Quantitative Evaluation of Kidney and Gallbladder Stones by Texture Analysis Using Gray Level Co-Occurrence Matrix Based on Diagnostic Ultrasound Images
Abstract
1. Introduction
2. Materials and Methods
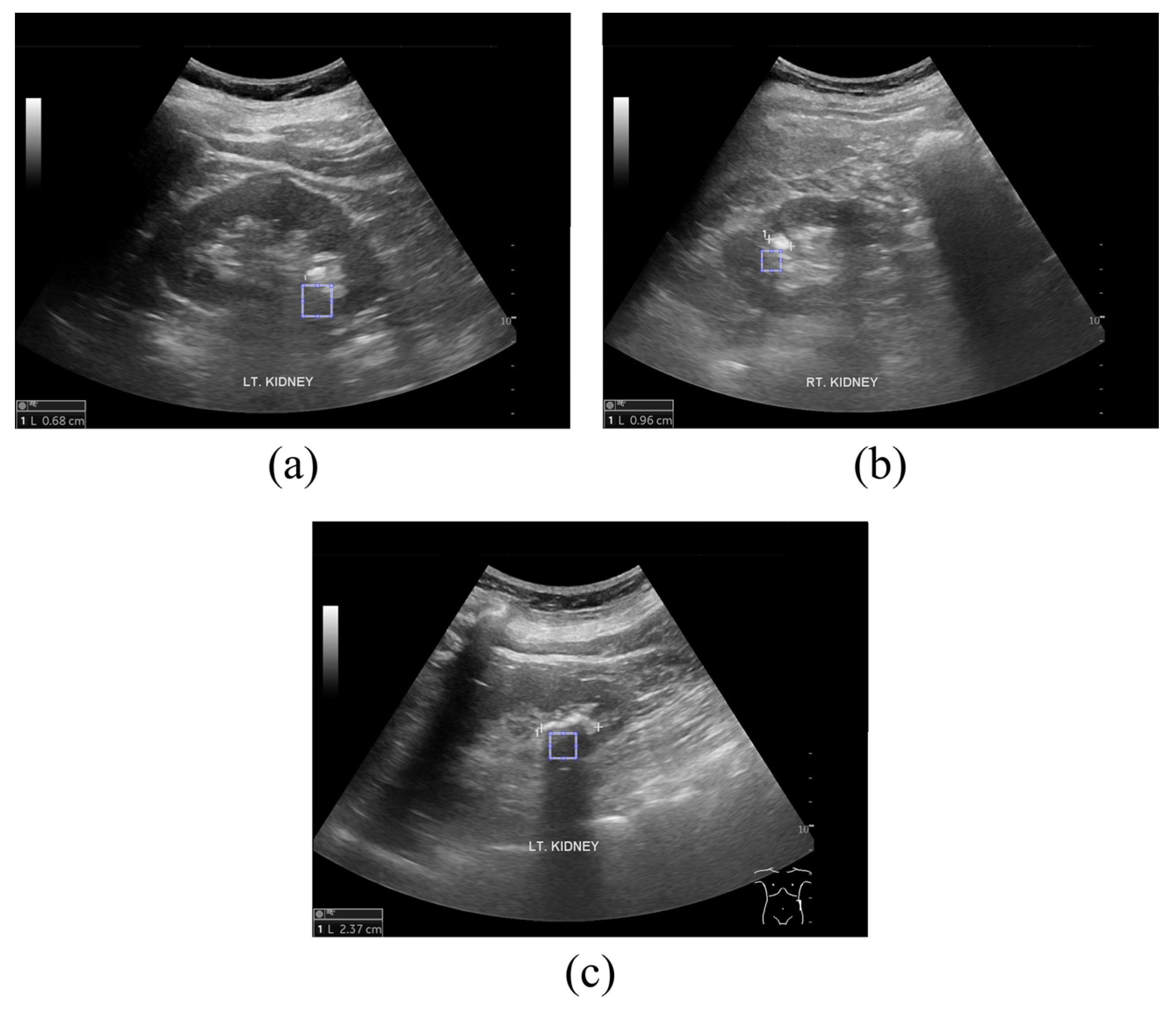
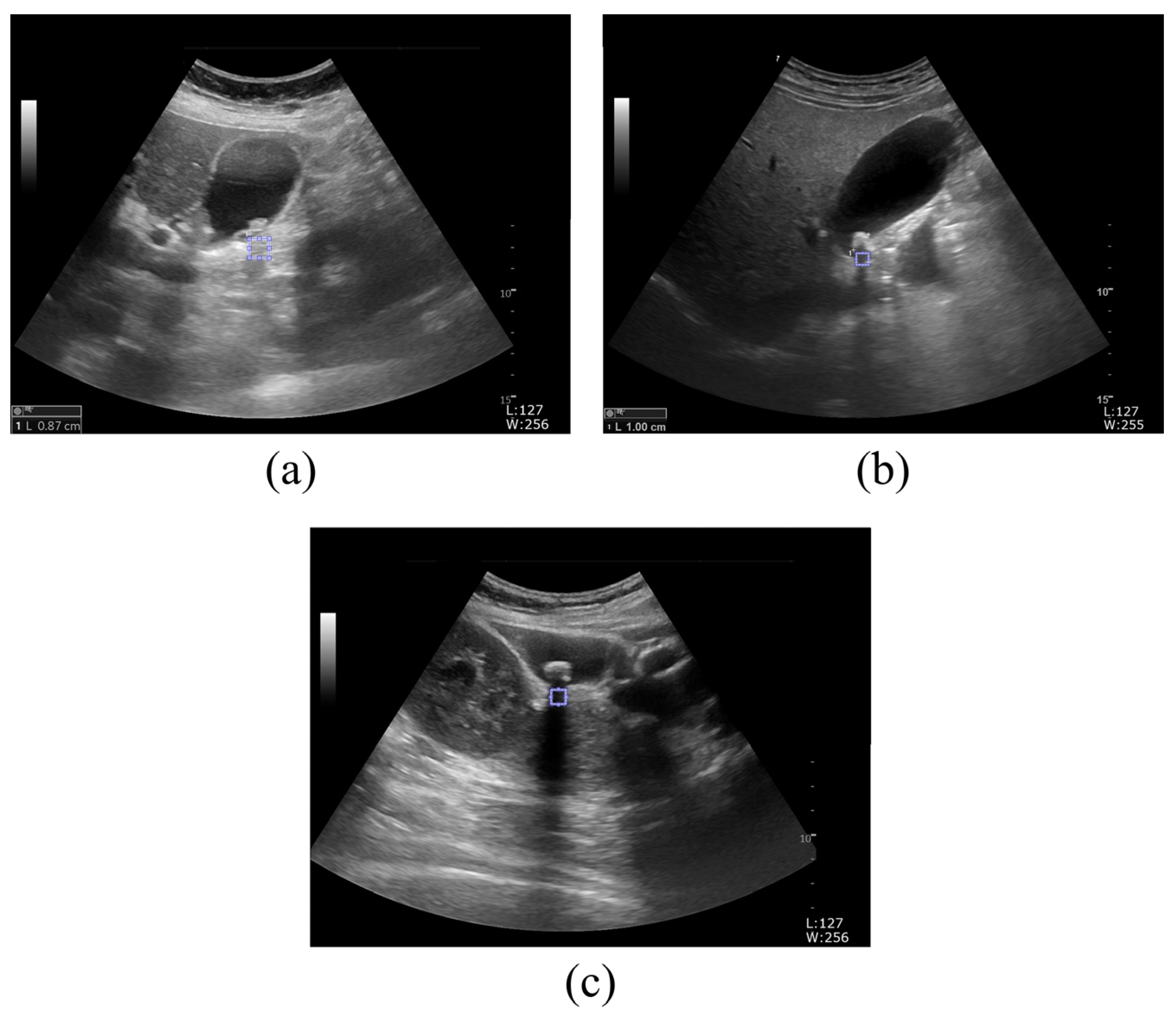
2.1. Ultrasound Image Acquisition
2.2. Proposed Framework to Determine Kidney and Gallbladder Stones Using the GLCM
2.3. Evaluation Method of Ultrasound Image Texture
