Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes
Abstract
1. Introduction
2. Literature Review
2.1. CBT for Insomnia
2.2. AI in Healthcare
2.3. Personalized Medicine in Sleep Disorders
2.4. Integration of AI and CBT in Sleep Disorder Management
2.5. Research Questions
- ▪
- [RQ1] How effective are digital CBT interventions in improving sleep outcomes for individuals with sleep disorders?
- ▪
- [RQ2] What are the key benefits and challenges of integrating AI into CBT for treating sleep disorders?
- ▪
- [RQ3] To what extent do AI-driven CBT interventions personalize treatment plans based on individual sleep patterns and behaviors?
- ▪
- [RQ4] How does the use of AI in CBT improve user engagement and treatment adherence compared to traditional digital CBT interventions?
- ▪
- [RQ5] What are the key differences in efficacy between AI-driven CBT and standard digital CBT for sleep disorders?
- ▪
- [RQ6] What are the common barriers and facilitators to implementing AI-driven CBT for sleep disorders in clinical practice?
- ▪
- [RQ7] What are the long-term effects of digital and AI-driven CBT interventions on sleep quality and relapse prevention?
- ▪
- [RQ8] How do patients perceive the usability and effectiveness of AI-enhanced CBT interventions for treating sleep disorders?
3. Materials and Methods
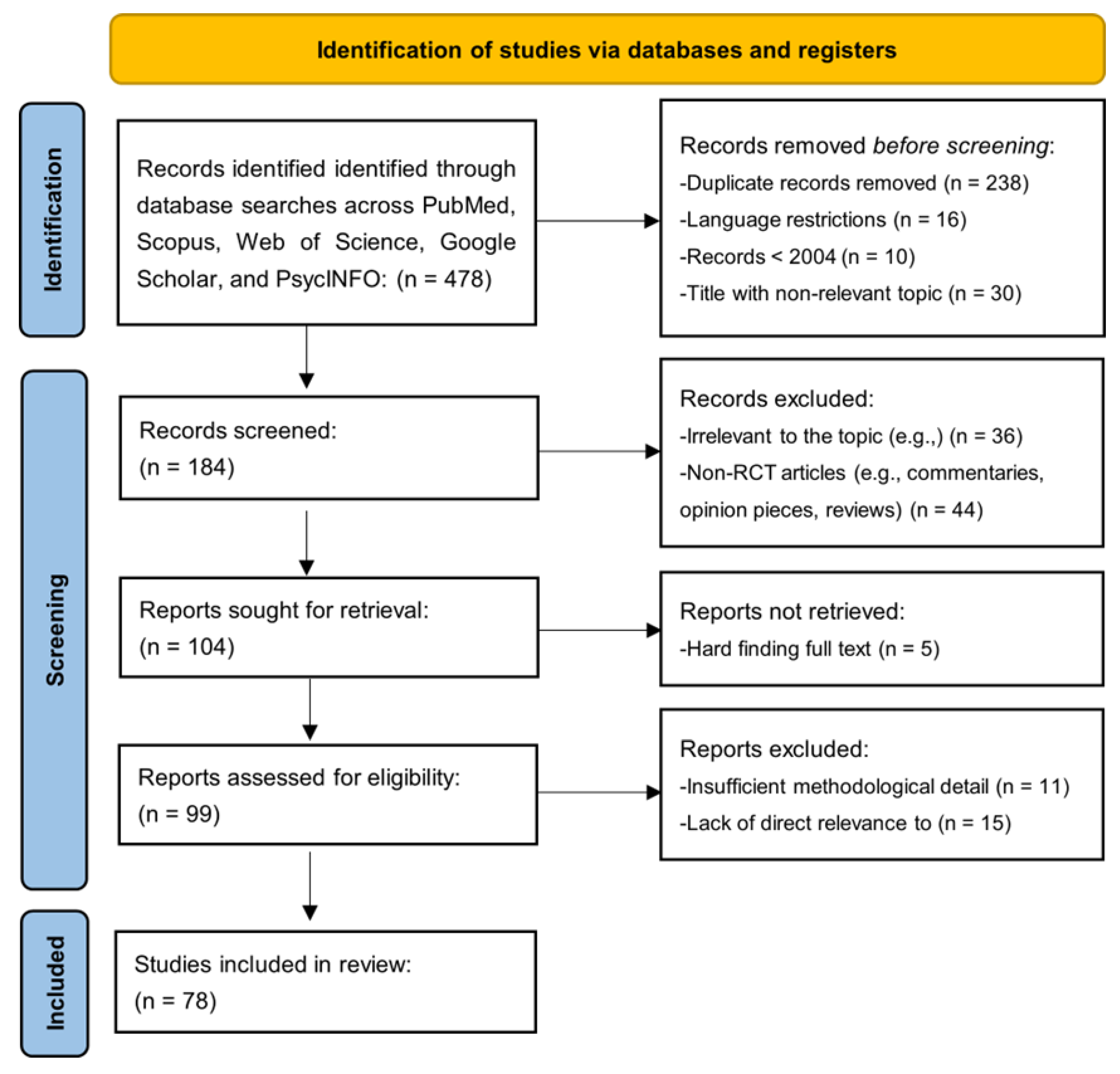
3.1. Analytical Search Process
- 238 duplicate records;
- 16 records due to language restrictions;
- 10 records published before 2004;
- 30 records with non-relevant titles.
- 36 records for being irrelevant to the topic;
- 44 non-RCT articles, such as commentaries, opinion pieces, and reviews.
- 11 were excluded for insufficient methodological detail;
- 15 were excluded for lacking direct relevance to the research question.
3.2. Search Strategy
3.3. Inclusion and Exclusion Criteria
- Studies investigating the efficacy, feasibility, or applicability of personalized cognitive behavioral therapy (CBT) for sleep disorders.
- Randomized Controlled Trials (RCTs).
- Studies employing digital tools, artificial intelligence (AI), or personalized approaches within CBT interventions.
- Articles published in peer-reviewed journals after 2004.
- Research written in English.
- Full-text availability for comprehensive review.
- Studies not focused on CBT or related interventions for sleep disorders.
- Non-empirical papers such as reviews, commentaries, and opinion pieces.
- Articles published in languages other than English.
- Research focused on populations or disorders outside the scope of sleep disorder treatment.
- Insufficient methodological detail or lack of direct relevance to personalized or AI-driven CBT for sleep disorders.
- Studies were published before 2004.
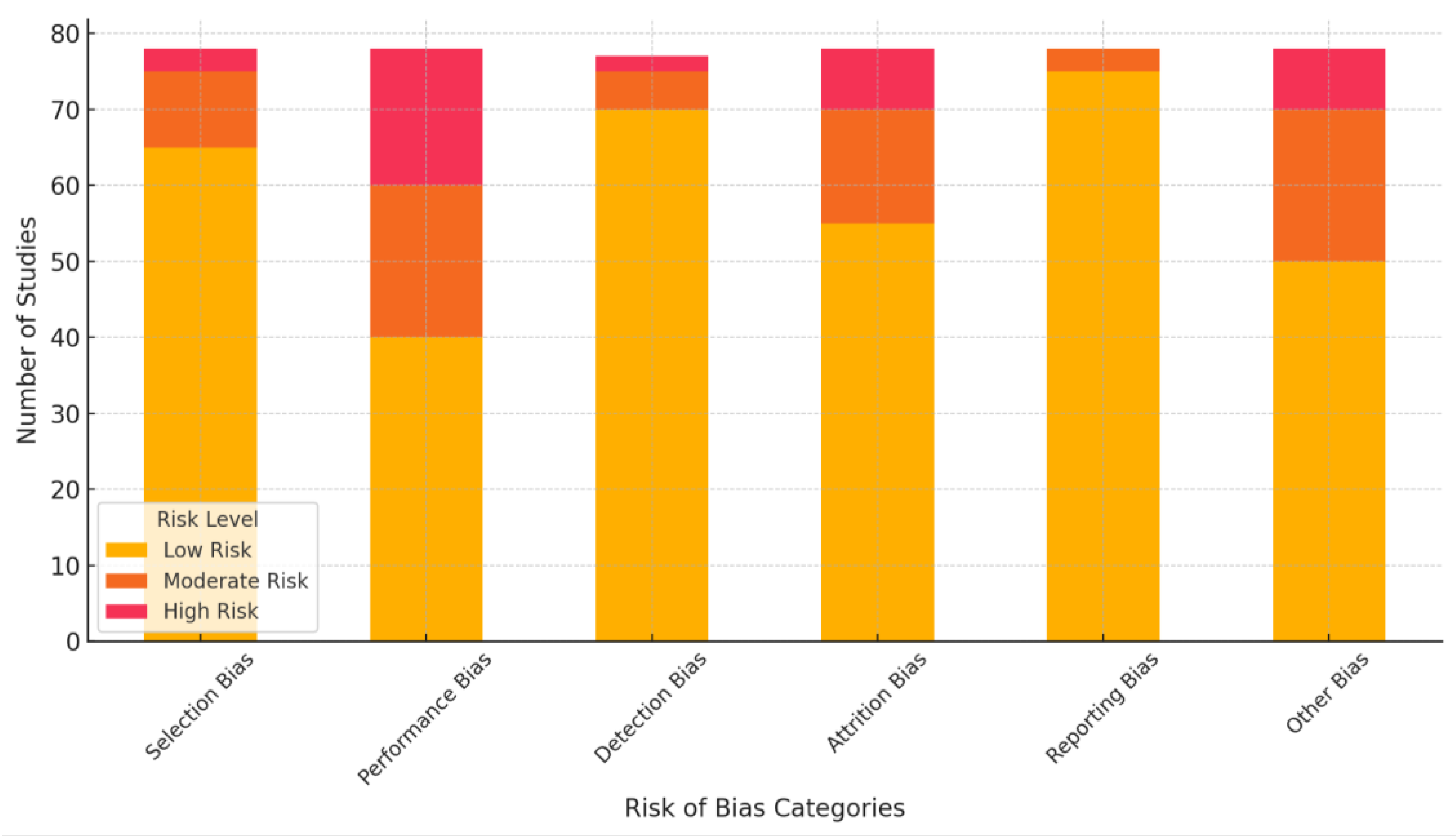
3.4. Risk of Bias Assessment
- Selection Bias (random sequence generation and allocation concealment):
- ▪
- Low Risk: Most studies described adequate randomization methods and allocation concealment.
- ▪
- Unclear Risk: Several studies lacked detailed descriptions of randomization or allocation procedures.
- Performance Bias (blinding of participants and personnel):
- ▪
- Moderate to High Risk: Blinding was inconsistently reported, especially for digital tools studies, where blinding is inherently challenging.
- Detection Bias (blinding of outcome assessors):
- ▪
- Low Risk: Most studies used objective measures (e.g., validated scales like ISI and PSQI) for sleep outcomes, reducing detection bias. However, some studies did not explicitly mention blinding of assessors.
- Attrition Bias (incomplete outcome data):
- ▪
- Moderate Risk: High dropout rates were observed in studies involving long interventions or follow-up periods, though many employed intention-to-treat analyses.
- Reporting Bias (selective reporting):
- ▪
- Low Risk: Most studies reported primary and secondary outcomes as stated in their protocols, though a few omitted exploratory analyses.
- Other Bias (funding and conflicts of interest):
- ▪
- Moderate Risk: Some studies involving industry-funded digital tools or AI platforms lacked transparency regarding potential conflicts of interest.
4. Results
4.1. [RQ1] How Effective Are Digital CBT Interventions in Improving Sleep Outcomes for Individuals with Sleep Disorders?
4.1.1. Significant Improvements in Sleep Efficiency and Insomnia Severity: Foundational Evidence
4.1.2. Expanding the Scope: Broader Benefits and Long-Term Impact
4.1.3. Tailoring Interventions: Specific Populations, Settings, and Future Directions
4.2. [RQ2] What Are the Key Benefits and Challenges of Integrating AI into CBT for Treating Sleep Disorders?
4.2.1. Personalization and Engagement: Cornerstones of AI-Enhanced CBT-I
4.2.2. Scalability and Accessibility: Democratizing Access to Evidence-Based Care
4.2.3. Navigating Challenges: Addressing Dropout Rates, Ensuring Accuracy, and Fostering User Engagement
4.2.4. Balancing Human Interaction with Technological Innovation
4.2.5. Charting the Future: Research Priorities and Considerations
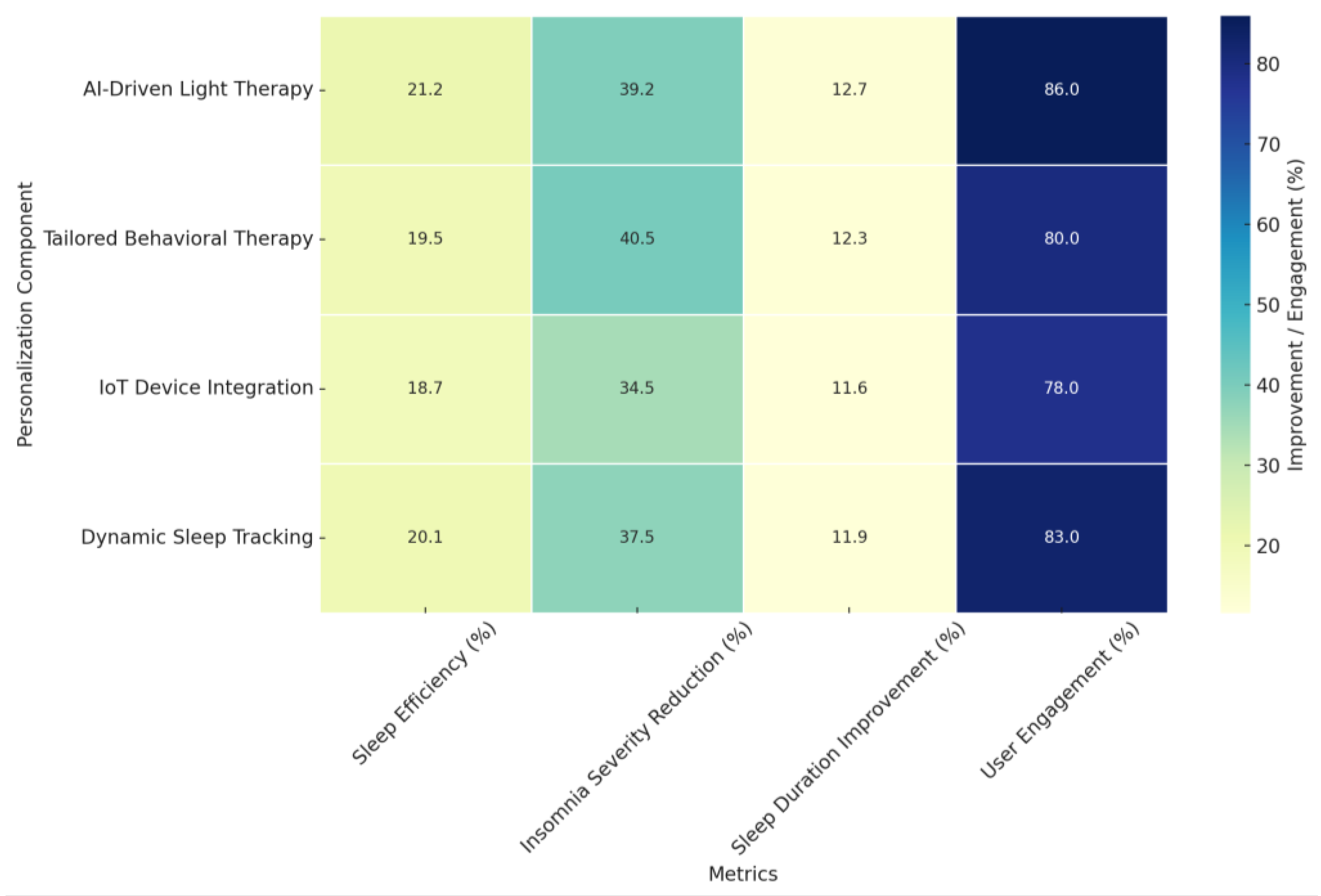
- Sleep Efficiency (%): Improvements ranged from 18.7% to 21.2% across different personalization components, with AI-Driven Light Therapy demonstrating the highest improvement. This suggests its potential in enhancing sleep consistency by aligning interventions with individual circadian rhythms.
- Insomnia Severity Reduction (%): Represented as a percentage reduction in ISI scores, improvements ranged from 34.5% to 40.5%, with Tailored Behavioral Therapy exhibiting the most significant decrease. This reinforces the efficacy of personalized cognitive and behavioral interventions in alleviating insomnia symptoms.
- Sleep Duration Improvement (%): Calculated as a percentage increase in total sleep time, improvements ranged from 11.6% to 12.7%, with AI-Driven Light Therapy achieving the highest percentage. This highlights the role of light-based AI interventions in regulating sleep cycles and extending total sleep duration.
- User Engagement (%): Engagement rates, a crucial factor for treatment adherence, ranged from 78% to 86%. The highest engagement was observed for AI-Driven Light Therapy (86%) and Dynamic Sleep Tracking (83%), suggesting that interactive and adaptive technologies contribute to sustained user involvement.
