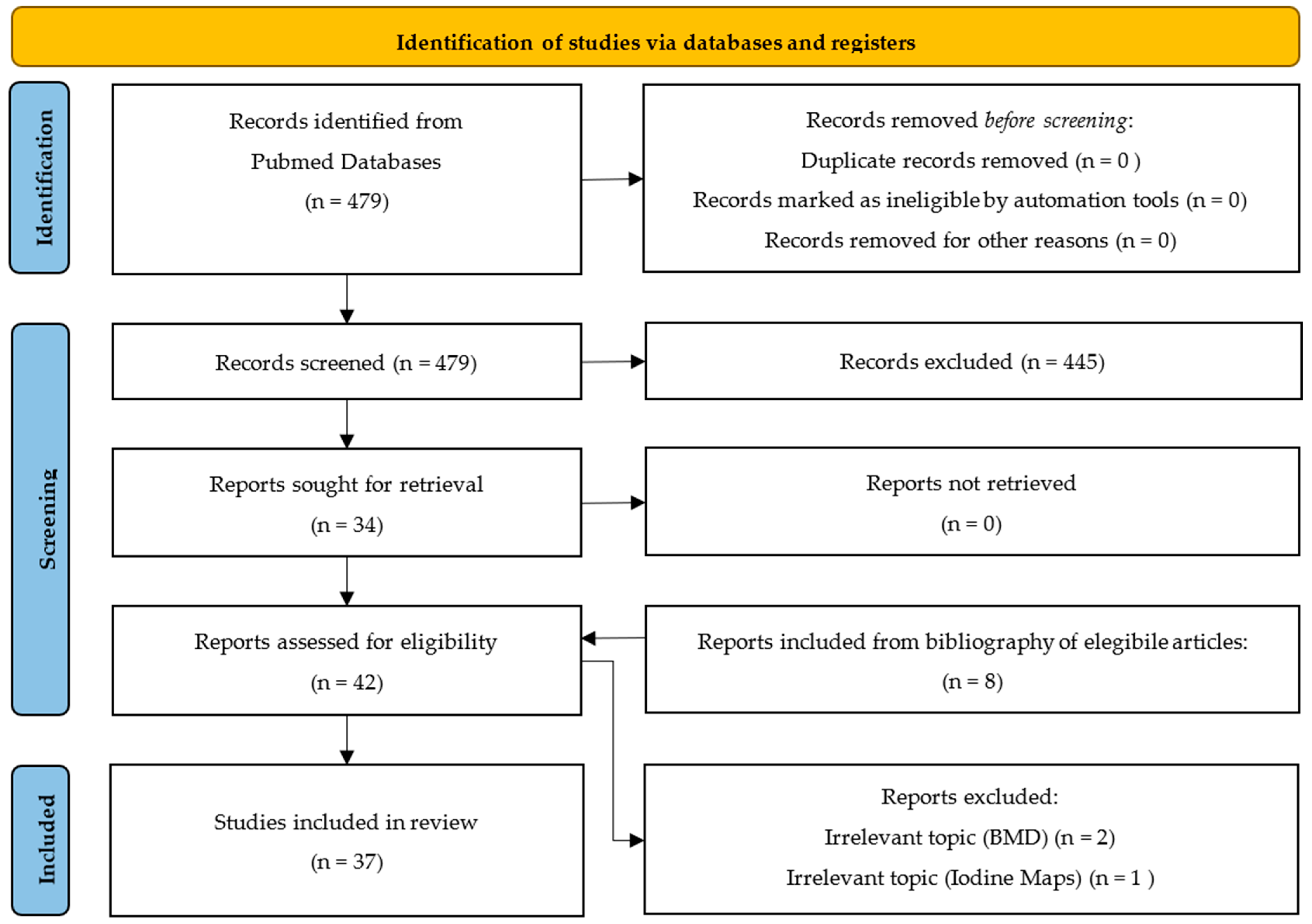
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Visualization of Periprosthetic Bone
4.1.1. Ex Vivo
4.1.2. In Vivo
4.2. Visualization of Pelvic Organs/Periprosthetic Soft Tissue and Vascularization
4.2.1. Ex Vivo
4.2.2. In Vivo
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Conventional images |
| CT | Computed tomography |
| DECT | Dual-energy CT |
| Fe | Iron |
| iMAR | Iterative metal artifact reduction |
| KeV | Kilo-electronVolts |
| MAR | Metal artifact reduction |
| PMI | Pseudo-monochromatic imaging |
| Sn | Tin (Stannum) |
| THA | Total hip arthroplasty |
| Ti | Titanium |
| VMI | Virtual monochromatic imaging |
References
- Piacentino, F.; Fontana, F.; Zorzetto, G.; Saccomanno, A.; Gatta, T.; Recaldini, C.; Franzi, F.; Imperatori, A.; Rotolo, N.; Coppola, A.; et al. Dual-Layer Spectral CT as Innovative Imaging Guidance in Lung Biopsies: Could Color-Coded Z-Effective Images Allow More Diagnostic Samplings and Biomarkers Information? J. Clin. Med. 2023, 12, 7426. [Google Scholar] [CrossRef]
- Curti, M.; Fontana, F.; Piacentino, F.; Ossola, C.; Coppola, A.; Carcano, G.; Venturini, M. Dual-Layer Spectral CT Fusion Imaging for Lung Biopsies: More Accurate Targets, Diagnostic Samplings, and Biomarker Information? Eur. Radiol. Exp. 2022, 6, 34. [Google Scholar] [CrossRef]
- Fontana, F.; Piacentino, F.; Gnesutta, A.; Macchi, E.; Coppola, A.; Saccomanno, A.; Gatta, T.; Recaldini, C.; Minenna, M.; Tamborini, C.; et al. Transcatheter Aortic Valve Implantation (TAVI) Planning with Dual-Layer Spectral CT Using Virtual Monoenergetic Image (VMI) Reconstructions and 20 mL of Contrast Media. J. Clin. Med. 2024, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Zorzetto, G.; Coppola, A.; Molinelli, V.; Angeretti, M.G.; Casarin, J.; Fontana, F.; Piacentino, F.; Carcano, G.; Ghezzi, F.; Venturini, M. Spectral CT in Peritoneal Carcinomatosis from Ovarian Cancer: A Tool for Differential Diagnosis of Small Nodules? Eur. Radiol. Exp. 2022, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, G.M.; Ascenti, V.; Barbera, S.; Fontana, F.; Aricò, F.M.; Piacentino, F.; Coppola, A.; Cicero, G.; Marino, M.A.; Booz, C.; et al. Virtual Non-Contrast Spectral CT in Renal Masses: Is It Time to Discard Conventional Unenhanced Phase? J. Clin. Med. 2023, 12, 4718. [Google Scholar] [CrossRef] [PubMed]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? AJR Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, Y.; Li, F.; Li, X.; Qin, L.; Huang, W. Dual-Energy Computed Tomography Iodine Maps: Application in the Diagnosis of Periprosthetic Joint Infection in Total Hip Arthroplasty. J. Arthroplast. 2025, 40, 499–505. [Google Scholar] [CrossRef]
- Cheong, S.C.W.; Yan, Y.Y.; Sheikh, A.; Ouellette, H.A.; Munk, P.L.; Murray, N.; Mallinson, P.I. Dual-Energy CT Applications in Musculoskeletal Disorders. Br. J. Radiol. 2024, 97, 705–715. [Google Scholar] [CrossRef]