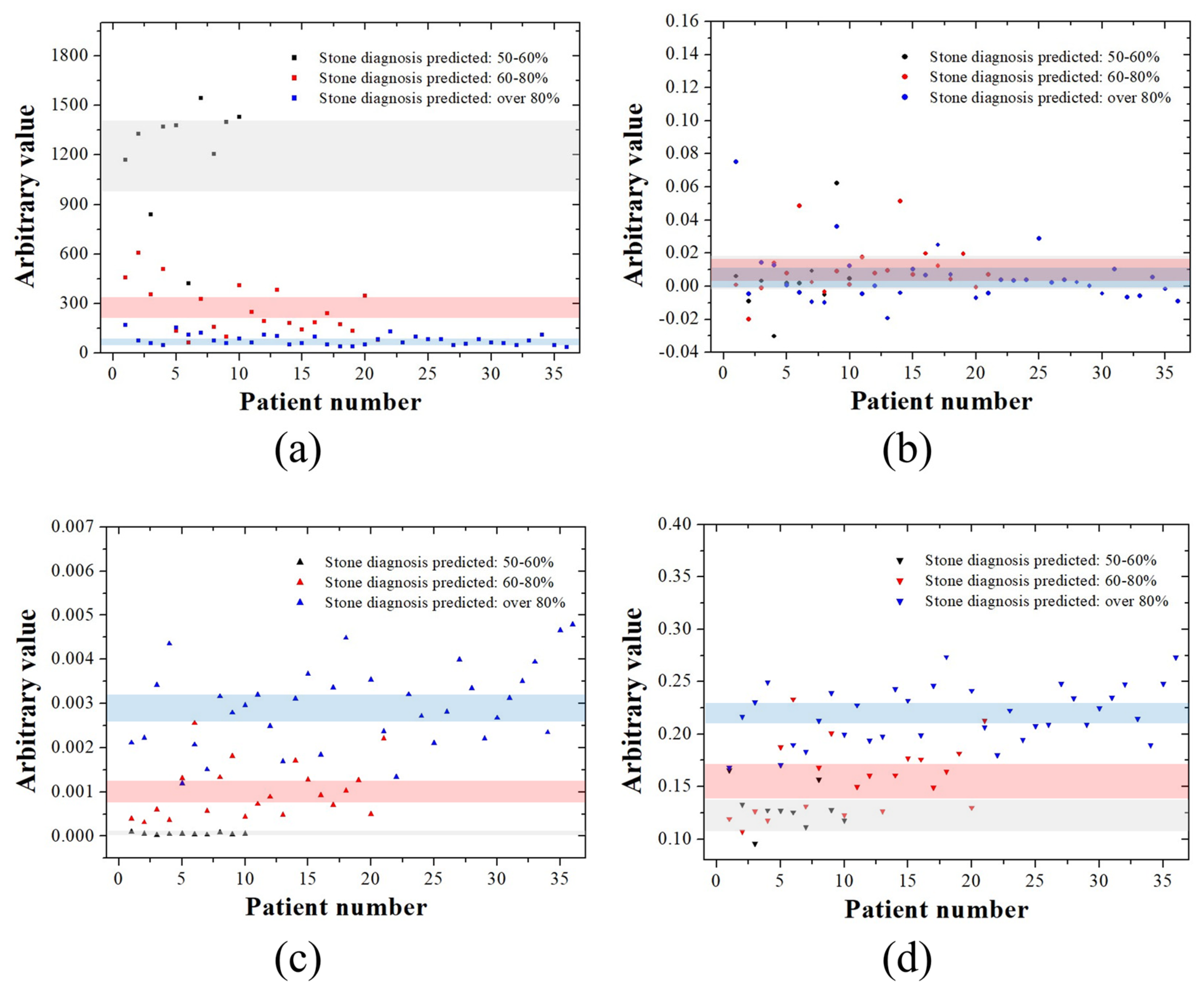
3. Results
4. Discussion
- (1)
- As a retrospective study, it is subject to inherent biases, including selection bias and variability in imaging protocols.
