A Systematic Comparison of Age, Comorbidity and Frailty of Two Defined ICU Populations in the German Helios Hospital Group from 2016–2021
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
- (1)
- Due to the changes in pseudonymization from 2016 onwards, the data prior to this are no longer comparable; thus, the analysis was limited to the 6-year study period. This limitation may prevent important trends prior to this point in time from being recorded. Nevertheless, we conclude that this observation period is sufficient to show significant age trends because we used our total population of 6,204,093 cases as the basis. The measurement interval was based on the patient’s individual, daily admission date, which we included in the study as a mean value per year; this is considered consistent and in accordance with best practices using the analytical methods described. Furthermore, a longer observation period (>2 years) of the age distribution of cases with and without COVID-19 would show the longitudinal trend and effect of COVID-19 more clearly.
- (2)
- The CodeBased and BedBased ICU definitions have limitations. In the BedBased ICU definition, bed occupancy includes patients who may not have a hard ICU indication. The CodeBased ICU definition reflects only part of the reality because it includes patients with intensive medical therapy but excludes those with intensive monitoring (as is common in IMCs).
- (3)
- The selected definitions are not clearly replicable and controllable. The list of approaches is not necessarily finite. Due to the lack of an ICU definition, the CodeBased ICU definition was adopted as the first-quality definition, and the BedBased ICU definition was taken from the Helios Hospital Group’s own bed classification. According to this, hospitals are required to provide information on their completed services; here, there are challenges in coming to the same understanding of what is meant by “ICU”—disparities are to be expected [67].
- (4)
- The weighting of age as a determining variable within the HFS is not definitive. The use of the two scores is based on administrative data and may be influenced by coding practices. The accuracy of HFS is being discussed in the scientific community, and attempts are being made to optimize it [68,69].
- (5)
- At present, there is no uniform (inter)national definition of what is meant by “ICU” or “IMC”. The introduction of a uniform national ICU definition is the responsibility of legislators. Initial steps can be taken at the level of individual hospitals, but only a legal requirement can create a common definition. The introduction of a uniform definition of ICUs with clear criteria will not replace or override clinical assessments or the individual needs of patients.
- (6)
- Additionally, a differentiated approach should be used to assess the admission behavior, therapeutic value, and quality of outcomes for elderly patients in ICUs, acknowledging the challenges in balancing patient-centered care with ICU admission, such as discharge regulations and coding behavior.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1
| CodeBased ICU All Cases | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Groups/Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend (a) | Trend (n) | p Value |
| 18–39 years | 2416 (4.2%) | 2238 (4.1%) | 2028 (4.0%) | 1721 (4.3%) | 1565 (3.8%) | 1776 (4.6%) | → | 1.01 | 0.12 |
| 40−59 years | 10,733 (19%) | 9745 (18%) | 9015 (18%) | 7166 (18%) | 7153 (18%) | 7075 (18%) | ↓ | 0.99 | 0.025 |
| 60−69 years | 11,882 (21%) | 11,694 (22%) | 11,121 (22%) | 9412 (23%) | 9645 (24%) | 9186 (24%) | ↑ | 1.04 | <0.001 |