- Mushtaq, N.; To, K.; Gooding, C.; Khan, W. Radiological Imaging Evaluation of the Failing Total Hip Replacement. Front. Surg. 2019, 6, 35. [Google Scholar] [CrossRef]
- Ferguson, R.J.; Palmer, A.J.; Taylor, A.; Porter, M.L.; Malchau, H.; Glyn-Jones, S. Hip Replacement. Lancet 2018, 392, 1662–1671. [Google Scholar] [CrossRef]
- Conti, D.; Baruffaldi, F.; Erani, P.; Festa, A.; Durante, S.; Santoro, M. Dual-Energy Computed Tomography Applications to Reduce Metal Artifacts in Hip Prostheses: A Phantom Study. Diagnostics 2022, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Kuchenbecker, S.; Faby, S.; Sawall, S.; Lell, M.; Kachelrieß, M. Dual Energy CT: How Well Can Pseudo-Monochromatic Imaging Reduce Metal Artifacts? Med. Phys. 2015, 42, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Pawałowski, B.; Panek, R.; Szweda, H.; Piotrowski, T. Combination of Dual-Energy Computed Tomography and Iterative Metal Artefact Reduction to Increase General Quality of Imaging for Radiotherapy Patients with High Dense Materials. Phantom Study. Phys. Med. 2020, 77, 92–99. [Google Scholar] [CrossRef]
- Dwyer, A.; Korlaet, M.; Callary, S.A.; Robertson, T.; Smitham, P.; Solomon, L.B. Impact of Computed Tomography Metal Artifact Reduction Protocol on Periprosthetic Tissue Characterization after Total Hip Arthroplasty: A Cadaveric Study. J. Orthop. Res. 2023, 41, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Barreto, I.; Pepin, E.; Davis, I.; Dean, C.; Massini, T.; Rees, J.; Olguin, C.; Quails, N.; Correa, N.; Rill, L.; et al. Comparison of Metal Artifact Reduction Using Single-Energy CT and Dual-Energy CT with Various Metallic Implants in Cadavers. Eur. J. Radiol. 2020, 133, 109357. [Google Scholar] [CrossRef]
- Ishikawa, T.; Suzuki, S.; Harashima, S.; Fukui, R.; Kaiume, M.; Katada, Y. Metal Artifacts Reduction in Computed Tomography: A Phantom Study to Compare the Effectiveness of Metal Artifact Reduction Algorithm, Model-Based Iterative Reconstruction, and Virtual Monochromatic Imaging. Medicine 2020, 99, e23692. [Google Scholar] [CrossRef]
- Higashigaito, K.; Angst, F.; Runge, V.M.; Alkadhi, H.; Donati, O.F. Metal Artifact Reduction in Pelvic Computed Tomography With Hip Prostheses: Comparison of Virtual Monoenergetic Extrapolations From Dual-Energy Computed Tomography and an Iterative Metal Artifact Reduction Algorithm in a Phantom Study. Investig. Radiol. 2015, 50, 828–834. [Google Scholar] [CrossRef]
- Huflage, H.; Grunz, J.-P.; Hackenbroch, C.; Halt, D.; Luetkens, K.S.; Alfred Schmidt, A.M.; Patzer, T.S.; Ergün, S.; Bley, T.A.; Kunz, A.S. Metal Artefact Reduction in Low-Dose Computed Tomography: Benefits of Tin Prefiltration versus Postprocessing of Dual-Energy Datasets over Conventional CT Imaging. Radiography 2022, 28, 690–696. [Google Scholar] [CrossRef]