- (2)
- There may be potential variability in image quality and operator-dependent factors inherent to ultrasound imaging.
- (3)
- The feature analysis was performed using only GLCM, and further research based on various texture analysis matrices is needed.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamaguchi, A.; Kato, N.; Sugata, S.; Hamada, T.; Furuya, N.; Mizumoto, T.; Tamaru, Y.; Kusunoki, R.; Kuwai, T.; Kouno, H.; et al. Effectiveness of abdominal ultrasonography for improving the prognosis of pancreatic cancer during medical checkup: A Single Center retrospective analysis. Diagnostics 2022, 12, 2913. [Google Scholar] [CrossRef] [PubMed]
- Arienti, V.; Aluigi, L.; Pretolani, S.; Accogli, E.; Polimeni, L.; Domanico, A.; Violi, F. Ultrasonography (US) and non-invasive diagnostic methods for non-alcoholic fatty liver disease (NAFLD) and early vascular damage. Possible application in a population study on the metabolic syndrome (MS). Intern. Emerg. Med. 2012, 7 (Suppl. S3), S283–S290. [Google Scholar] [CrossRef] [PubMed]
- El-Koofy, N.; El-Karaksy, H.; El-Akel, W.; Helmy, H.; Anwar, G.; El-Sayed, R.; El-Hennawy, A. Ultrasonography as a non-invasive tool for detection of nonalcoholic fatty liver disease in overweight/obese Egyptian children. Eur. J. Radiol. 2012, 81, 3120–3123. [Google Scholar] [CrossRef] [PubMed]
- Moftakhar, L.; Jafari, F.; Johari, M.G.; Rezaeianzadeh, R.; Hosseini, S.V.; Rezaianzadeh, A. Prevalence and risk factors of kidney stone disease in population aged 40-70 years old in Kharameh cohort study: A cross-sectional population-based study in southern Iran. BMC Urol. 2022, 22, 205. [Google Scholar] [CrossRef]
- Dunmire, B.; Harper, J.D.; Cunitz, B.W.; Lee, F.C.; His, R.; Liu, Z.; Bailey, M.R.; Sorensen, M.D. Use of the acoustic shadow width to determine kidney stone size with ultrasound. J. Urol. 2016, 195, 171–177. [Google Scholar] [CrossRef]
- Sim, K.C. Ultrasonography of acute flank pain: A focus on renal stones and acute pyelonephritis. Ultrasonography 2018, 37, 345–354. [Google Scholar] [CrossRef]
- Mencarini, L.; Vestito, A.; Zagari, R.M.; Montagnani, M. New developments in the ultrasonography diagnosis of gallbladder diseases. Gastroenterol. Insights 2024, 15, 42–68. [Google Scholar] [CrossRef]
- Yu, J.; Tan, J.; Wang, Y. Ultrasound speckle reduction by a SUSAN-controlled anisotropic diffusion method. Pattern Recognit. 2010, 43, 3083–3092. [Google Scholar] [CrossRef]
- Sudeep, P.V.; Palanisamy, P.; Rajan, J.; Baradaran, H.; Saba, L.; Gupta, A.; Suri, J.S. Speckle reduction in medical ultrasound images using an unbiased non-local means method. Biomed. Signal Process. Control 2016, 28, 1–8. [Google Scholar] [CrossRef]
- Vimala, B.B.; Srinivasan, S.; Mathivanan, S.K.; Muthukumaran, V.; Babu, J.C.; Herencsar, N.; Vilcekova, L. Image noise removal in ultrasound breast images based on hybrid deep learning technique. Sensors 2023, 23, 1167. [Google Scholar] [CrossRef]
- Noble, J.A.; Boukerroui, D. Ultrasound image segmentation: A survey. IEEE Trans. Med. Imaging 2006, 25, 987–1010. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cheng, H.D.; Huang, J.; Tian, J.; Tang, X.; Liu, J. Probability density difference-based active contour for ultrasound image segmentation. Pattern Recognit. 2010, 43, 2028–2042. [Google Scholar] [CrossRef]