| 70−79 years | 17,770 (31%) | 16,551 (30%) | 14,850 (30%) | 11,355 (28%) | 11,127 (27%) | 10,207 (27%) | ↓ | 0.96 | <0.001 |
| ≥80 years | 14,613 (25%) | 14,091 (26%) | 13,230 (26%) | 10,775 (27%) | 11,317 (28%) | 10,080 (26%) | ↑ | 1.02 | <0.001 |
| Mean Age | 69.3 (14.4) | 69.5 (14.3) | 69.5 (14.2) | 69.4 (14.2) | 69.5 (14.1) | 68.7 (14.4) | ↓ | −0.06 | <0.001 |
| Total Cases | 57,414 | 54,319 | 50,244 | 40,429 | 40,807 | 38,324 | ↓ | x | x |
| CodeBased ICU All Cases Without COVID-19 | |||||||||
| Age Groups/Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend (a) | Trend (n) | p Value |
| 18–39 years | 2416 (4.2%) | 2238 (4.1%) | 2028 (4.0%) | 1721 (4.3%) | 1489 (3.9%) | 1495 (4.6%) | → | 1.01 | 0.2 |
| 40−59 years | 10,733 (19%) | 9745 (18%) | 9015 (18%) | 7166 (18%) | 6648 (17%) | 5764 (18%) | ↓ | 0.99 | <0.001 |
| 60−69 years | 11,882 (21%) | 11,694 (22%) | 11,121 (22%) | 9412 (23%) | 8997 (24%) | 7729 (24%) | ↑ | 1.04 | <0.001 |
| 70−79 years | 17,770 (31%) | 16,551 (30%) | 14,850 (30%) | 11,355 (28%) | 10,302 (27%) | 8755 (27%) | ↓ | 0.96 | <0.001 |
| ≥80 years | 14,613 (25%) | 14,091 (26%) | 13,230 (26%) | 10,775 (27%) | 10,583 (28%) | 8972 (27%) | ↑ | 1.02 | <0.001 |
| Mean Age | 69.3 (14.4) | 69.5 (14.3) | 69.5 (14.2) | 69.4 (14.2) | 69.5 (14.1) | 69.1 (14.4) | ↓ | −0.01 | 0.7 |
| Total Cases | 57,414 | 54,319 | 50,244 | 40,429 | 38,019 | 32,715 | ↓ | x | x |
| BedBased ICU All Cases | |||||||||
| Age Groups/Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend (a) | Trend (n) | p Value |
| 18–39 years | 5588 (6.3%) | 5844 (6.4%) | 5314 (6.5%) | 4874 (6.4%) | 4012 (6.3%) | 3447 (6.2%) | → | 1.00 | 0.3 |
| 40−59 years | 16,695 (19%) | 16,920 (18%) | 14,810 (18%) | 13,752 (18%) | 11,341 (18%) | 9977 (18%) | ↓ | 0.99 | <0.001 |
| 60−69 years | 16,520 (19%) | 17,428 (19%) | 15,888 (19%) | 15,340 (20%) | 12,959 (20%) | 11,619 (21%) | ↑ | 1.03 | <0.001 |
| 70−79 years | 24,984 (28%) | 25,425 (28%) | 21,856 (27%) | 19,502 (26%) | 15,292 (24%) | 13,624 (24%) | ↓ | 0.95 | <0.001 |
| ≥80 years | 24,530 (28%) | 26,285 (29%) | 23,954 (29%) | 22,945 (30%) | 19,741 (31%) | 17,251 (31%) | ↑ | 1.03 | <0.001 |
| Mean Age | 68.7 (16.0) | 68.9 (16.1) | 69.0 (16.1) | 69.0 (16.0) | 69.1 (16.0) | 69.1 (15.9) | ↑ | 0.07 | <0.001 |
| Total Cases | 88,317 | 91,902 | 81,822 | 76,413 | 63,345 | 55,918 | ↓ | x | x |
| BedBased ICU All Cases Without COVID-19 | |||||||||
| Age Groups/Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend (a) | Trend (n) | p Value |
| 18–39 years | 5588 (6.3%) | 5844 (6.4%) | 5314 (6.5%) | 4874 (6.4%) | 3971 (6.4%) | 3314 (6.2%) | → | 1.00 | 0.8 |
| 40−59 years | 16,695 (19%) | 16,920 (18%) | 14,810 (18%) | 13,752 (18%) | 11,111 (18%) | 9482 (18%) | ↓ | 0.99 | <0.001 |
| 60−69 years | 16,520 (19%) | 17,428 (19%) | 15,888 (19%) | 15,340 (20%) | 12,683 (21%) | 11,058 (21%) | ↑ | 1.03 | <0.001 |
| 70−79 years | 24,984 (28%) | 25,425 (28%) | 21,856 (27%) | 19,502 (26%) | 14,853 (24%) | 12,964 (24%) | ↓ | 0.95 | <0.001 |
| ≥80 years | 24,530 (28%) | 26,285 (29%) | 23,954 (29%) | 22,945 (30%) | 19,183 (31%) | 16,480 (31%) | ↑ | 1.03 | <0.001 |
| Mean Age | 68.7 (16.0) | 68.9 (16.1) | 69.0 (16.1) | 69.0 (16.0) | 69.0 (16.1) | 69.1 (15.9) | ↑ | 0.07 | <0.001 |
| Total Cases | 88,317 | 91,902 | 81,822 | 76,413 | 61,801 | 53,298 | ↓ | x | x |
References
- Ageing and Health. 16 January 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 17 January 2023).
- European Commission. Demographic Change in Europe: Eurobarometer Survey. 2023. Available online: https://europa.eu/eurobarometer/surveys/detail/3112 (accessed on 29 April 2024).
- OECD. Demography—Old-Age Dependency Ratio (Indicator). 2024. Available online: https://data.oecd.org/pop/old-age-dependency-ratio.htm (accessed on 21 June 2024).