- Schwarz, G.M.; Huber, S.; Wassipaul, C.; Kasparek, M.; Hirtler, L.; Hofstaetter, J.G.; Bader, T.; Ringl, H. Influence of Scan Parameters of Single and Dual-Energy CT Protocols in Combination with Metal Artifact Suppression Algorithms for THA: An Ex Vivo Study. J. Bone Jt. Surg. Am. 2023, 105, 620–629. [Google Scholar] [CrossRef]
- Selles, M.; Stuivenberg, V.H.; Wellenberg, R.H.H.; van de Riet, L.; Nijholt, I.M.; van Osch, J.A.C.; van Hamersvelt, R.W.; Leiner, T.; Boomsma, M.F. Quantitative Analysis of Metal Artifact Reduction in Total Hip Arthroplasty Using Virtual Monochromatic Imaging and Orthopedic Metal Artifact Reduction, a Phantom Study. Insights Imaging 2021, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K.M.; Nowik, P.; Persliden, J.; Thunberg, P.; Norrman, E. Metal Artefact Reduction in CT Imaging of Hip Prostheses—An Evaluation of Commercial Techniques Provided by Four Vendors. Br. J. Radiol. 2015, 88, 20140473. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, E.; Bäck, A.; Thilander-Klang, A. Comparison of metal artefacts for different dual energy CT techniques. Radiat. Prot. Dosim. 2021, 195, 232–245. [Google Scholar] [CrossRef]
- Wellenberg, R.H.H.; Boomsma, M.F.; van Osch, J.a.C.; Vlassenbroek, A.; Milles, J.; Edens, M.A.; Streekstra, G.J.; Slump, C.H.; Maas, M. Quantifying Metal Artefact Reduction Using Virtual Monochromatic Dual-Layer Detector Spectral CT Imaging in Unilateral and Bilateral Total Hip Prostheses. Eur. J. Radiol. 2017, 88, 61–70. [Google Scholar] [CrossRef]
- Lewis, M.; Reid, K.; Toms, A.P. Reducing the Effects of Metal Artefact Using High keV Monoenergetic Reconstruction of Dual Energy CT (DECT) in Hip Replacements. Skelet. Radiol. 2013, 42, 275–282. [Google Scholar] [CrossRef]
- Vellarackal, A.J.; Kaim, A.H. Metal Artefact Reduction of Different Alloys with Dual Energy Computed Tomography (DECT). Sci. Rep. 2021, 11, 2211. [Google Scholar] [CrossRef]
- Yoo, H.J.; Hong, S.H.; Choi, J.-Y.; Chae, H.D. Comparison of Metal Artifact Reduction Algorithms in Patients with Hip Prostheses: Virtual Monoenergetic Images vs. Orthopedic Metal Artifact Reduction. J. Korean Soc. Radiol. 2022, 83, 1286–1297. [Google Scholar] [CrossRef]
- Yue, D.; Fan Rong, C.; Ning, C.; Liang, H.; Ai Lian, L.; Ru Xin, W.; Ya Hong, L. Reduction of Metal Artifacts from Unilateral Hip Arthroplasty on Dual-Energy CT with Metal Artifact Reduction Software. Acta Radiol. 2018, 59, 853–860. [Google Scholar] [CrossRef]
- Laukamp, K.R.; Lennartz, S.; Neuhaus, V.-F.; Große Hokamp, N.; Rau, R.; Le Blanc, M.; Abdullayev, N.; Mpotsaris, A.; Maintz, D.; Borggrefe, J. CT Metal Artifacts in Patients with Total Hip Replacements: For Artifact Reduction Monoenergetic Reconstructions and Post-Processing Algorithms Are Both Efficient but Not Similar. Eur. Radiol. 2018, 28, 4524–4533. [Google Scholar] [CrossRef]
- Neuhaus, V.; Grosse Hokamp, N.; Zopfs, D.; Laukamp, K.; Lennartz, S.; Abdullayev, N.; Maintz, D.; Borggrefe, J. Reducing Artifacts from Total Hip Replacements in Dual Layer Detector CT: Combination of Virtual Monoenergetic Images and Orthopedic Metal Artifact Reduction. Eur. J. Radiol. 2019, 111, 14–20. [Google Scholar] [CrossRef]