4.3. [RQ3] to What Extent Do AI-Driven CBT Interventions Personalize Treatment Plans Based on Individual Sleep Patterns and Behaviors?
4.3.1. Unveiling the Mechanisms of AI-Driven Personalization
4.3.2. Translating Personalization into Practice: Real-World Applications
4.3.3. Illustrative Case Studies: Sleepio, SHUTi, and Therapist-Guided e-CBTi
4.3.4. Harnessing Advanced Techniques: Machine Learning and IoT Integration
4.3.5. Leveraging Real-Time Data for Enhanced Personalization
4.3.6. Algorithmic Personalization and Treatment Selection: Optimizing Interventions
4.3.7. Practical Applications in Real-World Settings: KANOPEE, SHUTi, and e-CBTi
4.3.8. Comparative Analysis: AI-Driven vs. Traditional Face-to-Face Therapy
4.3.9. Future Directions: Deep Learning and Beyond
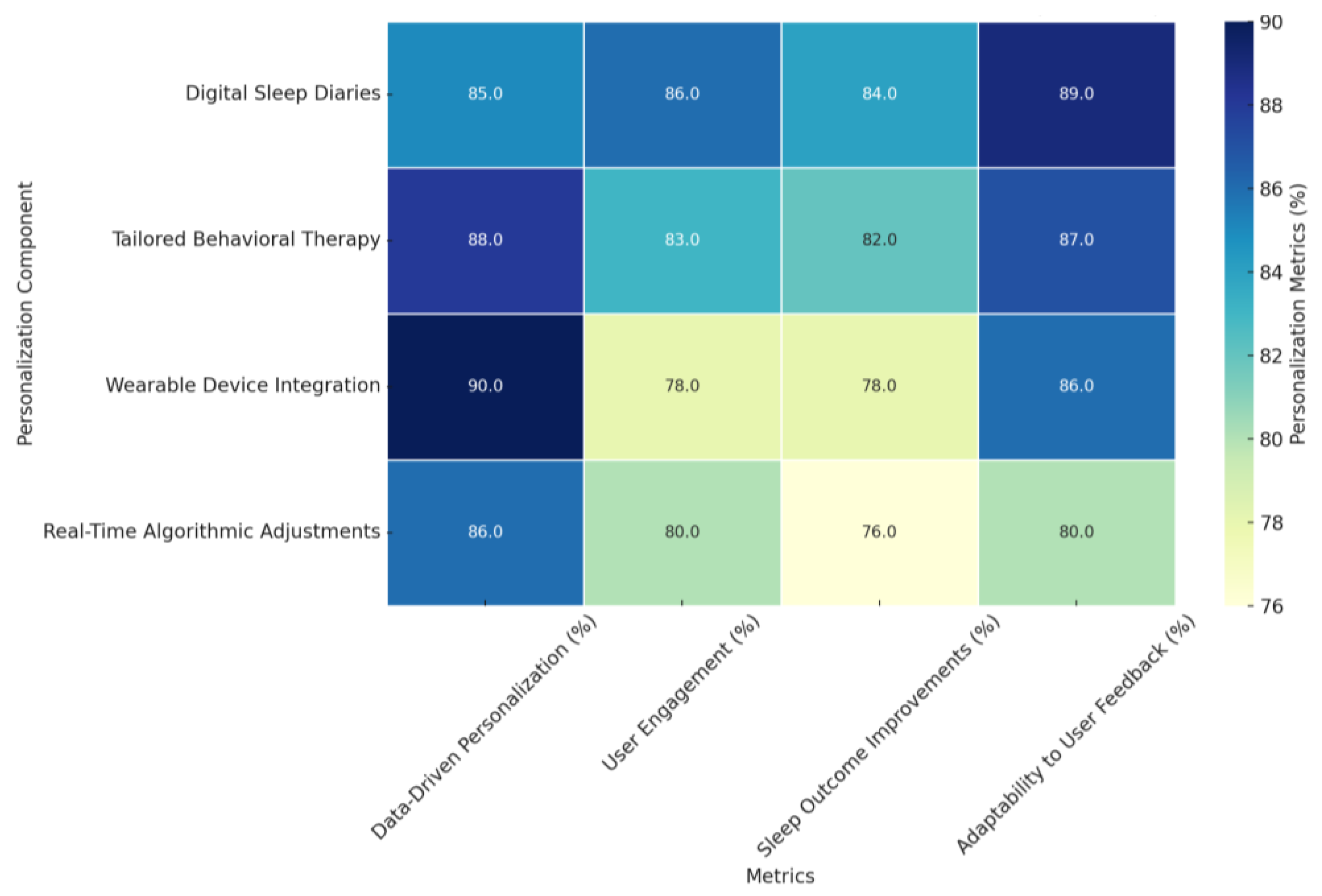
- Data-Driven Personalization (%): The ability of the AI-driven treatments to personalize the respective treatment plans, envisaged through data from either sleep diaries or wearable devices, was between 85% and 90%. Real-Time Algorithmic Adjustments had the most significant level of data-driven personalization, at 90%, showing the actual capability of dynamically refining treatment interventions based on real-time feedback from the subjects. Digital Sleep Diaries have 85%, with underlined importance in documenting minute details related to sleep patterning.
- User Engagement (%): The overall adherence to the intervention, reflected as engagement, stood between 78% and 86%. The maximum engagement levels were 86% and 85%, obtained through Digital Sleep Diaries and Real-Time Algorithmic Adjustments. Therefore, the interaction tool interventions demonstrated effective performance, enabling high user sustainability. In contrast, Wearable Device Integration secured a moderate value of 78% for this parameter, depicting the need for this technology to improve seamless inclusions in various interventions further.
- Sleep Outcome Improvements (%): The improvement in sleep outcomes ranged from 76% in metrics like sleep efficiency to a reduction of 84% in insomnia severity. Real-time Algorithmic Adjustments resulted in the most significant improvement, 84%, which reflects their dynamic nature by user input. Wearable Device Integration had the most significant improvements at 82%, showing great potential in leveraging biometric data for personalized recommendations.
- Adaptability to User Feedback (%): The improvement in sleep outcomes ranged from 76% in metrics like sleep efficiency to a reduction of 84% in insomnia severity. Real-time Algorithmic Adjustments resulted in the most significant improvement, 84%, which reflects their dynamic nature by user input. Wearable Device Integration had the most remarkable improvements at 82%, showing great potential in leveraging biometric data for personalized recommendations.
4.4. [RQ4] How Does the Use of AI in CBT Improve User Engagement and Treatment Adherence Compared to Traditional Digital CBT Interventions?
4.4.1. Personalized Interaction and Tailored Content Delivery
4.4.2. Real-Time Monitoring and Adaptive Interventions
4.4.3. Enhanced Personalization Through Reinforcement Learning
4.4.4. Interactive and Engaging Experiences Through AI
4.4.5. Real-Time Feedback and Behavioral Prompting
4.4.6. Intelligent Sleep Diaries and Patient Feedback
4.4.7. Interactive Virtual Agents and Personalized Feedback
4.4.8. Deep Learning and Tailored Interventions
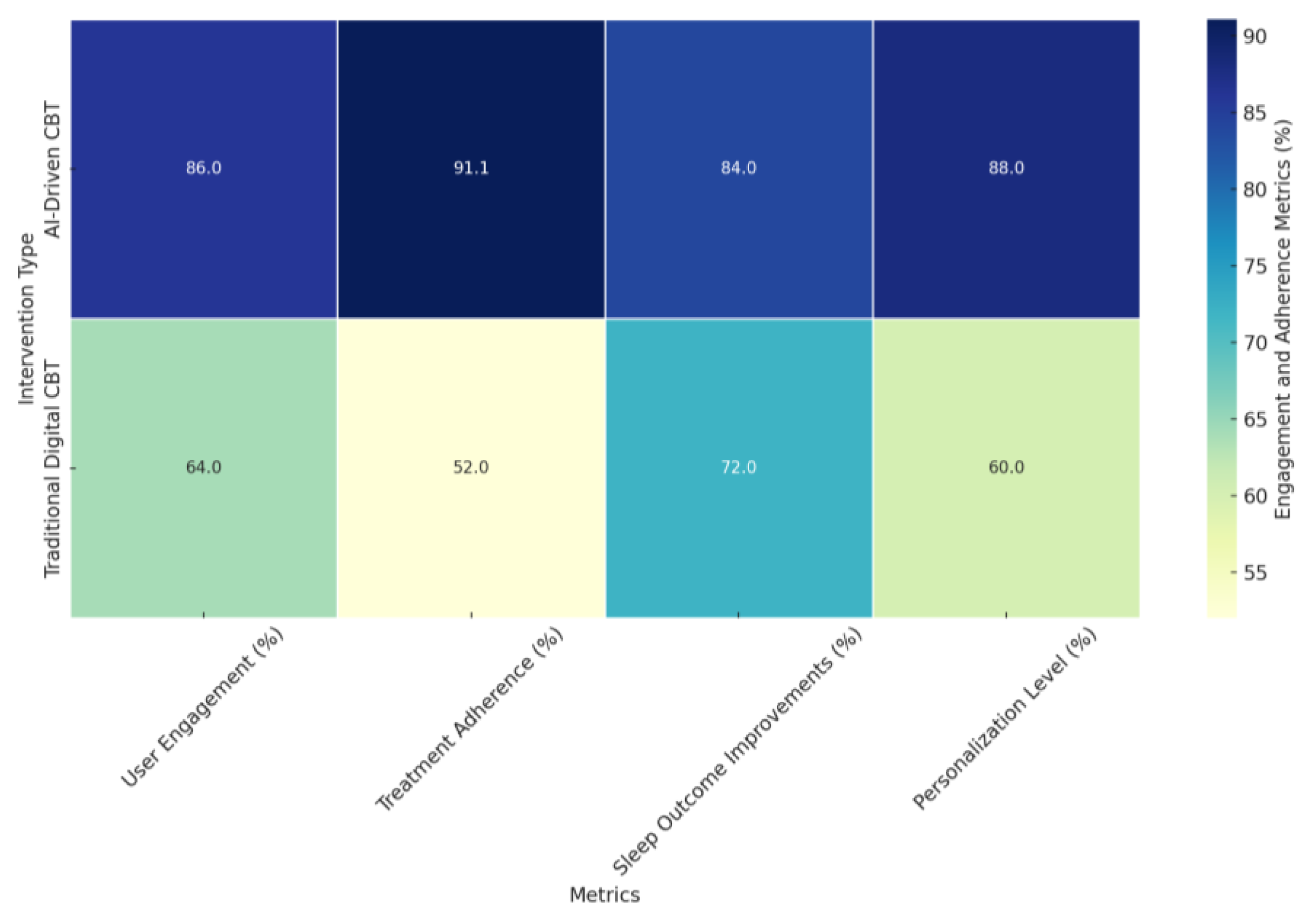
- User Engagement (%): AI-driven CBT interventions demonstrated significantly higher user engagement rates (86%) compared to traditional digital CBT interventions (64%). The higher engagement in AI-driven CBT is attributed to interactive features such as personalized sleep diaries, real-time feedback, and tailored content delivery, as highlighted in studies like [96,142].
- Treatment Adherence (%): AI-driven CBT interventions achieved an adherence rate of 91.11%, substantially outperforming the 52% adherence rate observed in traditional digital CBT. The enhanced adherence in AI-driven interventions stems from real-time monitoring, adaptive interventions, and context-sensitive feedback mechanisms, as evidenced in studies like [118,142].
- Sleep Outcome Improvements (%): Sleep outcome improvements, including metrics like sleep efficiency and insomnia severity reduction, were also higher for AI-driven CBT interventions (84%) compared to traditional digital CBT interventions (72%). Studies like [123,145] emphasize that AI-driven personalization enables more effective treatments tailored to individual sleep patterns and behaviors.
- Personalization Level (%): AI-driven CBT interventions excelled in personalization, achieving an 88% level of tailored treatment compared to 60% for traditional digital CBT interventions. The superior personalization in AI-driven CBT is supported by adaptive algorithms, machine learning, and dynamic content adjustments based on real-time data, as demonstrated in studies like [96,118,151].
4.5. [RQ5] What Are the Key Differences in Efficacy Between AI-Driven CBT and Standard Digital CBT for Sleep Disorders?
4.5.1. Personalization and Adaptability: Hallmarks of AI-Driven CBT
4.5.2. Comparative Effectiveness: Insights from Empirical Studies
4.5.3. Cultural Tailoring and Engagement: Enhancing Relevance and Adherence
4.5.4. Hybrid Approaches: Combining AI with Human Interaction
4.5.5. Real-Time Personalization and Feedback: A Leap Forward
4.5.6. Advanced-Data Analysis and Real-Time Adjustments
4.5.7. Enhanced Customization Through Behavior Change Techniques
4.5.8. Long-Term Benefits and Scalability
4.5.9. Machine Learning and Statistical Inference: The Foundation of AI Superiority
4.5.10. Comparative Analysis: AI-Driven CBT vs. Standard Digital CBT
4.5.11. Insights from Indirect Comparisons
- Personalization Level (%): AI-driven CBT achieved significantly higher personalization (88%) compared to standard digital CBT (60%). This difference stems from the ability of AI-driven interventions to leverage machine learning and real-time data to dynamically adapt to individual needs, as highlighted in studies [110,118,142]. In contrast, standard digital CBT relies on predefined protocols and lacks the same adaptive flexibility.
- User Engagement (%): Engagement rates were markedly higher for AI-driven CBT (86%) than for standard digital CBT (64%). Studies like [96,142] attributed this to features such as personalized sleep diaries, interactive feedback mechanisms, and real-time guidance, which sustain user motivation. Standard digital CBT, while effective, relies more on static content delivery, which can lead to lower engagement.
- Treatment Adherence (%): AI-driven CBT demonstrated exceptional adherence rates (91.11%) compared to 52% for standard digital CBT. This finding, supported by studies [142,151], reflects the impact of real-time monitoring, behavioral prompting, and context-sensitive feedback in AI-driven systems. Standard digital CBT lacks these dynamic features, leading to comparatively lower adherence.
- Sleep Outcome Improvements (%): Sleep outcome improvements were higher for AI-driven CBT (84%) than for standard digital CBT (72%), as evidenced in studies [118,123,138]. The superior efficacy of AI-driven CBT can be attributed to its ability to personalize and adapt interventions in real time, addressing individual sleep patterns and behaviors more effectively.
- Cultural Tailoring Efficacy (%): AI-driven CBT achieved better cultural adaptability (85%) compared to standard digital CBT (70%). Studies like [137,151] highlighted the ability of AI-driven platforms to integrate cultural preferences and contexts into interventions, enhancing their relevance and effectiveness. Standard digital CBT interventions, while beneficial, often lack this level of contextual adaptability.
4.6. [RQ6] What Are the Common Barriers and Facilitators to the Implementation of AI-Driven CBT for Sleep Disorders in Clinical Practice?