- Viswanath, K.; Gunasundari, R.; Hussan, S.A. VLSI Implementation and Analysis of Kidney Stone Detection by Level Set Segmentation and ANN Classification. Procedia Comput. Sci. 2015, 48, 612–622. [Google Scholar] [CrossRef]
- Huang, Y.L.; Chen, D.R.; Jiang, Y.R.; Kuo, S.J.; Wu, H.K.; Moon, W.K. Computer-aided diagnosis using morphological features for classifying breast lesions on ultrasound. Ultrasound Obstet. Gynecol. 2008, 32, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Yusufiyah, H.K.N.; Nugroho, H.A.; Adji, T.B.; Nugroho, A. Feature extraction for classifying lesion’s shape of breast ultrasound images. In Proceedings of the (ICITACEE) 2nd International Conference on Information Technology, Computer, and Electrical Engineering, Semarang, Indonesia, 16–18 October 2015. [Google Scholar] [CrossRef]
- Wu, W.-J.; Lin, S.-W.; Moon, W.K. Combining support vector machine with genetic algorithm to classify ultrasound breast tumor images. Comput. Med. Imaging Graph. 2012, 36, 627–633. [Google Scholar] [CrossRef]
- Sudarshan, V.K.; Mookiah, M.R.K.; Acharya, U.R.; Chandran, V.; Molinari, F.; Fujita, H.; Ng, K.H. Application of wavelet techniques for cancer diagnosis using ultrasound images: A review. Comput. Biol. Med. 2016, 69, 97–111. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural features for image classification. IEEE Trans. Syst. Man Cybern. 1973, SMC–3, 610–621. [Google Scholar] [CrossRef]
- Kiswanto, K.; Hadiyanto, H.; Sediyono, E. Meat texture image classification using the haar wavelet approach and a gray-level co-occurrence matrix. Appl. Syst. Innov. 2024, 7, 49. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Kidney stone prevention. Adv. Nutr. 2023, 14, 555–569. [Google Scholar] [CrossRef]
- Morgan, M.S.C.; Pearle, M.S. Medical management of renal stones. BMJ 2016, 352, i52. [Google Scholar] [CrossRef]
- Chaussy, C.; Eisenberger, F.; Forssmann, B. Extracorporeal shock wave lithotripsy (ESWL®): A Chronology. J. Endourol. 2007, 21, 1249–1253. [Google Scholar] [CrossRef]
- Qiang, Y.-C.; Guo, Y.-G.; Wang, Y.-Q. The effectiveness and safety of extracorporeal shock wave lithotripsy for the management of kidney stones: A protocol of systematic review and meta-analysis. Medicine 2020, 99, e19915. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.D.; Sohng, W.; Jang, E.; Choi, D.; Chung, H. Feasibility of discrimination of gall bladder (GB) stone and GB polyp using voltage-applied SERS measurement of bile juice samples in conjunction with two-trace two-dimensional (2T2D) correlation analysis. Analyst 2021, 146, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- McCain, R.S.; Diamond, A.; Jones, C.; Coleman, H.G. Current practices and future prospects for the management of gallbladder polyps: A topical review. World J. Gastroenterol. 2018, 24, 2844–2852. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Di Ciaula, A.; de Bari, O.; Garruti, G.; Palmieri, V.; Wang, D.H. Management of gallstones and its related complications. Expert Rev. Gastroenterol. Hepatol. 2015, 10, 93–112. [Google Scholar]