- Bongers, M.N.; Schabel, C.; Thomas, C.; Raupach, R.; Notohamiprodjo, M.; Nikolaou, K.; Bamberg, F. Comparison and Combination of Dual-Energy- and Iterative-Based Metal Artefact Reduction on Hip Prosthesis and Dental Implants. PLoS ONE 2015, 10, e0143584. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, K.K.; Song, H.-T.; Kim, S.; Suh, J.-S. Metal Artefact Reduction in Gemstone Spectral Imaging Dual-Energy CT with and without Metal Artefact Reduction Software. Eur. Radiol. 2012, 22, 1331–1340. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, H.-J.; Oh, E.; Cha, J.G.; Hwang, J.; Hong, S.S.; Chang, Y.W. Visibility of Bony Structures around Hip Prostheses in Dual-Energy CT: With or without Metal Artefact Reduction Software. J. Med. Imaging Radiat. Oncol. 2018, 62, 634–641. [Google Scholar] [CrossRef]
- Guziński, M.; Kubicki, K.; Waszczuk, Ł.; Morawska-Kochman, M.; Kochman, A.; Sąsiadek, M. Dual-Energy Computed Tomography in Loosening of Revision Hip Prosthesis: A Comparison Between MARS and Non-MARS Images. J. Comput. Assist. Tomogr. 2019, 43, 379–385. [Google Scholar] [CrossRef]
- Meinel, F.G.; Bischoff, B.; Zhang, Q.; Bamberg, F.; Reiser, M.F.; Johnson, T.R.C. Metal Artifact Reduction by Dual-Energy Computed Tomography Using Energetic Extrapolation: A Systematically Optimized Protocol. Investig. Radiol. 2012, 47, 406–414. [Google Scholar] [CrossRef]
- Kosmas, C.; Hojjati, M.; Young, P.C.; Abedi, A.; Gholamrezanezhad, A.; Rajiah, P. Dual-Layer Spectral Computerized Tomography for Metal Artifact Reduction: Small versus Large Orthopedic Devices. Skelet. Radiol. 2019, 48, 1981–1990. [Google Scholar] [CrossRef]
- Magarelli, N.; De Santis, V.; Marziali, G.; Menghi, A.; Burrofato, A.; Pedone, L.; Del Prete, D.; Iezzi, R.; de Waure, C.; D’andrea, M.; et al. Application and Advantages of Monoenergetic Reconstruction Images for the Reduction of Metallic Artifacts Using Dual-Energy CT in Knee and Hip Prostheses. Radiol. Med. 2018, 123, 593–600. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, Y.E.; Luo, S.; Shi, H.; Li, L.; Zheng, L.; Zhang, L.J.; Lu, G. Monoenergetic Imaging of Dual-Energy CT Reduces Artifacts from Implanted Metal Orthopedic Devices in Patients with Factures. Acad. Radiol. 2011, 18, 1252–1257. [Google Scholar] [CrossRef]
- Foti, G.; Fighera, A.; Campacci, A.; Natali, S.; Guerriero, M.; Zorzi, C.; Carbognin, G. Diagnostic Performance of Dual-Energy CT for Detecting Painful Hip Prosthesis Loosening. Radiology 2021, 300, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.G.; Rechner, L.A.; Appelt, A.L.; Berthelsen, A.K.; Costa, J.C.; Friborg, J.; Persson, G.F.; Bangsgaard, J.P.; Specht, L.; Aznar, M.C. Metal Artefact Reduction for Accurate Tumour Delineation in Radiotherapy. Radiother. Oncol. 2018, 126, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Filograna, L.; Magarelli, N.; Leone, A.; Guggenberger, R.; Winklhofer, S.; Thali, M.J.; Bonomo, L. Value of Monoenergetic Dual-Energy CT (DECT) for Artefact Reduction from Metallic Orthopedic Implants in Post-Mortem Studies. Skelet. Radiol. 2015, 44, 1287–1294. [Google Scholar] [CrossRef]