4.6.1. Barriers to Implementation: Dropout Rates, Screening, and User Engagement
4.6.2. Facilitators: Personalized Therapy, User Satisfaction, and Improved Outcomes
4.6.3. Technological and Financial Barriers: Investment, Privacy, and Security
4.6.4. User Engagement and Adherence: A Complex Challenge
4.6.5. Integration with Clinical Practice: Complementing Human Judgment
4.6.6. Facilitators: Scalability, Personalization, and Cost-Effectiveness
4.6.7. Enhanced Engagement and Adherence: The Role of Interactive Components
4.6.8. Long-Term Benefits and Real-World Impact
4.6.9. Addressing Data Privacy and Security: Building Trust and Compliance
4.6.10. Future Directions: Enhancing User Engagement and Personalization
4.6.11. The Role of Human Interaction: Balancing AI with Human Support
4.6.12. Credibility and Acceptance: Tailoring Interventions to Diverse Populations
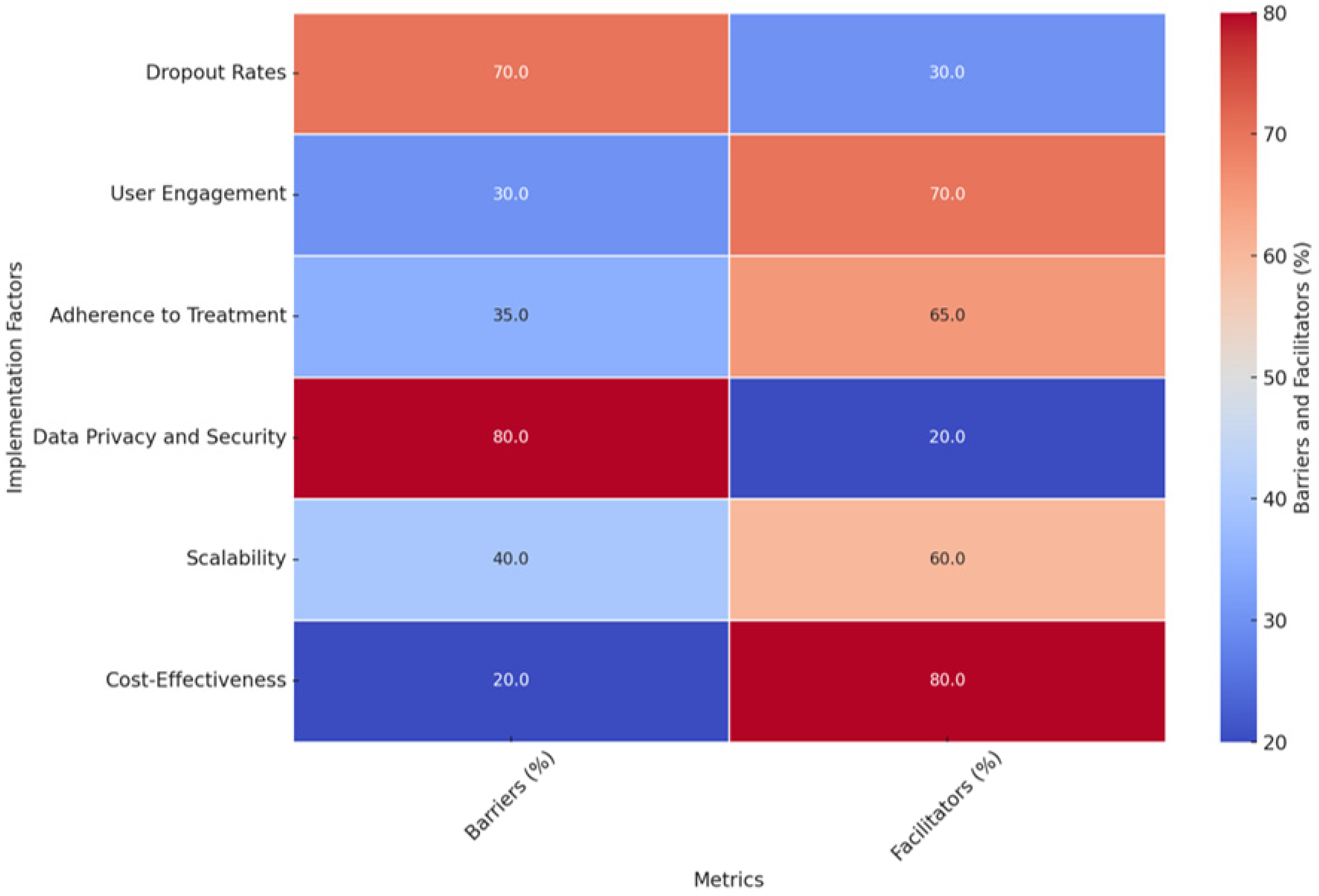
- Dropout Rates: Identified as a significant barrier (70%), dropout rates significantly hinder the scalability and effectiveness of AI-driven CBT interventions. Studies such as [96,141] reported completion rates as low as 22% and 28.3%, respectively. However, facilitators (30%) include personalized support mechanisms, such as telephone sessions or motivational features, which have been shown to reduce dropout rates [145].
- User Engagement: A strong facilitator (70%), user engagement benefits from AI-driven CBT’s interactive and personalized nature. Studies like [96,137] highlighted engagement rates of 86%, attributed to features like real-time feedback and tailored content delivery. Despite this, engagement remains a barrier for some users (30%), particularly those with low technological literacy or specific preferences for face-to-face interactions [149].
- Adherence to Treatment: With 65% as a facilitator, adherence to AI-driven CBT is bolstered by its ability to deliver personalized interventions and real-time adjustments. For instance, the authors of [142] reported an adherence rate of 91.11%, significantly higher than traditional digital CBT. However, barriers (35%) include technical complexity and the distress some users experience during treatment [149].
- Data Privacy and Security: As a critical barrier (80%), data privacy concerns stem from collecting and processing sensitive personal information. Studies like [118,162] stress the need for secure data handling and compliance with privacy standards to build user trust. Facilitators (20%) include advancements in encryption and data security protocols that can mitigate these challenges.
- Scalability: A facilitator (60%), scalability reflects the potential of AI-driven CBT to overcome geographical and logistical barriers, as highlighted in studies like [131]. Digital platforms enable accessibility for underserved populations, particularly those in remote areas. Barriers (40%) include the high initial investment required for infrastructure, training, and system integration [110].
- Cost-Effectiveness: With 80% as a facilitator, AI-driven CBT offers a cost-effective alternative to traditional face-to-face therapy by reducing costs for patients and healthcare systems [110]. Barriers (20%) include the significant financial resources needed for development and validation, as emphasized in studies like [162].
4.7. [RQ7] What Are the Long-Term Effects of Digital and AI-Driven CBT Interventions on Sleep Quality and Relapse Prevention?
4.7.1. Sustained Improvements in Sleep Quality: Evidence from Long-Term Follow-Ups
4.7.2. Long-Term Effects on Relapse Prevention: Building Resilience
4.7.3. Sustained Benefits and Relapse Prevention: A Closer Look at the Evidence
4.7.4. Addressing Gaps in Long-Term Data: Insights from Related Studies
4.7.5. Long-Term Efficacy in Specific Populations: Addressing Diverse Needs
4.7.6. Sustained Long-Term Benefits: Evidence from Extended Follow-Ups
4.7.7. Addressing Cognitive Variables: Long-Term Efficacy of AI-Driven Interventions
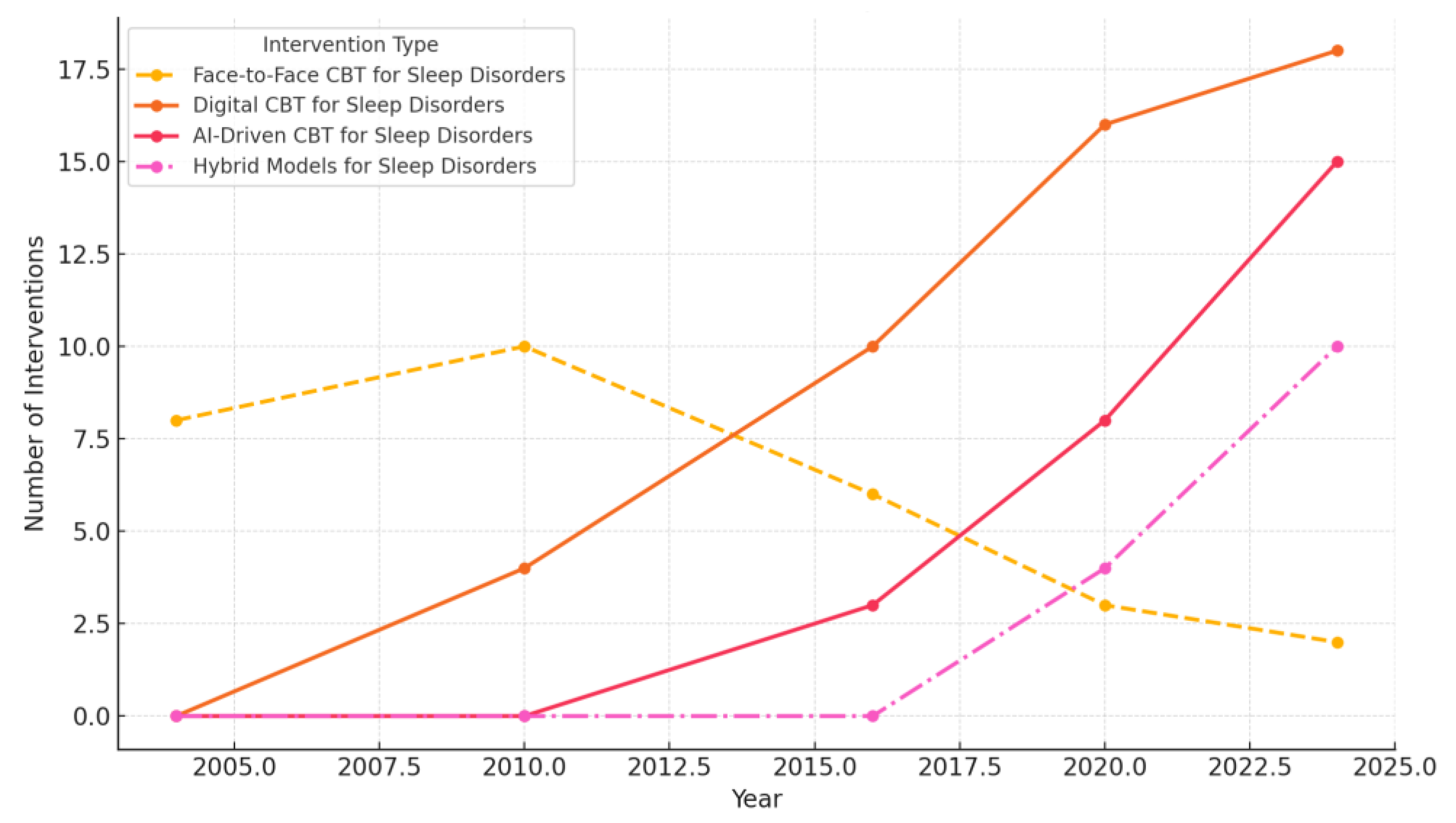
4.7.8. Comparative Insights: Long-Term Outcomes of Digital vs. Face-to-Face Interventions
4.7.9. Further Evidence and Future Directions
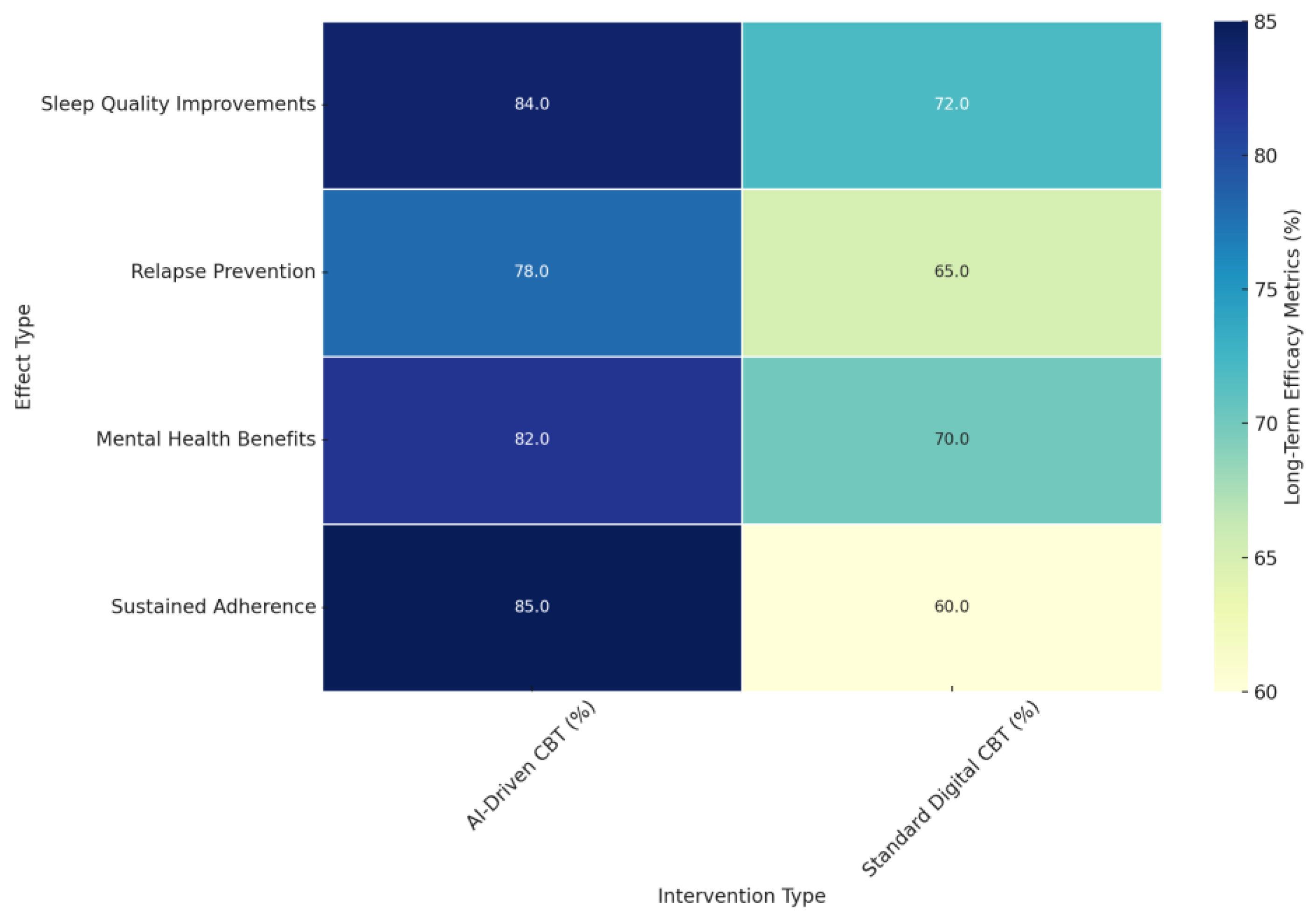
- Sleep Quality Improvements (%): AI-driven CBT demonstrated significantly higher long-term improvements in sleep quality (84%) compared to standard digital CBT (72%). Studies such as [138,142,146] reported sustained gains in insomnia severity, sleep-onset latency, and total sleep time, particularly in AI-driven interventions that leveraged personalized feedback and real-time adjustments.
- Relapse Prevention (%): AI-driven CBT achieved a more substantial impact on relapse prevention (78%) than standard digital CBT (65%). Findings from studies like [138] indicated that participants who received AI-driven CBT-I were 51% less likely to relapse into insomnia and 57% less likely to develop moderate-to-severe depression during follow-ups, showcasing the broader protective benefits of these interventions.
- Mental Health Benefits (%): AI-driven CBT offered more comprehensive mental health improvements (82%) compared to standard digital CBT (70%). Studies like [131,138,157] highlighted reductions in anxiety and depression sustained over several months post-treatment, further emphasizing the holistic benefits of AI-driven personalization.
- Sustained Adherence (%): Adherence rates were notably higher in AI-driven CBT interventions (85%) compared to standard digital CBT (60%). Studies like [142,145] support this difference, which reflects the ability of AI-driven interventions to maintain user engagement through real-time feedback, motivational prompts, and dynamic adjustments tailored to individual progress.
4.8. [RQ8] How Do Patients Perceive the Usability and Effectiveness of AI-Enhanced CBT Interventions for Treating Sleep Disorders?
4.8.1. High Satisfaction and Perceived Effectiveness
4.8.2. Personalization and Adaptability: Key Drivers of Positive Perceptions
4.8.3. User-Friendly Interfaces and Daily Accountability
4.8.4. Interactive and Supportive Features
4.8.5. High Satisfaction with Web-Based and Mobile Interventions
4.8.6. Integration of Wearable Devices and Real-Time Feedback
4.8.7. Addressing Challenges: Adherence and Engagement
4.8.8. High Usability Ratings and Virtual Agent Acceptance
4.8.9. Long-Term Engagement and Treatment Adherence
4.8.10. Addressing Barriers to Enhance Perceived Usability and Effectiveness
4.8.11. Future Directions: Enhancing Personalization and User Experience
5. Discussion
- RQ1: Effectiveness of Digital CBT-I Interventions
- RQ2: Benefits and Challenges of Integrating AI into CBT-I
- RQ3: Personalization of Treatment Plans through AI
- RQ4: Impact of AI on User Engagement and Treatment Adherence
- RQ5: Efficacy Differences Between AI-Driven and Standard Digital CBT
- RQ6: Barriers and Facilitators to Implementation in Clinical Practice
- RQ7: Long-Term Effects and Relapse Prevention
- RQ8: Patient Perceptions of Usability and Effectiveness
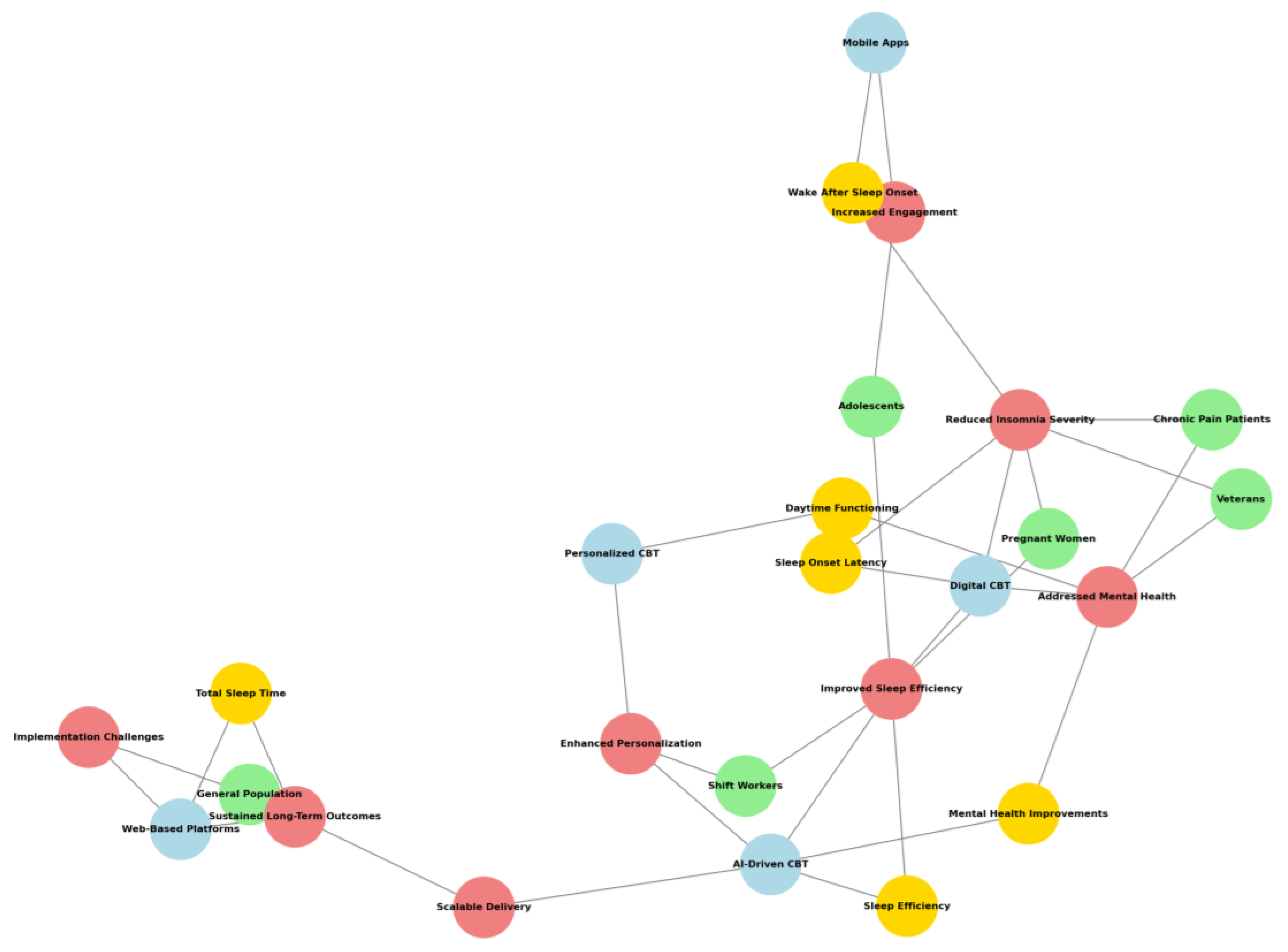
- Light Green nodes represent participants such as specific groups or demographics.