- Soleymani, Y.; Valibeiglou, Z.; Fazel Ghaziani, M.F.; Jahanshahi, A.; Khezerloo, D. Radiomics reproducibility in computed tomography through changes of ROI size, resolution, and hounsfield unit: A phantom study [Resolution]. Radiography 2024, 30, 1629–1636. [Google Scholar] [CrossRef]
- Moon, J.H.; Hwang, J.-Y.; Park, J.S.; Koh, S.H.; Park, S.-Y. Impact of region of interest (ROI) size on the diagnostic performance of shear wave elastography in differentiating solid breast lesions. Acta Radiol. 2018, 59, 657–663. [Google Scholar] [CrossRef]
- Renard-Penna, R.; Martin, A.; Conort, P.; Mozer, P.; Grenier, P. Kidney stones and imaging: What can your radiologist do for you? World J. Urol. 2015, 33, 193–202. [Google Scholar] [CrossRef]
- Pinto, A.; Reginelli, A.; Cagini, L.; Coppolino, F.; Stabile Ianora, A.A.S.; Bracale, R.; Giganti, M.; Romano, L. Accuracy of ultrasonography in the diagnosis of acute calculous cholecystitis: Review of the literature. Crit. Ultrasound J. 2013, 5, S11. [Google Scholar] [CrossRef]
- Al-Kadi, O.S. Combined statistical and model based texture features for improved image classification. In Proceedings of the 4th IET International Conference on Advances in Medical, Signal and Information Processing—MEDSIP, Santa Margherita Ligure, Italy, 14–16 July 2008. [Google Scholar] [CrossRef][Green Version]
- Durgamahanthi, V.; Christaline, J.A.; Edward, A.S. GLCM and GLRLM based texture analysis: Application to brain cancer diagnosis using histopathology images. Intelligent computing and applications. Adv. Intell. Syst. Comput. 2021, 1172, 691–706. [Google Scholar]


| Disease Type | Expected Probability of Diagnosis | ||
|---|---|---|---|
| Number of Images with 50–60% Probability | Number of Images with 60–80% Probability | Number of Images with ≥80% Probability | |
| Kidney stone | 14 | 23 | 15 |
| Gallbladder stone | 10 | 21 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, K.; Jeong, H.-W.; Lee, Y. Quantitative Evaluation of Kidney and Gallbladder Stones by Texture Analysis Using Gray Level Co-Occurrence Matrix Based on Diagnostic Ultrasound Images. J. Clin. Med. 2025, 14, 2268. https://doi.org/10.3390/jcm14072268
Kim M, Kim K, Jeong H-W, Lee Y. Quantitative Evaluation of Kidney and Gallbladder Stones by Texture Analysis Using Gray Level Co-Occurrence Matrix Based on Diagnostic Ultrasound Images. Journal of Clinical Medicine. 2025; 14(7):2268. https://doi.org/10.3390/jcm14072268
Chicago/Turabian StyleKim, Minkyoung, Kyuseok Kim, Hyun-Woo Jeong, and Youngjin Lee. 2025. "Quantitative Evaluation of Kidney and Gallbladder Stones by Texture Analysis Using Gray Level Co-Occurrence Matrix Based on Diagnostic Ultrasound Images" Journal of Clinical Medicine 14, no. 7: 2268. https://doi.org/10.3390/jcm14072268
APA StyleKim, M., Kim, K., Jeong, H.-W., & Lee, Y. (2025). Quantitative Evaluation of Kidney and Gallbladder Stones by Texture Analysis Using Gray Level Co-Occurrence Matrix Based on Diagnostic Ultrasound Images. Journal of Clinical Medicine, 14(7), 2268. https://doi.org/10.3390/jcm14072268