- Wichtmann, H.M.; Laukamp, K.R.; Manneck, S.; Appelt, K.; Stieltjes, B.; Boll, D.T.; Benz, M.R.; Obmann, M.M. Metal Implants on Abdominal CT: Does Split-Filter Dual-Energy CT Provide Additional Value over Iterative Metal Artifact Reduction? Abdom. Radiol. 2023, 48, 424–435. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, Q.; Liu, C.; Wang, Q.; Lv, Y.; Tang, Z.; Luo, Y.; Yang, H. Optimal Combination Periprosthetic Vasculature Visualization and Metal Artifact Reduction by Spectral Computed Tomography Using Virtual Monoenergetic Images in Total Hip Arthroplasty. Insights Imaging 2023, 14, 181. [Google Scholar] [CrossRef]
- Han, S.C.; Chung, Y.E.; Lee, Y.H.; Park, K.K.; Kim, M.J.; Kim, K.W. Metal Artifact Reduction Software Used with Abdominopelvic Dual-Energy CT of Patients with Metal Hip Prostheses: Assessment of Image Quality and Clinical Feasibility. AJR Am. J. Roentgenol. 2014, 203, 788–795. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.H.; Han, J.K. Combined Application of Virtual Monoenergetic High keV Images and the Orthopedic Metal Artifact Reduction Algorithm (O-MAR): Effect on Image Quality. Abdom. Radiol. 2019, 44, 756–765. [Google Scholar] [CrossRef]
- Reynoso, E.; Capunay, C.; Rasumoff, A.; Vallejos, J.; Carpio, J.; Lago, K.; Carrascosa, P. Periprosthetic Artifact Reduction Using Virtual Monochromatic Imaging Derived From Gemstone Dual-Energy Computed Tomography and Dedicated Software. J. Comput. Assist. Tomogr. 2016, 40, 649–657. [Google Scholar] [CrossRef]
- Neuhaus, V.; Große Hokamp, N.; Abdullayev, N.; Rau, R.; Mpotsaris, A.; Maintz, D.; Borggrefe, J. Metal Artifact Reduction by Dual-Layer Computed Tomography Using Virtual Monoenergetic Images. Eur. J. Radiol. 2017, 93, 143–148. [Google Scholar] [CrossRef]
- Horat, L.; Hamie, M.Q.; Huber, F.A.; Guggenberger, R. Optimization of Monoenergetic Extrapolations in Dual-Energy CT for Metal Artifact Reduction in Different Body Regions and Orthopedic Implants. Acad. Radiol. 2019, 26, e67–e74. [Google Scholar] [CrossRef]
| Technique | Description | Main Clinical Applications |
|---|---|---|
| Conventional Imaging (CI) | Standard imaging obtained with conventional CT without energy modifications. | Standard CT scan. General evaluation of anatomical structures; however, it may be limited by the presence of metal artifacts and suboptimal contrast in certain applications. |
| Metal Artifact Reduction (MAR) | Algorithms that are used to improve CT image quality in patients with metalware. MAR algorithms can be associated with either CI, VMI, or other acquisition techniques. | Enhancement of the visualization of anatomical structures adjacent to metalware. MAR algorithms are available on most CT scanners. |