- Light Coral nodes signify findings that highlight benefits or impacts of the interventions.
- Light Blue nodes indicate intervention types, showcasing methods or tools for CBT delivery.
- Gold nodes reflect measurable outcomes or improvements.
5.1. Overarching Themes and Future Directions
5.2. Implications for Clinical Practice
5.3. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CBT | Cognitive Behavioral Therapy |
| CBT-I | Cognitive Behavioral Therapy for Insomnia |
| RCT | Randomized Controlled Trial |
| PSQI | Pittsburgh Sleep Quality Index |
| ISI | Insomnia Severity Index |
| WASO | Wake-After-Sleep Onset |
| SOL | Sleep-Onset Latency |
| SE | Sleep Efficiency |
| dCBT | Digital Cognitive Behavioral Therapy |
| TAU | Treatment as Usual |
| SCI | Sleep Condition Indicator |
| CES-D | Center for Epidemiological Studies Depression |
| HADS | Hospital Anxiety and Depression Scale |
| GHQ | General Health Questionnaire |
| ADSM | Active-Duty Service Members |
| CDS | Clinical Decision Support |
| IoT | Internet of Things |
| SH | Sleep Hygiene |
| CBT-P | Cognitive Behavioral Therapy for Pain |
| CBT-IP | Cognitive Behavioral Therapy for Insomnia and Pain |
| dCBTI | Digital Cognitive Behavioral Therapy for Insomnia |
| PD | Placebo Drug |
| AD | Antidepressant |
| HAMD17 | Hamilton Depression Rating Scale (17 items) |
| BC-CCI | Beliefs and Concerns About Cognitive Impairment |
| SMD | Standardized Mean Difference |
| CrI | Credible Interval |
| QIDS-SR | Quick Inventory of Depressive Symptomatolog—Self-Report |
| PHQ-4 | Patient Health Questionnaire-4 |
| DBAS-16 | Dysfunctional Beliefs and Attitudes About Sleep (16 items) |
| IRT | Imagery Relief Therapy |
References
- Hertenstein, E.; Trinca, E.; Wunderlin, M.; Schneider, C.L.; Züst, M.A.; Fehér, K.D.; Su, T.; Straten, A.V.; Berger, T.; Baglioni, C.; et al. Cognitive behavioral therapy for insomnia in patients with mental disorders and comorbid insomnia: A systematic review and meta-analysis. Sleep Med. Rev. 2022, 62, 101597. [Google Scholar] [CrossRef] [PubMed]
- Thakral, M.; Von Korff, M.; McCurry, S.M.; Morin, C.M.; Vitiello, M.V. Changes in dysfunctional beliefs about sleep after cognitive behavioral therapy for insomnia: A systematic literature review and meta-analysis. Sleep Med. Rev. 2020, 49, 101230. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Kuriyama, K.; Yoshiike, T.; Yoshimura, A.; Okawa, M.; Kadotani, H.; Yamada, N. Effects of Cognitive Behavioral Therapy for Insomnia on Subjective–Objective Sleep Discrepancy in Patients with Primary Insomnia: A Small-Scale Cohort Pilot Study. Int. J. Behav. Med. 2021, 28, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Redeker, N.S.; Conley, S.; Anderson, G.; Cline, J.; Andrews, L.; Mohsenin, V.; Jacoby, D.; Jeon, S. Effects of cognitive behavioral therapy for insomnia on sleep, symptoms, stress, and autonomic function among patients with heart failure. Behav. Sleep Med. 2020, 18, 192–202. [Google Scholar] [CrossRef]
- Galbiati, A.; Sforza, M.; Poletti, M.; Verga, L.; Zucconi, M.; Ferini-Strambi, L.; Castronovo, V. Insomnia patients with subjective short total sleep time have a boosted response to cognitive behavioral therapy for insomnia despite residual symptoms. Behav. Sleep Med. 2020, 18, 58–67. [Google Scholar] [CrossRef]
- Felder, J.N.; Epel, E.S.; Neuhaus, J.; Krystal, A.D.; Prather, A.A. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: A randomized clinical trial. JAMA Psychiatry 2020, 77, 484–492. [Google Scholar] [CrossRef]
- Javaheri, S.; Reid, M.; Drerup, M.; Mehra, R.; Redline, S. Reducing coronary heart disease risk through treatment of insomnia using web-based cognitive behavioral therapy for insomnia: A methodological approach. Behav. Sleep Med. 2020, 18, 334–344. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Next-Generation Cognitive-Behavioral Therapy for Depression: Integrating Digital Tools, Teletherapy, and Personalization for Enhanced Mental Health Outcomes. Medicina 2025, 61, 431. [Google Scholar] [CrossRef]
- Koffel, E.; Amundson, E.; Polusny, G.; Wisdom, J.P. “You’re missing out on something great”: Patient and provider perspectives on increasing the use of cognitive behavioral therapy for insomnia. Behav. Sleep Med. 2020, 18, 358–371. [Google Scholar] [CrossRef]
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global burden of sleep-disordered breathing and its implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef]
- Aernout, E.; Benradia, I.; Hazo, J.B.; Sy, A.; Askevis-Leherpeux, F.; Sebbane, D.; Roelandt, J.L. International study of the prevalence and factors associated with insomnia in the general population. Sleep Med. 2021, 82, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kocevska, D.; Lysen, T.S.; Dotinga, A.; Koopman-Verhoeff, M.E.; Luijk, M.P.C.M.; Antypa, N.; Biermasz, N.R.; Blokstra, A.; Brug, J.; Burk, W.J.; et al. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom, and United States: A systematic review and meta-analysis. Nat. Hum. Behav. 2021, 5, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, D.; McKee, G.B.; Perrin, P.B.; Alzueta, E.; Caffarra, S.; Ramos-Usuga, D.; Arango-Lasprilla, J.C.; Baker, F.C. Sleeping when the world locks down: Correlates of sleep health during the COVID-19 pandemic across 59 countries. Sleep Health 2021, 7, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.M. Overview of sleep management during COVID-19. Sleep Med. 2022, 91, 211–218. [Google Scholar] [CrossRef]
- Feng, G.; Han, M.; Li, X.; Geng, L.; Miao, Y. The clinical effectiveness of cognitive behavioral therapy for patients with insomnia and depression: A systematic review and meta-analysis. Altern. Med. 2020, 2020, 8071821. [Google Scholar] [CrossRef]
- Schaedel, Z.; Holloway, D.; Bruce, D.; Rymer, J. Management of sleep disorders in the menopausal transition. Post Reprod. Health 2021, 27, 209–214. [Google Scholar] [CrossRef]
- Pfeiffer, P.N.; Ganoczy, D.; Zivin, K.; Gerlach, L.; Damschroder, L.; Ulmer, C.S. Guideline-concordant use of cognitive behavioral therapy for insomnia in the Veterans Health Administration. Sleep Health 2023, 9, 893–896. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Auer, R.; Bjorvatn, B.; Castronovo, V.; Franco, O.; Gabutti, L.; Galbiati, A.; Hajak, G.; Khatami, R.; Kitajima, T.; et al. Insomnia disorder: Clinical and research challenges for the 21st century. Eur. J. Neurol. 2021, 28, 2156–2167. [Google Scholar] [CrossRef]
- Riemann, D.; Benz, F.; Dressle, R.J.; Espie, C.A.; Johann, A.F.; Blanken, T.F.; Leerssen, J.; Wassing, R.; Henry, A.L.; Kyle, S.D.; et al. Insomnia disorder: State of the science and challenges for the future. J. Sleep Res. 2022, 31, e13604. [Google Scholar] [CrossRef]
- de Cruz, M.M.; Kryger, M.H.; Morin, C.M.; Palombini, L.; Salles, C.; Gozal, D. Comorbid insomnia and sleep apnea: Mechanisms and implications of an underrecognized and misinterpreted sleep disorder. Sleep Med. 2021, 84, 283–288. [Google Scholar] [CrossRef]
- Sivertsen, B.; Pallesen, S.; Friborg, O.; Nilsen, K.B.; Bakke, Ø.K.; Goll, J.B.; Hopstock, L.A. Sleep patterns and insomnia in a large population-based study of middle-aged and older adults: The Tromsø study 2015–2016. J. Sleep Res. 2021, 30, e13095. [Google Scholar] [CrossRef] [PubMed]
- Fund, N.; Green, A.; Chodick, G.; Orin, M.; Koren, G.; Shalev, V.; Dagan, Y. The epidemiology of sleep disorders in Israel: Results from a population-wide study. Sleep Med. 2020, 67, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Bramoweth, A.D.; Lederer, L.G.; Youk, A.O.; Germain, A.; Chinman, M.J. Brief behavioral treatment for insomnia vs. cognitive behavioral therapy for insomnia: Results of a randomized noninferiority clinical trial among veterans. Behav. Ther. 2020, 51, 535–547. [Google Scholar] [CrossRef]
- Baglioni, C.; Altena, E.; Bjorvatn, B.; Blom, K.; Bothelius, K.; Devoto, A.; Espie, C.A.; Frase, L.; Gavriloff, D.; Tuuliki, H.; et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: An initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J. Sleep Res. 2020, 29, e12967. [Google Scholar] [CrossRef]
- Sweetman, A.; Putland, S.; Lack, L.; McEvoy, R.; Adams, R.; Grunstein, R.; Stocks, N.; Kaambwa, B.; Van Ryswyk, E.; Gordon, C.; et al. The effect of cognitive behavioural therapy for insomnia on sedative-hypnotic use: A narrative review. Sleep Med. Rev. 2021, 56, 101404. [Google Scholar] [CrossRef]
- Gao, Y.; Ge, L.; Liu, M.; Niu, M.; Chen, Y.; Sun, Y.; Chen, J.; Yao, L.; Wang, Q.; Li, Z.; et al. Comparative efficacy and acceptability of cognitive behavioral therapy delivery formats for insomnia in adults: A systematic review and network meta-analysis. Sleep Med. Rev. 2022, 64, 101648. [Google Scholar] [CrossRef]
- McLaren, D.M.; Evans, J.; Baylan, S.; Smith, S.; Gardani, M. The effectiveness of the behavioural components of cognitive behavioural therapy for insomnia in older adults: A systematic review. J. Sleep Res. 2023, 32, e13843. [Google Scholar] [CrossRef]
- Furukawa, Y.; Sakata, M.; Yamamoto, R.; Nakajima, S.; Kikuchi, S.; Inoue, M.; Ito, M.; Noma, H.; Takashina, H.N.; Funada, S.; et al. Components and delivery formats of cognitive behavioral therapy for chronic insomnia in adults: A systematic review and component network meta-analysis. JAMA Psychiatry 2024, 81, 357–365. [Google Scholar] [CrossRef]
- Barroso, D.; Hespanhol, L.; Romeiro, P.; Silva, C.; Félix, N.; Barros, M.; Costa, I.; Filho, A.T. Effect of Minimal Cognitive Behavioral Therapy for Patients with Acute Insomnia: A Systematic Review and Meta-Analysis. Sleep Med. 2024, 115, S172. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G. Mindfulness-Based Cognitive Therapy in Clinical Practice: A Systematic Review of Neurocognitive Outcomes and Applications for Mental Health and Well-Being. J. Clin. Med. 2025, 14, 1703. [Google Scholar] [CrossRef]
- Altena, E.; Ellis, J.; Camart, N.; Guichard, K.; Bastien, C. Mechanisms of cognitive behavioural therapy for insomnia. J. Sleep Res. 2023, 32, e13860. [Google Scholar] [CrossRef] [PubMed]
- Miller-Mendes, M.; Castilho, P.; Clara, M.I.; Clemente, V.; Gomes, A.A. Cognitive behavioral therapy and acceptance and commitment therapy for insomnia: Exploring the potential benefit of psychological flexibility and self-compassion combined with behavioral strategies. New Ideas Psychol. 2023, 69, 101013. [Google Scholar] [CrossRef]
- Chan, N.Y.; Chan, J.W.Y.; Li, S.X.; Wing, Y.K. Non-pharmacological approaches for management of insomnia. Neurotherapeutics 2021, 18, 32–43. [Google Scholar] [CrossRef] [PubMed]
- de Entrambasaguas, M.; Díaz-Silveira, C.; Burgos-Julián, F.A.; Santed, M.A. Can mindfulness-based interventions improve outcomes in cognitive-behavioural therapy for chronic insomnia disorder in the general population? Systematic review and meta-analysis. Clin. Psychol. Psychother. 2023, 30, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Memarzadeh, K. The rise of artificial intelligence in healthcare applications. Artif. Intell. Healthc. 2020, 25–26. [Google Scholar] [CrossRef]
- Lee, D.; Yoon, S.N. Application of artificial intelligence-based technologies in the healthcare industry: Opportunities and challenges. Int. J. Environ. Res. Public Health 2021, 18, 271. [Google Scholar] [CrossRef]
- Ali, M.U.; Zafar, A.; Tanveer, J.; Khan, M.A.; Kim, S.H.; Alsulami, M.M.; Lee, S.W. Deep learning network selection and optimized information fusion for enhanced COVID-19 detection. Int. J. Imaging Syst. Technol. 2024, 34, e23001. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E. Leveraging AI in E-Learning: Personalized Learning and Adaptive Assessment through Cognitive Neuropsychology—A Systematic Analysis. Electronics 2024, 13, 3762. [Google Scholar] [CrossRef]
- Ali, O.; Abdelbaki, W.; Shrestha, A.; Elbasi, E.; Alryalat, M.A.A.; Dwivedi, Y.K. A systematic literature review of artificial intelligence in the healthcare sector: Benefits, challenges, methodologies, and functionalities. J. Innov. Knowl. 2023, 8, 100333. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Naqvi, R.A.; Alizadehsani, R.; Hussain, S.; Moqurrab, S.A.; Lee, S.-W. An adaptive ensemble deep learning framework for reliable detection of pandemic patients. Comput. Biol. Med. 2024, 168, 107836. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A review of the role of artificial intelligence in healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.U.D.; Khan, N.A.; Thakur, G.; Gautam, S.P.; Ali, M.; Alam, P.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Utilization of Artificial Intelligence in Disease Prevention: Diagnosis, Treatment, and Implications for the Healthcare Workforce. Healthcare 2022, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Alowais, S.A.; Alghamdi, S.S.; Alsuhebany, N.; Alqahtani, T.; Alshaya, A.I.; Almohareb, S.N.; Aldairem, A.; Alrashed, M.; Bin Saleh, K.; Badreldin, H.A.; et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med. Educ. 2023, 23, 689. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Goldstein, C. Clinical applications of artificial intelligence in sleep medicine: A sleep clinician's perspective. Sleep Breath. 2023, 27, 39–55. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E.; Aroutzidis, A.; Antonopoulou, H. Advances in Neuroimaging and Deep Learning for Emotion Detection: A Systematic Review of Cognitive Neuroscience and Algorithmic Innovations. Diagnostics 2025, 15, 456. [Google Scholar] [CrossRef]
- Perez-Pozuelo, I.; Zhai, B.; Palotti, J.; Mall, R.; Aupetit, M.; Garcia-Gomez, J.M.; Taheri, S.; Guan, Y.; Fernandez-Luque, L. The future of sleep health: A data-driven revolution in sleep science and medicine. NPJ Digit. Med. 2020, 3, 42. [Google Scholar] [CrossRef]
- Tutun, S.; Johnson, M.E.; Ahmed, A.; Albizri, A.; Irgil, S.; Yesilkaya, I.; Ucar, E.N.; Sengun, T.; Harfouche, A. An AI-based decision support system for predicting mental health disorders. Inf. Syst. Front. 2023, 25, 1261–1276. [Google Scholar] [CrossRef]
- Zidaru, T.; Morrow, E.M.; Stockley, R. Ensuring patient and public involvement in the transition to AI-assisted mental health care: A systematic scoping review and agenda for design justice. Health Expect. 2021, 24, 1072–1124. [Google Scholar] [CrossRef]
- Lüdtke, S.; Hermann, W.; Kirste, T.; Beneš, H.; Teipel, S. An algorithm for actigraphy-based sleep/wake scoring: Comparison with polysomnography. Clin. Neurophysiol. 2021, 132, 137–145. [Google Scholar] [CrossRef]
- Halkiopoulos, C.; Gkintoni, E. The Role of Machine Learning in AR/VR-Based Cognitive Therapies: A Systematic Review for Mental Health Disorders. Electronics 2025, 14, 1110. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Jiang, F.; Zhao, H. A novel machine learning unsupervised algorithm for sleep/wake identification using actigraphy. Chronobiol. Int. 2020, 37, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Regalia, G.; Gerboni, G.; Migliorini, M.; Lai, M.; Pham, J.; Puri, N.; Pavlova, M.K.; Picard, R.W.; Sarkis, R.A.; Onorati, F. Sleep assessment by means of a wrist actigraphy-based algorithm: Agreement with polysomnography in an ambulatory study on older adults. Chronobiol. Int. 2021, 38, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R. Application of deep learning to improve sleep scoring of wrist actigraphy. Sleep Med. 2022, 74, 235. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.; Oh, J. Automation of classification of sleep stages and estimation of sleep efficiency using actigraphy. Front. Public Health 2023, 10, 1092222. [Google Scholar] [CrossRef]