- Eurostat. Ageing Europe—Statistics on Population Developments. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Ageing_Europe_-_statistics_on_population_developments (accessed on 11 February 2023).
- Eurostat. Ageing Europe—Statistics on Population Developments: Where Do Old People Live and Where Do They Come from? 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Ageing_Europe_-_statistics_on_population_developments#Older_people_.E2.80.94_increasingly_old_and_with_growing_dependency (accessed on 11 February 2023).
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259, Erratum in Lancet 2017, 390, e38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuchs, Z.; Blumstein, T.; Novikov, I.; Walter-Ginzburg, A.; Lyanders, M.; Gindin, J.; Habot, B.; Modan, B. Morbidity, comorbidity, and their association with disability among community-dwelling oldest-old in Israel. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, M447–M455. [Google Scholar] [CrossRef] [PubMed]
- Lehnert, T.; Heider, D.; Leicht, H.; Heinrich, S.; Corrieri, S.; Luppa, M.; Riedel-Heller, S.; König, H.-H. Review: Health care utilization and costs of elderly persons with multiple chronic conditions. Med. Care Res. Rev. 2011, 68, 387–420. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Taljaard, M.; Bryson, G.L.; Beaulé, P.E.; Gagné, S.; Hamilton, G.; Hladkowicz, E.; Huang, A.; Joanisse, J.A.; Lavallée, L.T.; et al. Frailty as a Predictor of Death or New Disability After Surgery: A Prospective Cohort Study. Ann. Surg. 2020, 271, 283–289. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Biasio, J.C.d.; Mittel, A.M.; Mueller, A.L.; Ferrante, L.E.; Kim, D.H.; Shaefi, S. Frailty in Critical Care Medicine: A Review. Anesth. Analg. 2020, 130, 1462–1473. [Google Scholar] [CrossRef]
- Muscedere, J.; Waters, B.; Varambally, A.; Bagshaw, S.M.; Boyd, J.G.; Maslove, D.; Sibley, S.; Rockwood, K. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 1105–1122. [Google Scholar] [CrossRef]
- Cecconi, M.; Leaver, S.; Jung, C. Caring for frail patients in the ICU: A multidimensional approach. Intensive Care Med. 2024, 50, 583–586. [Google Scholar] [CrossRef]
- Flaatten, H.; Lange, D.W.d.; Morandi, A.; Andersen, F.H.; Artigas, A.; Bertolini, G.; Boumendil, A.; Cecconi, M.; Christensen, S.; Faraldi, L.; et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med. 2017, 43, 1820–1828. [Google Scholar] [CrossRef]
- Guidet, B.; Lange, D.W.d.; Boumendil, A.; Leaver, S.; Watson, X.; Boulanger, C.; Szczeklik, W.; Artigas, A.; Morandi, A.; Andersen, F.; et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: The VIP2 study. Intensive Care Med. 2020, 46, 57–69. [Google Scholar] [CrossRef]
- American Thoracic Society. Fair allocation of intensive care unit resources. Am. J. Respir. Crit. Care Med. 1997, 156, 1282–1301, Erratum in Am. J. Respir. Crit. Care Med. 1998, 157, 671. [Google Scholar]
- Badawi, O.; Brennan, T.; Celi, L.A.; Feng, M.; Ghassemi, M.; Ippolito, A.; Johnson, A.; Mark, R.G.; Mayaud, L.; Moody, G.; et al. Making big data useful for health care: A summary of the inaugural mit critical data conference. JMIR Med. Inform. 2014, 2, e22. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Winkelmann, J.; Eckhardt, H.; Nimptsch, U.; Panteli, D.; Reichebner, C.; Rombey, T.; Busse, R. A country-level analysis comparing hospital capacity and utilisation during the first COVID-19 wave across Europe. Health Policy 2022, 126, 373–381. [Google Scholar] [CrossRef]
- Dongelmans, D.A.; Pilcher, D.; Beane, A.; Soares, M.; Del Pilar Arias Lopez, M.; Fernandez, A.; Guidet, B.; Haniffa, R.; Salluh, J.I. Linking of global intensive care (LOGIC): An international benchmarking in critical care initiative. J. Crit. Care 2020, 60, 305–310. [Google Scholar] [CrossRef]
- Bogdanov, C.; Hohenstein, S.; Brederlau, J.; Groesdonk, H.V.; Bollmann, A.; Kuhlen, R. A Comparison of Different Intensive Care Unit Definitions Derived from the German Administrative Data Set: A Methodological, Real-World Data Analysis from 86 Helios Hospitals. J. Clin. Med. 2024, 13, 3393. [Google Scholar] [CrossRef]