| Virtual Monochromatic Imaging (VMI) | Images generated at different energies (keV) using DECT to reduce artifacts and improve contrast. | Bone evaluation, metal artifact reduction, periprosthetic structure assessment, enhanced contrast conditions in oncology and vascular imaging. VMI requires DECT scanner. |
| Technique | Advantages | Disadvantages |
|---|---|---|
| Metal Artifact Reduction (MAR) | Significantly reduces metal artifacts, improving the visualization of bones and soft tissues; it is compatible with a wide range of CT scanners. | It may introduce new distortions or secondary artifacts; effectiveness may vary depending on the type of metal implant and the specific technique used. |
| Virtual Monochromatic Imaging (VMI) | Allows selection of the optimal energy level (keV) to improve image quality and reduce artifacts; may reduce the amount of contrast medium required or radiation dose. | The optimal keV selection varies depending on the type of prosthesis and diagnostic objective; it requires expertise in interpretation; effectiveness may be influenced by the presence of significant metal artifacts. |
| Pseudo-Monochromatic Imaging (PMI) | Reduces beam hardening and metal artifacts in certain cases. | Reduced contrast-to-noise ratio (CNR); limited effectiveness with intense metal artifacts. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, A.; Tessitore, L.; Macina, C.; Piacentino, F.; Fontana, F.; Pautasso, A.; Ascenti, V.; Minici, R.; Laganà, D.; Catania, T.; et al. Dual-Energy Computed Tomography, a New Metal Artifact Reduction Technique for Total Hip Arthroplasty: Is There a Light in the Darkness? J. Clin. Med. 2025, 14, 2258. https://doi.org/10.3390/jcm14072258
Coppola A, Tessitore L, Macina C, Piacentino F, Fontana F, Pautasso A, Ascenti V, Minici R, Laganà D, Catania T, et al. Dual-Energy Computed Tomography, a New Metal Artifact Reduction Technique for Total Hip Arthroplasty: Is There a Light in the Darkness? Journal of Clinical Medicine. 2025; 14(7):2258. https://doi.org/10.3390/jcm14072258
Chicago/Turabian StyleCoppola, Andrea, Luigi Tessitore, Chiara Macina, Filippo Piacentino, Federico Fontana, Andrea Pautasso, Velio Ascenti, Roberto Minici, Domenico Laganà, Tommasa Catania, and et al. 2025. "Dual-Energy Computed Tomography, a New Metal Artifact Reduction Technique for Total Hip Arthroplasty: Is There a Light in the Darkness?" Journal of Clinical Medicine 14, no. 7: 2258. https://doi.org/10.3390/jcm14072258
APA StyleCoppola, A., Tessitore, L., Macina, C., Piacentino, F., Fontana, F., Pautasso, A., Ascenti, V., Minici, R., Laganà, D., Catania, T., Ascenti, G., Venturini, M., & D’Angelo, F. (2025). Dual-Energy Computed Tomography, a New Metal Artifact Reduction Technique for Total Hip Arthroplasty: Is There a Light in the Darkness? Journal of Clinical Medicine, 14(7), 2258. https://doi.org/10.3390/jcm14072258