- Cay, G.; Ravichandran, V.; Sadhu, S.; Zisk, A.H.; Salisbury, A.L.; Solanki, D.; Mankodiya, K. Recent advancement in sleep technologies: A literature review on clinical standards, sensors, apps, and AI methods. IEEE Access 2022, 10, 104737–104756. [Google Scholar] [CrossRef]
- Shamim-Uzzaman, Q.A.; Bae, C.J.; Ehsan, Z.; Setty, A.R.; Devine, M.; Dhankikar, S.; Donskoy, I.; Fields, B.; Hearn, H.; Hwang, D.; et al. The use of telemedicine for the diagnosis and treatment of sleep disorders: An American Academy of Sleep Medicine update. J. Clin. Sleep Med. 2021, 17, 1103–1107. [Google Scholar] [CrossRef]
- Al-Shawwa, B.; Glynn, E.; Hoffman, M.A.; Ehsan, Z.; Ingram, D.G. Outpatient health care utilization for sleep disorders in the Cerner Health Facts database. J. Clin. Sleep Med. 2021, 17, 203–209. [Google Scholar] [CrossRef]
- Bazoukis, G.; Bollepalli, S.C.; Chung, C.T.; Li, X.; Tse, G.; Bartley, B.L.; Batool-Anwar, S.; Quan, S.F.; Armoundas, A.A. Application of artificial intelligence in the diagnosis of sleep apnea. J. Clin. Sleep Med. 2023, 19, 1337–1363. [Google Scholar] [CrossRef]
- Tramonti, F.; Giorgi, F.; Fanali, A. Systems thinking and the biopsychosocial approach: A multilevel framework for patient-centred care. Syst. Res. Behav. Sci. 2021, 38, 215–230. [Google Scholar] [CrossRef]
- Rosignoli, C.; Ornello, R.; Onofri, A.; Caponnetto, V.; Grazzi, L.; Raggi, A.; Leonardi, M.; Sacco, S. Applying a biopsychosocial model to migraine: Rationale and clinical implications. J. Headache Pain 2022, 23, 100. [Google Scholar] [CrossRef]
- Gkintoni, E.; Kourkoutas, E.; Vassilopoulos, S.; Mousi, M. Clinical Intervention Strategies and Family Dynamics in Adolescent Eating Disorders: Enhancing Early Detection and Outcomes. J. Clin. Med. 2024, 13, 4084. [Google Scholar] [CrossRef]
- Gkintoni, E.; Nikolaou, G. The Cross-Cultural Validation of Neuropsychological Assessments and Their Clinical Applications in Cognitive Behavioral Therapy: A Scoping Analysis. Int. J. Environ. Res. Public Health 2024, 21, 1110. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Breckons, M.; Lambelet, B.B.; Chung, J.; List, T.; Lobbezoo, F.; Nixdorf, D.R.; Oyarzo, J.F.; Peck, C.; Tsukiyama, Y.; et al. Challenges in the clinical implementation of a biopsychosocial model for assessment and management of orofacial pain. J. Oral Rehabil. 2020, 47, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, A. Back pain treatment: A new perspective. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1–13. [Google Scholar] [CrossRef]
- Richter, K.; Baumgärtner, L.; Niklewski, G.; Peter, L.; Köck, M.; Kellner, S.; Hillemacher, T.; Büttner-Teleaga, A. Sleep disorders in migrants and refugees: A systematic review with implications for personalized medical approach. Epma J. 2020, 11, 251–260. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Garvey, J.F.; McNicholas, W.T. Technologic advances in the assessment and management of obstructive sleep apnoea beyond the apnoea-hypopnoea index: A narrative review. J. Thorac. Dis. 2020, 12, 5020. [Google Scholar] [CrossRef]
- Ong, J.C.; Crawford, M.R.; Wallace, D.M. Sleep apnea and insomnia: Emerging evidence for effective clinical management. Chest 2021, 159, 2020–2028. [Google Scholar] [CrossRef]
- Lee, J.J.; Sundar, K.M. Evaluation and management of adults with obstructive sleep apnea syndrome. Lung 2021, 199, 87–101. [Google Scholar] [CrossRef]
- Mediano, O.; Cano-Pumarega, I.; Sánchez-De-La-Torre, M.; Alonso-Álvarez, M.L.; Troncoso, M.F.; García-Río, F.; Egea, C.; Durán-Cantolla, J.; Terán-Santos, J.; Barbé, F.; et al. Spanish Sleep Network. Upcoming scenarios for the comprehensive management of obstructive sleep apnea: An overview of the Spanish Sleep Network. Arch. Bronconeumol. 2020, 56, 35–41. [Google Scholar] [CrossRef]
- Scott, J.; Kallestad, H.; Vedaa, O.; Sivertsen, B.; Etain, B. Sleep disturbances and first onset of major mental disorders in adolescence and early adulthood: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 57, 101429. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, A.J.; Lamkaddem, A.; Benouajjit, A.; Ben El Bouaazzaoui, M.; El Houari, F.; Alami, M.; Labyad, S.; Chahidi, A.; Benjelloun, M.; Rabhi, S.; et al. Sleep quality and mental health in the context of the COVID-19 pandemic and lockdown in Morocco. Sleep Med. 2020, 74, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Schipper, S.B.; Van Veen, M.M.; Elders, P.J.; van Straten, A.; Van Der Werf, Y.D.; Knutson, K.L.; Rutters, F. Sleep disorders in people with type 2 diabetes and associated health outcomes: A review of the literature. Diabetologia 2021, 64, 2367–2377. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Ding, W.; Meng, Y.; Hu, H.; Liu, Z.; Zeng, X.; Wang, M. Psychological distress and sleep problems when people are under interpersonal isolation during an epidemic: A nationwide multicenter cross-sectional study. Eur. Psychiatry 2020, 63, e77. [Google Scholar] [CrossRef]
- Sun, Y.; Laksono, I.; Selvanathan, J.; Saripella, A.; Nagappa, M.; Pham, C.; Englesakis, M.; Peng, P.; Morin, C.M.; Chung, F. Prevalence of sleep disturbances in patients with chronic non-cancer pain: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 57, 101467. [Google Scholar] [CrossRef]
- Mandelkorn, U.; Genzer, S.; Choshen-Hillel, S.; Reiter, J.; Meira e Cruz, M.; Hochner, H.; Kheirandish-Gozal, L.; Gozal, D.; Gileles-Hillel, A. Escalation of sleep disturbances amid the COVID-19 pandemic: A cross-sectional international study. J. Clin. Sleep Med. 2021, 17, 45–53. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, Q.; Li, J.; Wang, F.; He, T.; Cheng, X.; Yang, B.X.; Ho, G.W.; Fu, G. A Generic Review of Integrating Artificial Intelligence in Cognitive Behavioral Therapy. arXiv 2024, arXiv:2407.19422. [Google Scholar]
- Olawade, D.B.; Wada, O.Z.; Odetayo, A.; David-Olawade, A.C.; Asaolu, F.; Eberhardt, J. Enhancing mental health with Artificial Intelligence: Current trends and future prospects. J. Med. Surg. Public Health 2024, 3, 100099. [Google Scholar] [CrossRef]
- Gkintoni, E.; Vantaraki, F.; Skoulidi, C.; Anastassopoulos, P.; Vantarakis, A. Gamified Health Promotion in Schools: The Integration of Neuropsychological Aspects and CBT—A Systematic Review. Medicina 2024, 60, 2085. [Google Scholar] [CrossRef]
- Rubin, M.; Arnon, H.; Huppert, J.D.; Perry, A. Considering the Role of Human Empathy in AI-Driven Therapy. JMIR Ment. Health 2024, 11, e56529. [Google Scholar] [CrossRef]
- Mathin, S.; Chandra, D.S.; Sunkireddy, A.R.; Varma, B.J.V.; Hariharan, S.; Kukreja, V. Personalized Mental Health Analysis Using Artificial Intelligence Approach. In Proceedings of the 2024 International Conference on Advances in Data Engineering and Intelligent Computing Systems (ADICS), Chennai, India, 18–19 April 2024. [Google Scholar] [CrossRef]
- Alotaibi, A.; Sas, C. Review of AI-Based Mental Health Apps. In Proceedings of the 36th International BCS Human-Computer Interaction Conference, Lancaster, UK, 28–29 August 2023; BCS Learning & Development: Swindon, UK, 2023. [Google Scholar] [CrossRef]
- Thieme, A.; Hanratty, M.; Lyons, M.; Palacios, J.; Marques, R.F.; Morrison, C.; Doherty, G. Designing human-centered AI for mental health: Developing clinically relevant applications for online CBT treatment. ACM Trans. Comput.-Hum. Interact. 2023, 30, 1–50. [Google Scholar] [CrossRef]
- Lin, A.; Espay, A.J. Remote delivery of cognitive behavioral therapy to patients with functional neurological disorders: Promise and challenges. Epilepsy Behav. Rep. 2021, 16, 100496. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- van den Akker, O.R.; Peters, G.-J.Y.; Bakker, C.J.; Carlsson, R.; Coles, N.A.; Corker, K.S.; Feldman, G.; Moreau, D.; Nordström, T.; Pickering, J.S.; et al. Increasing the transparency of systematic reviews: Presenting a generalized registration form. Syst. Rev. 2023, 12, 170. [Google Scholar] [CrossRef]
- A Alessi, C.; Fung, C.H.; Dzierzewski, J.M.; Fiorentino, L.; Stepnowsky, C.; Tapia, J.C.R.; Song, Y.; Zeidler, M.R.; Josephson, K.; Mitchell, M.N.; et al. Randomized controlled trial of an integrated approach to treating insomnia and improving the use of positive airway pressure therapy in veterans with comorbid insomnia disorder and obstructive sleep apnea. Sleep 2021, 44, zsaa235. [Google Scholar] [CrossRef]
- Alessi, C.; Martin, J.L.; Fiorentino, L.; Fung, C.H.; Dzierzewski, J.M.; Tapia, J.C.R.; Song, Y.; Josephson, K.; Jouldjian, S.; Mitchell, M.N. Cognitive Behavioral Therapy for Insomnia in Older Veterans Using Nonclinician Sleep Coaches: Randomized Controlled Trial. J. Am. Geriatr. Soc. 2016, 64, 1830–1838. [Google Scholar] [CrossRef]
- Anderson, K.; Goldsmith, P.; Gardiner, A. A pilot evaluation of an online cognitive behavioral therapy for insomnia disorder–targeted screening and interactive Web design lead to improved sleep in a community population. Nat. Sci. Sleep 2014, 6, 43–49. [Google Scholar] [CrossRef]
- Arnal, P.J.; Thorey, V.; Debellemaniere, E.; Mordret, E.; Llamosi, A.; Chouraki, A. Sleep Science at Home-Delivering Sleep Assessment and Digital CBT-i at Scale. Sleep 2020, 43, A209. [Google Scholar] [CrossRef]
- Bei, B.; Pinnington, D.; Quin, N.; Shen, L.; Blumfield, M.; Wiley, J.; Drummond, S.; Newman, L.; Manber, R. Improving perinatal sleep via a scalable cognitive behavioural intervention: Findings from a randomised controlled trial from pregnancy to 2 years postpartum. Psychol. Med. 2021, 53, 1–11. [Google Scholar] [CrossRef]
- Blom, K.; Tillgren, H.T.; Wiklund, T.; Danlycke, E.; Forssén, M.; Söderström, A.; Johansson, R.; Hesser, H.; Jernelöv, S.; Lindefors, N.; et al. Internet-vs. group-delivered cognitive behavior therapy for insomnia: A randomized controlled non-inferiority trial. Behav. Res. Ther. 2015, 70, 47–55. [Google Scholar] [CrossRef]
- Bostock, S.; Luik, A.; Espie, C. Sleep and Productivity Benefits of Digital Cognitive Behavioral Therapy for Insomnia: A Randomized Controlled Trial Conducted in the Workplace Environment. J. Occup. Environ. Med. 2016, 58, 683–689. [Google Scholar] [CrossRef]
- Brooks, A.; Tuason, R.; Chakravorty, S.; Raju, S.; Ritterband, L.; Thorndike, F.; Wallen, G. Online cognitive behavioral therapy for insomnia (CBT-I) for the treatment of insomnia among individuals with alcohol use disorder: Study protocol for a randomized controlled trial. Pilot Feasibility Stud. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Carney, C.; Edinger, J.; Kuchibhatla, M.; Lachowski, A.; Bogouslavsky, O.; Krystal, A.; Shapiro, C. Cognitive Behavioral Insomnia Therapy for Those With Insomnia and Depression: A Randomized Controlled Clinical Trial. Sleep 2017, 40, zsx019. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.; Baraças, L.; Hashioka, G.; Carvalho, A. 376 dCBT-I with Chatbot and Artificial Intelligence: A feasibility study in Brazil. Sleep 2021, 44, A149–A150. [Google Scholar] [CrossRef]
- Chan, C.; Wong, C.; Yu, B.; Hui, V.; Ho, F.; Cuijpers, P. Treating depression with a smartphone-delivered self-help cognitive behavioral therapy for insomnia: A parallel-group randomized controlled trial. Psychol. Med. 2021, 53, 1799–1813. [Google Scholar] [CrossRef]
- Cheng, P.; Casement, M.D.; A Kalmbach, D.; Castelan, A.C.; Drake, C.L. Digital cognitive behavioral therapy for insomnia promotes later health resilience during the coronavirus disease 19 (COVID-19) pandemic. Sleep 2020, 44, zsaa258. [Google Scholar] [CrossRef]
- Cheng, P.; A Kalmbach, D.; Tallent, G.; Joseph, C.L.; A Espie, C.; Drake, C.L. Depression prevention via digital cognitive behavioral therapy for insomnia: A randomized controlled trial. Sleep 2019, 42, zsz150. [Google Scholar] [CrossRef]
- Clarke, G.; McGlinchey, E.L.; Hein, K.; Gullion, C.M.; Dickerson, J.F.; Leo, M.C.; Harvey, A.G. Cognitive-behavioral treatment of insomnia and depression in adolescents: A pilot randomized trial. Behav. Res. Ther. 2015, 69, 111–118. [Google Scholar] [CrossRef]
- Cliffe, B.; Croker, A.; Denne, M.; Smith, J.; Stallard, P. Digital Cognitive Behavioral Therapy for Insomnia for Adolescents With Mental Health Problems: Feasibility Open Trial. JMIR Ment. Health 2020, 7, e14842. [Google Scholar] [CrossRef]
- Darden, M.; A Espie, C.; Carl, J.R.; Henry, A.L.; Kanady, J.C.; Krystal, A.D.; Miller, C.B. Cost-effectiveness of digital cognitive behavioral therapy (Sleepio) for insomnia: A Markov simulation model in the United States. Sleep 2020, 44, zsaa223. [Google Scholar] [CrossRef]
- Edinger, J.; Manber, R.; Simmons, B.; Johnson, R.; Horberg, R.; Depew, A.; Abraibesh, A.; Simpson, N.; Eldridge-Smith, E.D.; Strand, M.; et al. The Apnea and Insomnia Research (AIR) Trial: An Interim Report. Sleep 2022, 45, A207–A208. [Google Scholar] [CrossRef]
- Ellis, J.G.; Cushing, T.; Germain, A. Treating Acute Insomnia: A Randomized Controlled Trial of a “Single-Shot” of Cognitive Behavioral Therapy for Insomnia. Sleep 2015, 38, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, K.; Adachi, T.; Fujino, H.; Kugo, M.; Tatsumi, S.; Sasaki, J. Comparison of the effectiveness of cognitive behavioral therapy for insomnia, cognitive behavioral therapy for pain, and hybrid cognitive behavioral therapy for insomnia and pain in individuals with comorbid insomnia and chronic pain: A systematic review and network meta-analysis. Sleep Med. Rev. 2022, 66, 101693. [Google Scholar] [CrossRef] [PubMed]
- Espie, C.A.; Emsley, R.; Kyle, S.D.; Gordon, C.; Drake, C.L.; Siriwardena, A.N.; Cape, J.; Ong, J.C.; Sheaves, B.; Foster, R.; et al. Effect of Digital Cognitive Behavioral Therapy for Insomnia on Health, Psychological Well-being, and Sleep-Related Quality of Life: A Randomized Clinical Trial. JAMA Psychiatry 2019, 76, 21–30. [Google Scholar] [CrossRef]
- Espie, C.A.; Kyle, S.D.; Miller, C.B.; Ong, J.; Hames, P.; Fleming, L. Attribution, cognition and psychopathology in persistent insomnia disorder: Outcome and mediation analysis from a randomized placebo-controlled trial of online cognitive behavioural therapy. Sleep Med. 2014, 15, 913–917. [Google Scholar] [CrossRef]
- Eyal, S.; Altman, Y.; Baharav, A. Mobile Cognitive Behavioral Therapy is Efficient in Improving Sleep in Students. Sleep 2020, 43, A194. [Google Scholar] [CrossRef]
- Geagea, L.; Ghanimé, P.M.; El Hayek, S.; Kobeissy, F.; Tamim, H.; Elbejjani, M.; Talih, F. Assessing cognitive behavioral therapy for insomnia in individuals with cannabis use disorder utilizing actigraphy and serum biomarkers: A pilot study. Sleep Med. 2022, 100, 434–441. [Google Scholar] [CrossRef]
- Germain, A.; Wolfson, M.; Star, J.B.; Espejo, E.; Mysliwiec, V. 0395 Effectiveness of a Digital Clinical Decision Support Platform to Augment CBTI Capability Gaps in the DHA/DoD. Sleep 2024, 47, A170. [Google Scholar] [CrossRef]
- Grierson, A.; Hobbs, M.; Mason, E. Self-guided online cognitive behavioural therapy for insomnia: A naturalistic evaluation in patients with potential psychiatric comorbidities. J. Affect. Disord. 2020, 266, 305–310. [Google Scholar] [CrossRef]
- Haynes, J.; Talbert, M.; Fox, S.; Close, E. Cognitive Behavioral Therapy in the Treatment of Insomnia. South. Med. J. 2018, 111, 75–80. [Google Scholar] [CrossRef]
- Heenan, A.; Pipe, A.; Lemay, K.; Davidson, J.R.; Tulloch, H. Cognitive-Behavioral Therapy for Insomnia Tailored to Patients With Cardiovascular Disease: A Pre-Post Study. Behav. Sleep Med. 2020, 18, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.L.; Miller, C.B.; Emsley, R.; Sheaves, B.; Freeman, D.; Luik, A.I.; Littlewood, D.L.; Saunders, K.E.A.; Kanady, J.C.; Carl, J.R.; et al. Insomnia as a mediating therapeutic target for depressive symptoms: A sub-analysis of participant data from two large randomized controlled trials of a digital sleep intervention. J. Sleep Res. 2020, 30, e13140. [Google Scholar] [CrossRef] [PubMed]
- Horsch, C.H.; Lancee, J.; Griffioen-Both, F.; Spruit, S.; Fitrianie, S.; A Neerincx, M.; Beun, R.J.; Brinkman, W.-P. Mobile Phone-Delivered Cognitive Behavioral Therapy for Insomnia: A Randomized Waitlist Controlled Trial. J. Med. Internet Res. 2017, 19, e70. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, T.; Lee, M.R.G.; Stolee, J.D.; A Breitstein, J.; Kwon, H.P.; Mysliwiec, V. Cognitive Behavioral Therapy for Insomnia Among Active Duty Military Personnel Diagnosed With Obstructive Sleep Apnea. Mil. Med. 2022, 188, 2856–2861. [Google Scholar] [CrossRef]
- Hussaini, F.; Stachura, A.; Szalacha, L.; Canalejo, L. Insomnia Management Program in Primary Care Can Improve Subjective Sleep Quality and Insomnia Severity Index (ISI). Sleep 2024, 47, A181–A182. [Google Scholar] [CrossRef]
- Ito-Masui, A.; Sakamoto, R.; Matsuo, E.; Kawamoto, E.; Motomura, E.; Tanii, H.; Yu, H.; Sano, A.; Imai, H.; Shimaoka, M. Effect of an Internet–Delivered Cognitive Behavioral Therapy–Based Sleep Improvement App for Shift Workers at High Risk of Sleep Disorder: Single-Arm, Nonrandomized Trial. J. Med. Internet Res. 2023, 25, e45834. [Google Scholar] [CrossRef]
- Jernelöv, S.; Larsson, Y.; Llenas, M.; Nasri, B.; Kaldo, V. Effects and clinical feasibility of a behavioral treatment for sleep problems in adult attention deficit hyperactivity disorder (ADHD): A pragmatic within-group pilot evaluation. BMC Psychiatry 2019, 19, 226. [Google Scholar] [CrossRef]
- Kallestad, H.; Scott, J.; Vedaa, Ø.; Lydersen, S.; Vethe, D.; Morken, G.; Stiles, T.C.; Sivertsen, B.; Langsrud, K. Mode of delivery of Cognitive Behavioral Therapy for Insomnia: A randomized controlled non-inferiority trial of digital and face-to-face therapy. Sleep 2021, 44, zsab185. [Google Scholar] [CrossRef]
- A Kalmbach, D.; Cheng, P.; Ahmedani, B.K.; Peterson, E.L.; Reffi, A.N.; Sagong, C.; Seymour, G.M.; Ruprich, M.K.; Drake, C.L. Cognitive-behavioral therapy for insomnia prevents and alleviates suicidal ideation: Insomnia remission is a suicidolytic mechanism. Sleep 2022, 45, zsac251. [Google Scholar] [CrossRef]
- Kalmbach, D.A.; Cheng, P.; O’Brien, L.M.; Swanson, L.M.; Sangha, R.; Sen, S.; Guille, C.; Cuamatzi-Castelan, A.; Henry, A.L.; Roth, T.; et al. A randomized controlled trial of digital cognitive behavioral therapy for insomnia in pregnant women. Sleep Med. 2020, 72, 82–92. [Google Scholar] [CrossRef]
- Kim, J.; Gartenberg, D.; Bitonte, E.; Kushida, C. An Internet of Things Cognitive Behavioral Therapy-based Device Reduces Insomnia Severity and Increases Sleep Time. Sleep 2024, 47, A189. [Google Scholar] [CrossRef]
- Kuhn, E.; Miller, K.E.; Puran, D.; Wielgosz, J.; YorkWilliams, S.L.; Owen, J.E.; Jaworski, B.K.; Hallenbeck, H.W.; McCaslin, S.E.; Taylor, K.L. A Pilot Randomized Controlled Trial of the Insomnia Coach Mobile App to Assess Its Feasibility, Acceptability, and Potential Efficacy. Behav. Ther. 2021, 53, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Kyle, S.D.; Hurry, M.E.D.; Emsley, R.; Marsden, A.; Omlin, X.; Juss, A.; Spiegelhalder, K.; Bisdounis, L.; Luik, A.I.; Espie, C.A.; et al. The effects of digital cognitive behavioral therapy for insomnia on cognitive function: A randomized controlled trial. Sleep 2020, 43, zsaa034. [Google Scholar] [CrossRef]
- Liang, S.; Mao, H.; Yang, J.; Deng, W.; Cao, B.; Yu, Z.; Yang, L.; Xu, Y.; Hu, N.; Liu, W.; et al. Digital cognitive behavior therapy for insomnia improving sleep quality: A real-world study. BMC Psychiatry 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Lovato, N.; Lack, L.; Wright, H.; Kennaway, D.J. Evaluation of a Brief Treatment Program of Cognitive Behavior Therapy for Insomnia in Older Adults. Sleep 2014, 37, 117–126. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Zhang, J.; Huang, S.; Huang, F.; Wang, T.; Wu, F.; Mao, H.; Huang, Z. Comparative Effectiveness of Digital Cognitive Behavioral Therapy vs Medication Therapy Among Patients With Insomnia. JAMA Netw. Open 2023, 6, e237597. [Google Scholar] [CrossRef]
- Luik, A.I.; Machado, P.F.; Espie, C.A. Delivering digital cognitive behavioral therapy for insomnia at scale: Does using a wearable device to estimate sleep influence therapy? Digit. Med. 2018, 1, 3. [Google Scholar] [CrossRef]
- McCrae, C.S.; Chan, W.S.; Curtis, A.F.; Nair, N.; Deroche, C.B.; Munoz, M.; Takamatsu, S.; McLean, D.; Davenport, M.; E Muckerman, J.; et al. Telehealth cognitive behavioral therapy for insomnia in children with autism spectrum disorder: A pilot examining feasibility, satisfaction, and preliminary findings. Autism 2020, 25, 667–680. [Google Scholar] [CrossRef]
- McCurry, S.M.; Zhu, W.; Von Korff, M.; Wellman, R.; Morin, C.M.; Thakral, M.; Yeung, K.; Vitiello, M.V. Effect of Telephone Cognitive Behavioral Therapy for Insomnia in Older Adults With Osteoarthritis Pain: A Randomized Clinical Trial. JAMA Intern. Med. 2021, 181, 530–538. [Google Scholar] [CrossRef]
- Miller, C.; Waxmonsky, J.; Henry, A.; Emsley, R.; Espie, C. The Effectiveness of Digital Cognitive Behavioral Therapy for Insomnia, Fatigue, and Quality of Life in Cancer Patients. Sleep 2023, 46, A162. [Google Scholar] [CrossRef]
- Morin, C.M. Cognitive Behavioral Therapy for Chronic Insomnia: State of the Science Versus Current Clinical Practices. Ann. Intern. Med. 2015, 163, 236–237. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.; Thorndike, F.P.; Ojile, J.M.; Gerwien, R.; Wendorf, A.; A Maricich, Y. Digital CBT-I Treatment Improves Sleep and Reduces Anxiety and Depression Symptoms in Adults With Chronic Insomnia: Interim Analysis of DREAM Study. CNS Spectr. 2023, 28, 227–228. [Google Scholar] [CrossRef]
- Neuenschwander, E.; Spector, T.; Kalmbach, D.; Sagong, C.; Drake, C.; Cheng, P. Tracking circadian phase for personalized schedules: A precise medical approach to reduce circadian misalignment in shift workers. Sleep 2023, 46, A270–A271. [Google Scholar] [CrossRef]
- Nguyen, S.; McKenzie, D.; McKay, A.; Wong, D.; Rajaratnam, S.M.W.; Spitz, G.; Williams, G.; Mansfield, D.; Ponsford, J. Exploring predictors of treatment outcome in cognitive behavior therapy for sleep disturbance following acquired brain injury. Disabil. Rehabil. 2017, 40, 1906–1913. [Google Scholar] [CrossRef]
- Okajima, I.; Akitomi, J.; Kajiyama, I.; Ishii, M.; Murakami, H.; Yamaguchi, M. Effects of a Tailored Brief Behavioral Therapy Application on Insomnia Severity and Social Disabilities Among Workers With Insomnia in Japan. JAMA Netw. Open 2020, 3, e202775. [Google Scholar] [CrossRef]
- Paniccia, G.; Habash, S.; Drake, C.; Walch, O.; Cheng, P. Personalized Light Therapy for Night Shift Work: A Precision Medicine Approach to Reducing Insomnia and Sleepiness. Sleep 2024, 47, A11–A12. [Google Scholar] [CrossRef]
- Patel, S.; Ahmed, A.; Foldvary-Schaefer, N.; Ojo, O.; Genc, G.; Oravivattanakul, S.; Fernandez, H. A Computerized Cognitive Behavioral Therapy Randomized, Controlled, Pilot Trial for Insomnia in Parkinson’s Disease (ACCORD-PD Study) (P4.333). Neurology 2016, 86, P4-333. [Google Scholar] [CrossRef]
- Peter, L.; Reindl, R.; Zauter, S.; Hillemacher, T.; Richter, K. Effectiveness of an Online CBT-I Intervention and a Face-to-Face Treatment for Shift Work Sleep Disorder: A Comparison of Sleep Diary Data. Int. J. Environ. Res. Public Health 2019, 16, 3081. [Google Scholar] [CrossRef]
- Philip, P.; Dupuy, L.; Morin, C.M.; de Sevin, E.; Bioulac, S.; Taillard, J.; Serre, F.; Auriacombe, M.; Micoulaud-Franchi, J.-A. Smartphone-Based Virtual Agents to Help Individuals With Sleep Concerns During COVID-19 Confinement: Feasibility Study. J. Med. Internet Res. 2020, 22, e24268. [Google Scholar] [CrossRef]
- Pulantara, I.W.; Parmanto, B.; Germain, A. Clinical Feasibility of a Just-in-Time Adaptive Intervention App (iREST) as a Behavioral Sleep Treatment in a Military Population: Feasibility Comparative Effectiveness Study. J. Med. Internet Res. 2018, 20, e10124. [Google Scholar] [CrossRef]
- Reesen, J.; van der Zweerde, T.; Batelaan, N.; Fris, E.; Hoogendoorn, A.; Ikelaar, S.; Lakbila-Kamal, O.; Lancee, J.; Leerssen, J.; van Marle, H.; et al. Do better nights lead to better days? Guided internet-based cognitive behavioral therapy for insomnia in people suffering from a range of mental health problems: Protocol of a pragmatic randomized clinical trial. Contemp. Clin. Trials 2023, 127, 107122. [Google Scholar] [CrossRef] [PubMed]
- Reilly, E.D.; A Robinson, S.; Petrakis, B.A.; Gardner, M.M.; Wiener, R.S.; Castaneda-Sceppa, C.; Quigley, K.S. Mobile Intervention to Improve Sleep and Functional Health of Veterans With Insomnia: Randomized Controlled Trial. JMIR Form. Res. 2021, 5, e29573. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Zhang, Y.; Shi, Y.; Zhang, H.; Vitiello, M.V.; Tang, X. The beneficial effects of integrating a personalized telephone-delivered component into digital cognitive behavioral therapy for insomnia in a large, hospital-based population. Sleep Med. 2023, 106, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ritterband, L.M.; Thorndike, F.P.; Ingersoll, K.S.; Lord, H.R.; Gonder-Frederick, L.; Frederick, C.; Quigg, M.S.; Cohn, W.F.; Morin, C.M. Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up: A Randomized Clinical Trial. JAMA Psychiatry 2017, 74, 68–75. [Google Scholar] [CrossRef]
- Rötger, A.; Schuffelen, J.; Maurer, L.F.; Lorenz, N.; Pollok, B.; Gieselmann, A. The clinical effect of digital cognitive behavioural therapy for insomnia in subgroups with depressive and anxiety symptoms: A secondary analysis of a randomized–controlled trial. J. Sleep Res. 2024, 33, e14173. [Google Scholar] [CrossRef]
- Sadler, P.; McLaren, S.; Klein, B.; Harvey, J.; Jenkins, M. Cognitive behavior therapy for older adults with insomnia and depression: A randomized controlled trial in community mental health services. Sleep 2018, 41, zsy104. [Google Scholar] [CrossRef]
- Schuffelen, J.; Maurer, L.F.; Lorenz, N.; Rötger, A.; Pietrowsky, R.; Gieselmann, A. The clinical effects of digital cognitive behavioral therapy for insomnia in a heterogenous study sample: Results from a randomized controlled trial. Sleep 2023, 46, zsad184. [Google Scholar] [CrossRef]
- Schwartz, B.; Cohen, Z.D.; Rubel, J.A.; Zimmermann, D.; Wittmann, W.W.; Lutz, W. Personalized treatment selection in routine care: Integrating machine learning and statistical algorithms to recommend cognitive behavioral or psychodynamic therapy. Psychother. Res. 2020, 31, 33–51. [Google Scholar] [CrossRef]
- Selvanathan, J.; Pham, C.; Nagappa, M.; Peng, P.W.; Englesakis, M.; Espie, C.A.; Morin, C.M.; Chung, F. Cognitive behavioral therapy for insomnia in patients with chronic pain—A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2021, 60, 101460. [Google Scholar] [CrossRef]
- Siengsukon, C.F.; Beck, E.S.; Drerup, M. Feasibility and Treatment Effect of a Web-Based Cognitive Behavioral Therapy for Insomnia Program in Individuals with Multiple Sclerosis: A Pilot Randomized Controlled Trial. Int. J. MS Care 2020, 23, 107–113. [Google Scholar] [CrossRef]
- Speed, T.J.; Hanks, L.; Turner, G.; Gurule, E.; Kearson, A.; Buenaver, L.; Smith, M.T.; Antoine, D. A comparison of cognitive behavioral therapy for insomnia to standard of care in an outpatient substance use disorder clinic embedded within a therapeutic community: A RE-AIM framework evaluation. Trials 2022, 23, 1–15. [Google Scholar] [CrossRef]
- Stott, R.; Pimm, J.; Emsley, R.; Miller, C.B.; Espie, C.A. Does adjunctive digital CBT for insomnia improve clinical outcomes in an improving access to psychological therapies service? Behav. Res. Ther. 2021, 144, 103922. [Google Scholar] [CrossRef]
- Sweetman, A.; Lack, L.; Lambert, S.; Gradisar, M.; Harris, J. Does comorbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Med. 2017, 39, 38–46. [Google Scholar] [CrossRef]
- Sweetman, A.; McEvoy, R.D.; Smith, S.; Catcheside, P.G.; A Antic, N.; Chai-Coetzer, C.L.; Douglas, J.; O’grady, A.; Dunn, N.; Robinson, J.; et al. The effect of cognitive and behavioral therapy for insomnia on week-to-week changes in sleepiness and sleep parameters in patients with comorbid insomnia and sleep apnea: A randomized controlled trial. Sleep 2020, 43, zsaa002. [Google Scholar] [CrossRef] [PubMed]
- Talbot, L.S.; Maguen, S.; Metzler, T.J.; Schmitz, M.; McCaslin, S.E.; Richards, A.; Perlis, M.L.; Posner, D.A.; Weiss, B.; Ruoff, L.; et al. Cognitive Behavioral Therapy for Insomnia in Posttraumatic Stress Disorder: A Randomized Controlled Trial. Sleep 2014, 37, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Tomfohr-Madsen, L.M.; Clayborne, Z.M.; Rouleau, C.R.; Campbell, T.S. Sleeping for Two: An Open-Pilot Study of Cognitive Behavioral Therapy for Insomnia in Pregnancy. Behav. Sleep Med. 2016, 15, 377–393. [Google Scholar] [CrossRef]
- Tomfohr-Madsen, L.; Madsen, J.W.; Bonneville, D.B.; Virani, S.M.; Plourde, V.; Barlow, K.M.M.; Yeates, K.O.; Brooks, B.L. A Pilot Randomized Controlled Trial of Cognitive-Behavioral Therapy for Insomnia in Adolescents With Persistent Postconcussion Symptoms. J. Head Trauma Rehabil. 2020, 35, E103–E112. [Google Scholar] [CrossRef]
- Trauer, J.M.; Qian, M.Y.; Doyle, J.S.; Rajaratnam, S.M.W.; Cunnington, D. Cognitive Behavioral Therapy for Chronic Insomnia. Ann. Intern. Med. 2015, 163, 191–204. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kuroki, T.; Ichikawa, D.; Ozone, M.; Uchimura, N.; Ueno, T. Effect of smartphone-based cognitive behavioral therapy app on insomnia: A randomized, double-blind study. Sleep 2022, 46, zsac270. [Google Scholar] [CrossRef]
- Zachariae, R.; Lyby, M.S.; Ritterband, L.M.; O’Toole, M.S. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia–A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Guo, X.; Liu, Y.; Shen, Y.; Ma, J. Digital Cognitive Behavioral Therapy for Insomnia Using a Smartphone Application in China. JAMA Netw. Open 2023, 6, e234866. [Google Scholar] [CrossRef] [PubMed]
- Germain, A.; Markwald, R.R.; King, E.; Bramoweth, A.D.; Wolfson, M.; Seda, G.; Han, T.; Miggantz, E.; O’reilly, B.; Hungerford, L.; et al. Enhancing behavioral sleep care with digital technology: Study protocol for a hybrid type 3 implementation-effectiveness randomized trial. Trials 2021, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, V.; Gkintoni, E.; Halkiopoulos, C.; Karademas, E.C. Quality of Life and Incidence of Clinical Signs and Symptoms among Caregivers of Persons with Mental Disorders: A Cross-Sectional Study. Healthcare 2024, 12, 269. [Google Scholar] [CrossRef]
- McCradden, M.; Hui, K.; Buchman, D.Z. Evidence, ethics and the promise of artificial intelligence in psychiatry. J. Med. Ethics 2023, 49, 573–579. [Google Scholar] [CrossRef]
- Okpete, U.E.; Byeon, H. Challenges and prospects in bridging precision medicine and artificial intelligence in genomic psychiatric treatment. World J. Psychiatry 2024, 14, 1148–1164. [Google Scholar] [CrossRef]
- Rollwage, M.; Habicht, J.; Juechems, K.; Carrington, B.; Viswanathan, S.; Stylianou, M.; Hauser, T.U.; Harper, R. Using Conversational AI to Facilitate Mental Health Assessments and Improve Clinical Efficiency Within Psychotherapy Services: Real-World Observational Study. JMIR AI 2023, 2, e44358. [Google Scholar] [CrossRef]
- Ursin, F.; Timmermann, C.; Steger, F. Ethical Implications of Alzheimer’s Disease Prediction in Asymptomatic Individuals through Artificial Intelligence. Diagnostics 2021, 11, 440. [Google Scholar] [CrossRef]
- Naik, K.; Goyal, R.K.; Foschini, L.; Chak, C.W.; Thielscher, C.; Zhu, H.; Lu, J.; Lehár, J.; Pacanoswki, M.A.; Terranova, N.; et al. Current status and future directions: The application of artificial intelligence/machine learning for precision medicine. Clin. Pharmacol. Ther. 2024, 115, 673–686. [Google Scholar] [CrossRef]
- Schultebraucks, K.; Chang, B.P. The opportunities and challenges of machine learning in the acute care setting for precision prevention of posttraumatic stress sequelae. Exp. Neurol. 2021, 336, 113526. [Google Scholar] [CrossRef]
- Aggarwal, N.; Ahmed, M.; Basu, S.; Curtin, J.J.; Evans, B.J.; Matheny, M.E.; Nundy, S.; Sendak, M.P.; Shachar, C.; Shah, R.U.; et al. Advancing Artificial Intelligence in Health Settings Outside the Hospital and Clinic. NAM Perspect. 2020, 2020. [Google Scholar] [CrossRef]
- de Lacy, N.; Ramshaw, M.J.; McCauley, E.; Kerr, K.F.; Kaufman, J.; Nathan Kutz, J. Predicting individual cases of major adolescent psychiatric conditions with artificial intelligence. Transl. Psychiatry 2023, 13, 314. [Google Scholar] [CrossRef] [PubMed]


| Authors | Study Objectives | Participants (n) | Methodology | Main Findings |
|---|---|---|---|---|
| Alessi, C. et al. (2020) [87] | An integrated approach of cognitive behavioral therapy for insomnia and a PAP adherence program improved sleep and PAP use in adults with comorbid insomnia and obstructive sleep apnea. | 125 | The intervention consisted of a structured, manual-based treatment that integrated cognitive behavioral therapy for insomnia (CBTI) with a positive airway pressure (PAP) adherence program. The intervention was delivered in 5 weekly 1 h individual sessions by a “sleep coach” who had a master’s degree level of education but no clinical training or licensure. The CBTI components included stimulus control, sleep restriction, sleep hygiene, relaxation techniques, and cognitive therapy techniques. The PAP adherence components included education about OSA and PAP, reviewing the participant’s individual benefits and challenges with PAP use and providing individualized recommendations and strategies to address challenges. The sleep coach received weekly 1 h telephone supervision from a behavioral sleep medicine psychologist to review participant progress and problem-solve issues with CBTI and PAP adherence. |
|
| Alessi, C. et al. (2016) [88] | This paper tests a cognitive behavioral therapy for insomnia program designed for use by nonclinicians. | 159 | The intervention was a cognitive behavioral therapy for insomnia (CBT-I) program delivered by non-clinician “sleep coaches” with weekly telephone supervision by a psychologist with expertise in behavioral sleep medicine. The intervention was delivered either in a small group format (3–5 participants) or individually, with 5 one-hour sessions over 6 weeks. |
|
| Anderson, K. et al. (2014) [89] | Online cognitive behavioral therapy for insomnia can be personalized and effective, but screening for other sleep disorders is necessary. | 75 | The intervention was an online cognitive behavioral therapy (CBT) for insomnia disorder, delivered through a modern interactive video-based website. Participants underwent a rigorous screening process to exclude those with other sleep disorders or mental health conditions before starting the therapy, which was personalized based on their responses. |
|
| Arnal, P. et al. (2020) [90] | A personalized digital CBT-I program using hardware, software, and therapist support shows high engagement and effectiveness for treating insomnia. | 1304 | The intervention was a 6-week cognitive behavioral therapy for insomnia (CBT-i) program delivered through the Dreem platform. Participants had to complete at least one week of the program to be included in the analysis. |
|
| Bei, B. et al. (2021) [91] | A scalable cognitive behavioral therapy intervention improved sleep during pregnancy and up to 2 years postpartum, especially for those with elevated insomnia symptoms. | 163 | The intervention was a scalable cognitive behavioral therapy (CBT) sleep intervention. The intervention consisted of a 1 h telephone session and automated multimedia emails, delivered from the third trimester of pregnancy until 6 months postpartum. |
|
| Blom, K. et al. (2015) [92] | Internet-delivered and group-delivered cognitive behavioral therapy for insomnia were found to be equally effective. | 48 | Guided Internet-delivered CBT (ICBT) and group-delivered CBT (GCBT) for insomnia. |
|
| Bostock, S. et al. (2016) [93] | Digital cognitive behavioral therapy for insomnia improves sleep and work productivity in adults. | 270 | The intervention was a digital cognitive behavioral therapy (dCBT) for insomnia, consisting of 6 online sessions delivered by an animated therapist. A total of 135 participants received this dCBT intervention. |
|
| Brooks, A. et al. (2018) [94] | This study protocol examines the feasibility and efficacy of an online cognitive behavioral therapy for insomnia among individuals with alcohol use disorder. | 70 | The intervention is the SHUTi (Sleep Healthy Using The Internet) program, which is an Internet-based cognitive behavioral therapy for insomnia (CBT-I) intervention. The intervention will be delivered in two phases—a feasibility phase with 10 participants and then a larger RCT with 30 participants per group. | Not mentioned (the abstract does not provide any quantitative results or intervention effects from the study). |
| Carney, C. et al. (2017) [95] | This paper compares cognitive behavioral therapy for insomnia (CBT-I) plus antidepressant medication against treatments targeting solely depression or insomnia, finding that CBT-I groups improved on objective sleep measures. | 107 |
|
|
| Castro, L. et al. (2021) [96] | A fully automated digital cognitive behavioral therapy for insomnia using chatbot and AI shows feasibility in improving sleep parameters and engagement. | 3139 |
|
|
| Chan, C. et al. (2021) [97] | A smartphone-delivered self-help cognitive behavioral therapy for insomnia is effective in alleviating major depression and insomnia. | 320 | The intervention was a 6-week smartphone-delivered self-help cognitive behavioral therapy for insomnia (CBT-I) program called proACT-S. |
|
| Cheng, P. et al. (2020) [98] | Prior digital cognitive behavioral therapy for insomnia increased health resilience during the COVID-19 pandemic. | 208 | The intervention was 6 sessions of self-guided digital cognitive behavioral therapy for insomnia (dCBT-I), delivered via an animated “virtual therapist” who guided the participant’s progress. |
|
| Cheng, P. et al. (2019) [99] | Digital cognitive behavioral therapy for insomnia can prevent depression. | 1385 | The intervention was digital cognitive behavioral therapy for insomnia (dCBT-I) delivered via the Sleepio program. Participants received access to the program for 12 weeks and could complete the 6 core sessions on a weekly basis. The intervention covered behavioral, cognitive, and relaxation components, as well as sleep hygiene, and was delivered by an animated “virtual therapist”. |
|
| Clarke, G. et al. (2015) [100] | Cognitive behavioral treatment of insomnia and depression in adolescents shows promise but does not involve artificial intelligence. | 41 |
|
|
| Cliffe, B. et al. (2020) [101] | Digital cognitive behavioral therapy for insomnia is feasible and effective for improving sleep and mental health in adolescents with mental health problems. | 49 | The intervention was a 6-session, 20-min per session digital cognitive behavioral therapy for insomnia (digital CBTi) program called Sleepio, with additional weekly 15 min support telephone calls. |
|
| Darden, M. et al. (2020) [102] | Digital CBT is the most cost-effective insomnia treatment compared to other options in the US. | 100,000 |
|
|
| Edinger, J. et al. (2022) [103] | Digital and therapist-delivered cognitive behavioral therapies for insomnia are both effective for treating insomnia in sleep apnea patients. | 305 |
|
|
| Ellis, J. et al. (2015) [104] | A single session of cognitive behavioral therapy for insomnia is effective for treating acute insomnia. | 40 | The intervention consisted of a single 60 to 70 min session of cognitive behavioral therapy for insomnia (CBT-I), along with a self-help pamphlet that the participants were given. |
|
| Enomoto, K. et al. (2022) [105] | Cognitive behavioral therapy for insomnia is the most effective treatment option for individuals with comorbid insomnia and chronic pain. | 1094 |
|
|
| Espie, C. et al. (2019) [106] | Digital cognitive behavioral therapy for insomnia improves functional health, psychological well-being, and sleep-related quality of life. | 1711 | The intervention was digital cognitive behavioral therapy (dCBT) for insomnia, delivered using web and/or mobile channels, in addition to usual treatment. The control group received sleep hygiene education (SHE) through a website and a downloadable booklet in addition to usual treatment. |
|
| Espie, C. et al. (2014) [107] | Online cognitive behavioral therapy modifies sleep-related attributions, night-time thought content, and psychopathology, which partly mediates improvement in insomnia. | 164 |
|
|
| Eyal, S. et al. (2020) [108] | A mobile app providing personalized digital cognitive and behavioral therapy was effective in improving insomnia symptoms in students, but engagement remains a challenge. | 892 | The intervention was a mobile app called “Refresh by Sleeprate” that provided a sleep assessment followed by weekly cycles of personalized digital cognitive and behavioral reframing. The duration of the intervention was at least one week, with an average of 5.6 weekly nights and 28 total nights for those who completed the intervention. |
|
| Geagea, L. et al. (2022) [109] | This pilot study found that cognitive behavioral therapy for insomnia is effective in treating insomnia and comorbid conditions in individuals with cannabis use disorder. | 19 | The intervention was cognitive behavioral therapy for insomnia (CBTi), consisting of 4 sessions, with participants wearing an actigraphy device for 1 week before and 1 week after the 4 CBTi sessions. |
|
| Germain, A. et al. (2024) [110] | A digital clinical decision support platform can augment CBTI capabilities and yield rapid, clinically meaningful improvements in sleep among active-duty service members with insomnia. | 245 | The intervention was a digital clinical decision support (CDS) platform called COAST (NOCTEM®® Health, Inc., Pittsburgh, PA, USA) that was used by mental healthcare providers (MHCPs) to deliver cognitive behavioral therapy for insomnia (CBTI) to active-duty service members (ADSMs) with insomnia. The platform consisted of a clinician portal to remotely monitor and manage patients and a patient app to collect sleep diaries and display CBTI recommendations. The average treatment duration was 5 ± 1 weeks. |
|
| Grierson, A. et al. (2020) [111] | Unguided Internet-delivered cognitive behavioral therapy for insomnia is effective for individuals with potential psychiatric comorbidities. | 317 | The intervention was an unguided, self-guided online cognitive behavioral therapy program for insomnia (iCBT-I) consisting of 4 online lessons with automated web support but no human guidance or support. |
|
| Haynes, J. et al. (2018) [112] | Cognitive behavioral therapy is an effective treatment for insomnia, but the paper does not mention personalization using artificial intelligence. | The intervention is cognitive behavioral therapy for insomnia (CBTi), which includes the following components: sleep hygiene, stimulus control, sleep restriction, cognitive therapy, and relaxation training. |
| |
| Heenan, A. et al. (2019) [113] | A group-based cognitive behavioral therapy for insomnia tailored to patients with cardiovascular disease improved their sleep and reduced anxiety and depression. | 47 | The intervention was a 6-week group-based cognitive behavioral therapy for insomnia (CBT-I) program that was tailored for patients with cardiovascular disease (CVD). |
|
| Henry, A. et al. (2020) [114] | Cognitive behavioral therapy for insomnia can improve both insomnia and depressive symptoms, with insomnia improvement mediating effects on depression. | 3352 | The intervention was a fully-automated digital cognitive behavioral therapy (CBT) intervention for insomnia called Sleepio. |
|
| Horsch., C. et al. (2017) [115] | A fully automated mobile phone app delivering cognitive behavioral therapy for insomnia was found to be effective. | 151 |
|
|
| Hoyt, T. et al. (2022) [116] | Cognitive behavioral therapy for insomnia is effective in treating military personnel with comorbid insomnia and obstructive sleep apnea. | 73 | The intervention was individual cognitive behavioral therapy for insomnia (CBT-I) provided to all 73 participants at a specialty sleep clinic. |
|
| Hussaini, F. et al. (2024) [117] | A self-guided insomnia management program using a CBT-I mobile app can improve sleep quality and insomnia severity in primary care. | 23 | The intervention was a self-guided insomnia management program delivered through the CBT-I Coach mobile app, along with sleep hygiene education. Family medicine providers also received education on insomnia and recommended the use of CBT-I as the primary management approach. Participants were recruited over an 8-week period, including both referrals and patients identified through chart reviews. |
|
| Ito-Masui, A. et al. (2023) [118] | A 4-week, physician-assisted, Internet-delivered CBT program incorporating machine learning–based well-being prediction increased sleep duration and subjective sleep quality in shift workers at high risk of sleep disorders. | 61 |
|
|
| Jernelöv, S. et al. (2019) [119] | A CBT-i-based group treatment improved insomnia severity in adults with ADHD but does not involve personalized AI-based therapy. | 19 | Group-delivered CBT-i-based treatment, consisting of 10 weekly 90 min group sessions and scheduled telephone support, provided as an adjunct to usual care at the clinic for adult ADHD patients with self-reported sleep problems. |
|
| Kallestad, H. et al. (2021) [120] | This paper compares the efficacy of digital vs. face-to-face cognitive behavioral therapy for insomnia but does not involve personalization or artificial intelligence. | 101 |
|
|
| Kalmbach, D. et al. (2022) [121] | Cognitive behavioral therapy for insomnia can alleviate and prevent suicidal ideation by improving sleep. | 658 | The intervention was digital cognitive behavioral therapy for insomnia (digital CBTI). |
|
| Kalmbach, D. et al. (2020) [122] | Digital cognitive behavioral therapy for insomnia improves sleep quality and duration in pregnant women. | 91 | Digital cognitive behavioral therapy for insomnia (digital CBTI). |
|
| Kim, J. et al. (2024) [123] | An IoT device that delivers personalized CBT-I prompts can significantly reduce insomnia severity and increase sleep time. | 65 | The intervention was the “Full Sleep” Internet of Things (IoT) device that included an intelligence-sensing radar to passively track sleep patterns and deliver in-the-moment behavioral prompting to promote adherence to cognitive behavioral therapy for insomnia (CBT-I) directives. The device was used by participants as part of a 6–8-week program. |
|
| Kuhn, E. et al. (2021) [124] | This pilot study found that the Insomnia Coach mobile app, a CBT-I-based self-management tool, is feasible, and acceptable, and shows promise for improving insomnia and related outcomes in veterans. | 50 | The Intervention was the Insomnia Coach mobile app, a free, CBT-I-based self-management app that participants used for 6 weeks. |
|
| Kyle, S. et al. (2020) [125] | Digital cognitive behavioral therapy for insomnia decreases self-reported cognitive impairment but does not improve objective cognitive performance. | 410 | Digital cognitive behavioral therapy (dCBT) for insomnia. |
|
| Liang, S. et al. (2022) [126] | Digital cognitive behavioral therapy for insomnia is an effective treatment for improving sleep quality in a real-world clinical setting. | 6002 | The intervention was digital cognitive behavior therapy for insomnia (dCBT-I) delivered through a mobile app to patients with insomnia, anxiety disorders, or comorbid insomnia and depression. The intervention was delivered for at least 8 weeks and, in some cases, up to 12 weeks. |
|
| Lovato, N. et al. (2014) [127] | A brief 4-week group-based cognitive behavioral therapy program improved sleep quality and daytime functioning in older adults with insomnia. | 118 | The intervention was a 4-week, group-based treatment program of cognitive behavior therapy for insomnia (CBT-I) that included bedtime restriction therapy, sleep education, and cognitive restructuring. The sessions were 60 min long and delivered weekly to small groups of 4-5 participants. |
|
| Lu, M. et al. (2023) [128] | Digital cognitive behavioral therapy for insomnia is superior to medication therapy at 6-month follow-up, but the combination of the two is most effective long-term. | 4052 |
|
|
| Luik, A. et al. (2018) [129] | Using a wearable device to estimate sleep does not significantly affect the outcomes of digital cognitive behavioral therapy for insomnia. | 3551 | The intervention was digital cognitive behavioral therapy (dCBT) for insomnia. |
|
| McCrae, C. et al. (2020) [130] | Telehealth delivery of cognitive behavioral therapy for insomnia in children with autism spectrum disorder is feasible and may improve sleep, behavior, and arousal. | 17 | The intervention was an 8-session telehealth cognitive behavioral treatment for childhood insomnia (telehealth CBT-CI) delivered to 17 children (ages 6–12) with autism spectrum disorder and insomnia, along with their parents. The treatment integrity was assessed for delivery, receipt/understanding, and enactment, and parents found the intervention to be helpful, age-appropriate, and autism-friendly. |
|
| McCurry, S. et al. (2021) [131] | Telephone-delivered cognitive behavioral therapy for insomnia improved sleep, fatigue, and pain in older adults with osteoarthritis. | 327 | Telephone-delivered cognitive behavioral therapy for insomnia (CBT-I), consisting of 6 sessions of 20–30 min each, delivered over 8 weeks. The CBT-I sessions included sleep restriction, stimulus control, sleep hygiene, cognitive restructuring, and homework. |
|
| Miller, C. et al. (2023) [132] | Digital cognitive behavioral therapy was effective for reducing insomnia and fatigue symptoms in cancer patients. | 70 | The intervention was digital cognitive behavioral therapy (dCBT) for insomnia, delivered through the Sleepio digital therapeutic platform. |
|
| Morin, C. et al. (2015) [133] | Cognitive behavioral therapy for insomnia is an effective, non-pharmacological treatment, but personalized AI-based approaches are not discussed. | 1162 | Cognitive behavioral therapy for insomnia (CBT-i). |
|
| Morin, C. et al. (2023) [134] | Digital CBT-I treatment improved sleep and reduced anxiety and depression symptoms in adults with chronic insomnia. | 991 | The intervention is a 6–9-week prescription digital therapeutic (PDT) delivering cognitive behavioral therapy for insomnia (CBT-I), specifically the Somryst® (previously SHUTi, Nox Health, Atlanta, GA, USA) program. |
|
| Neuenschwander, E. et al. (2023) [135] | Personalized light therapy is more effective than non-personalized light therapy in correcting circadian misalignment in night shift workers. | 10 |
|
|
| Nguyen, S. et al. (2018) [136] | Cognitive behavioral therapy improves sleep quality over treatment as usual in persons with acquired brain injury, with better memory, younger age, and higher baseline depression predicting positive treatment response. | 32 | Cognitive behavior therapy (CBT) for sleep disturbance following acquired brain injury (ABI). |
|
| Okajima, I. et al. (2020) [137] | Tailored brief behavioral therapy for insomnia delivered via smartphone app improved insomnia severity, social disabilities, and work performance in workers. | 92 |
|
|
| Paniccia, G. et al. (2024) [138] | Personalized light therapy based on individual circadian rhythms is more effective than a one-size-fits-all approach for reducing insomnia and sleepiness in night shift workers. | 21 | The intervention was personalized light therapy, where participants received light therapy tailored to their individual circadian rhythms as determined by their dim light melatonin onset (DLMO). This was delivered through a mobile app (Arcashift) and a light box, along with light-blocking glasses. |
|
| Patel, S. et al. (2016) [139] | Computerized cognitive behavioral therapy can be an effective treatment option for insomnia in Parkinson’s disease patients. | 28 | The intervention was a 6-week computerized cognitive behavioral therapy for insomnia (CCBT-I) program, which involved a series of daily lessons, homework assignments, learnable skills, and appropriate recommendations. |
|
| Peter, L. et al. (2019) [140] | Online and face-to-face CBT-I interventions are both effective in improving sleep efficiency in shift workers. | Total: 33 |
|
|
| Philip, P. et al. (2020) [141] | A smartphone-based virtual agent can provide personalized cognitive behavioral interventions for sleep concerns during COVID-19 confinement. | 2069 |
|
|
| Pulantara, I. et al. (2018) [142] | A just-in-time adaptive intervention app (iREST) delivered personalized, evidence-based sleep recommendations and showed promising results for improving sleep in a military population. | 27 | The intervention was the iREST mobile health platform, which included a mobile app for participants to use for 4–6 weeks and a clinician portal through which clinicians provided evidence-based behavioral sleep treatment recommendations to participants during weeks 2–4. |
|
| Reesen, J. et al. (2023) [143] | This protocol describes a study evaluating whether guided Internet-delivered cognitive behavioral therapy for insomnia improves sleep and affects emotional distress in people with mental health problems. | 576 | The intervention is a guided Internet-based cognitive behavioral therapy for insomnia (iCBT-I) called “i-Sleep”. It consists of 5 online sessions delivered over 5–8 weeks, which include psychoeducation on sleep, establishing healthy sleeping behaviors through bedtime restriction and stimulus control, and addressing cognitive factors associated with insomnia. Participants also complete daily sleep diary monitoring using an app. |
|
| Reilly, E. et al. (2021) [144] | The use of a mobile app-delivered cognitive behavioral therapy for insomnia improves sleep outcomes in veterans, including those with comorbid conditions. | 33 |
|
|
| Ren, R. et al. (2023) [145] | Integrating personalized telephone sessions into digital cognitive behavioral therapy for insomnia provides increased clinical benefit, particularly for discontinuing sleep medications. | 572 |
|
|
| Ritterband, L. et al. (2017) [146] | A web-based cognitive behavioral therapy for insomnia intervention showed long-term effectiveness in improving sleep outcomes. | 303 | The intervention was a 6-week, fully automated, interactive, and tailored web-based cognitive behavior therapy for insomnia (CBT-I) program called “Sleep Healthy Using the Internet (SHUTi)”. It incorporated the primary tenets of face-to-face CBT-I and was compared to an online patient education program. |
|
| Rötger, A. et al. (2024) [147] | Digital cognitive behavioral therapy for insomnia reduces insomnia and comorbid symptoms of depression and anxiety. | 238 | 8 weeks of digital cognitive behavioral therapy for insomnia. |
|
| Sadler, P. et al. (2018) [148] | Cognitive behavioral therapy for insomnia, with or without positive mood strategies, effectively reduced insomnia and depression in older adults, but the advanced form was not significantly superior. | 72 |
|
|
| Schuffelen, J. et al. (2023) [149] | Digital cognitive behavioral therapy for insomnia is effective in reducing insomnia symptoms and improving daytime functioning in a heterogeneous study sample. | 238 | The intervention in this study was digital cognitive behavioral therapy for insomnia (dCBT-I) delivered through the “somnio” platform from the company mementor DE GmbH. Participants in the intervention group received access to the dCBT-I intervention over an 8-week period. |
|
| Schwartz, B. et al. (2020) [150] | This paper describes a treatment selection algorithm using machine learning and statistical inference to recommend cognitive behavioral or psychodynamic therapy for individual patients. | 1379 | The interventions were cognitive behavioral therapy (CBT) and psychodynamic therapy. The abstract does not provide any details on the frequency, duration, or amount/dose of these interventions. |
|
| Selvanathan, J. et al. (2021) [151] | Cognitive behavioral therapy for insomnia improves sleep, pain, and depressive symptoms in patients with chronic non-cancer pain. | 762 | The intervention was cognitive behavioral therapy for insomnia (CBT-I) for patients with comorbid insomnia and chronic non-cancer pain. |
|
| Siengsukon, C. et al. (2020) [152] | Web-based cognitive behavioral therapy for insomnia is feasible and effective for improving sleep outcomes in individuals with multiple sclerosis. | 41 |
|
|
| Speed, T. et al. (2022) [153] | This paper evaluates the feasibility of implementing cognitive behavioral therapy for insomnia in an outpatient substance use disorder treatment program but does not address personalized AI-based CBT-I. | 21 |
|
|
| Stott, R. et al. (2021) [154] | Offering a digital CBT-based sleep intervention alongside routine mental health treatment in an IAPT service improved clinical outcomes. | 510 | The intervention was a self-guided digital sleep intervention called Sleepio, which is based on the principles of cognitive behavioral therapy for insomnia (CBT-I). |
|
| Sweetman, A. et al. (2017) [155] | Cognitive behavioral therapy for insomnia is an effective treatment for insomnia in patients with comorbid obstructive sleep apnea. | 455 | Cognitive behavioral therapy for insomnia (CBT-i). |
|
| Sweetman, A. et al. (2020) [156] | Cognitive behavioral therapy for insomnia is safe and effective in patients with co-morbid moderate and severe sleep apnea, though they should be monitored for increased daytime sleepiness during initial bedtime restriction. | 145 | The intervention was a 4-week cognitive and behavioral therapy for insomnia (CBTi) program delivered by psychologists, with each session lasting 45 min and occurring either individually or in small groups. The key components of the CBTi program included sleep education, sleep hygiene, bedtime restriction therapy (BRT), feedback on sleep study results, cognitive therapy, and relapse prevention. BRT was initiated in the first week by restricting time in bed to match the patient’s average pretreatment total sleep time, with a minimum of 5.5 h. Psychologists reviewed the patients’ sleep diaries, sleepiness scores, and verbal reports each week to inform decisions about continuing, modifying, or discontinuing the BRT. |
|
| Talbot, L. et al. (2014) [157] | Cognitive behavioral therapy for insomnia improves sleep in individuals with post-traumatic stress disorder. | 45 | The intervention was an 8-session weekly individual cognitive behavioral therapy for insomnia (CBT-I) delivered by a licensed clinical psychologist or a board-certified psychiatrist. The key components of the CBT-I intervention were stimulus control therapy and sleep restriction therapy. |
|
| Tomfohr-Madsen, L. et al. (2017) [158] | Group cognitive behavioral therapy for insomnia delivered during pregnancy was associated with improvements in sleep and mood. | 13 | Group cognitive behavioral therapy for insomnia (CBT-I) delivered over 5 weekly sessions to pregnant women with insomnia. |
|
| Tomfohr-Madsen, L. et al. (2020) [159] | This pilot study found that 6 weeks of cognitive behavioral therapy for insomnia improved sleep in adolescents with persistent post-concussion symptoms. | 24 | The intervention was 6 weeks of cognitive behavioral therapy for insomnia (CBT-I). |
|
| Trauer, J. et al. (2015) [160] | Cognitive behavioral therapy is an effective nonpharmacologic treatment for chronic insomnia. | 1162 | The intervention was cognitive behavioral therapy for insomnia (CBT-i) that incorporated at least 2 of the following 5 components: cognitive therapy, stimulus control, sleep restriction, sleep hygiene, and relaxation therapy. The CBT-i was delivered in person on at least 2 occasions, but the frequency, duration, and amount/dose of the intervention are not specified. |
|
| Watanabe, Y. et al. (2022) [161] | Smartphone-based cognitive behavioral therapy app is effective for treating insomnia. | 175 | Smartphone-based cognitive behavioral therapy for insomnia (CBT-I) app. |
|
| Zachariae, R. et al. (2016) [162] | Internet-delivered cognitive behavioral therapy for insomnia is efficacious and can be considered a viable treatment option. | 1460 | The intervention was Internet-delivered cognitive behavioral therapy for insomnia (eCBT-I). |
|
| Zhang, C. et al. (2023) [163] | A smartphone-based, Chinese culture-adapted digital cognitive behavioral therapy for insomnia app reduced insomnia severity in the Chinese cultural context. | 82 | The intervention was a smartphone-based digital cognitive behavioral therapy for insomnia (DCBT-I) app that was tailored to the Chinese cultural context. Participants in the DCBT-I group used the app for approximately 10–15 min per day, with the app using a question-and-answer format to simulate a consultation process. The DCBT-I app included local language, Chinese cultural activities, and content consistent with Chinese lifestyles and cognition. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkintoni, E.; Vassilopoulos, S.P.; Nikolaou, G.; Boutsinas, B. Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes. J. Clin. Med. 2025, 14, 2265. https://doi.org/10.3390/jcm14072265
Gkintoni E, Vassilopoulos SP, Nikolaou G, Boutsinas B. Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes. Journal of Clinical Medicine. 2025; 14(7):2265. https://doi.org/10.3390/jcm14072265
Chicago/Turabian StyleGkintoni, Evgenia, Stephanos P. Vassilopoulos, Georgios Nikolaou, and Basilis Boutsinas. 2025. "Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes" Journal of Clinical Medicine 14, no. 7: 2265. https://doi.org/10.3390/jcm14072265
APA StyleGkintoni, E., Vassilopoulos, S. P., Nikolaou, G., & Boutsinas, B. (2025). Digital and AI-Enhanced Cognitive Behavioral Therapy for Insomnia: Neurocognitive Mechanisms and Clinical Outcomes. Journal of Clinical Medicine, 14(7), 2265. https://doi.org/10.3390/jcm14072265