- Verdonk, F.; Zacharowski, K.; Ahmed, A.; Orliaguet, G.; Pottecher, J. A multifaceted approach to intensive care unit capacity. Lancet Public Health 2021, 6, e448. [Google Scholar] [CrossRef]
- Abuhasira, R.; Anstey, M.; Novack, V.; Bose, S.; Talmor, D.; Fuchs, L. Intensive care unit capacity and mortality in older adults: A three nations retrospective observational cohort study. Ann. Intensive Care 2022, 12, 20. [Google Scholar] [CrossRef]
- Flaatten, H.; Beil, M.; Guidet, B. Elderly Patients in the Intensive Care Unit. Semin. Respir. Crit. Care Med. 2021, 42, 10–19. [Google Scholar] [CrossRef]
- O’Lynnger, T.M.; Zuckerman, S.L.; Morone, P.J.; Dewan, M.C.; Vasquez-Castellanos, R.A.; Cheng, J.S. Trends for Spine Surgery for the Elderly: Implications for Access to Healthcare in North America. Neurosurgery 2015, 77 (Suppl. S4), S136–S141. [Google Scholar] [CrossRef]
- Nielsson, M.S.; Christiansen, C.F.; Johansen, M.B.; Rasmussen, B.S.; Tønnesen, E.; Nørgaard, M. Mortality in elderly ICU patients: A cohort study. Acta Anaesthesiol. Scand. 2014, 58, 19–26. [Google Scholar] [CrossRef]
- König, S.; Pellissier, V.; Hohenstein, S.; Leiner, J.; Hindricks, G.; Kuhlen, R.; Bollmann, A. Hospitalization Rates and In-Hospital Mortality Before and During the COVID-19 Pandemic. Dtsch. Arztebl. Int. 2022, 119, 816–817. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, A.; Hohenstein, S.; Pellissier, V.; König, S.; Ueberham, L.; Hindricks, G.; Meier-Hellmann, A.; Kuhlen, R. Hospitalizations for emergency-sensitive conditions in Germany during the Covid-19 pandemic Insights from the German-wide Helios hospital network. Emerg. Med. J. 2021, 38, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Bundesministerium für Gesundheit. Verordnung zum Fallpauschalensystem für Krankenhäuser: KFPV; Bundesministerium für Gesundheit: Bonn, Germany, 2023. [Google Scholar]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef]
- Burbidge, J.B.; Magee, L.; Robb, A.L. Alternative Transformations to Handle Extreme Values of the Dependent Variable. J. Am. Stat. Assoc. 1988, 123–127. [Google Scholar] [CrossRef]
- Moore, B.J.; White, S.; Washington, R.; Coenen, N.; Elixhauser, A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med. Care 2017, 55, 698–705. [Google Scholar] [CrossRef]
- Gasparini, A. comorbidity: An R package for computing comorbidity scores. J. Open Source Softw. 2018, 3, 648. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 6 January 2022).
- Shumway, R.H. Time Series Analysis and Its Applications; Springer: New York, NY, USA, 2000. [Google Scholar]
- ECDC. Trend Analysis Guidance for Surveillance Data; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Daniels, R.; Müller, J.; Jafari, C.; Theile, P.; Kluge, S.; Roedl, K. Evolution of Clinical Characteristics and Outcomes of Critically Ill Patients 90 Years Old or Older Over a 12-Year Period: A Retrospective Cohort Study. Crit. Care Med. 2024, 52, e258–e267. [Google Scholar] [CrossRef]
- Anja Afentakis, T.M. Projektionen des Personalbedarfs und -angebots in Pflegeberufen bis 2025. Wirtsch. Stat. 2010, 62, 990–1002. [Google Scholar]
- Meara, J.G.; Leather, A.J.M.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030, evidence and solutions for achieving health, welfare, and economic development. Lancet 2015, 386, 569–624. [Google Scholar] [CrossRef]
- AAMC. Report Reinforces Mounting Physician Shortage; AAMC: Washington, DC, USA, 2021. [Google Scholar]
- Boniol, M.; Kunjumen, T.; Nair, T.S.; Siyam, A.; Campbell, J.; Diallo, K. The global health workforce stock and distribution in 2020 and 2030: A threat to equity and ’universal’ health coverage? BMJ Glob. Health 2022, 7, e009316. [Google Scholar] [CrossRef]
- Peters, M. Time to solve persistent, pernicious and widespread nursing workforce shortages. Int. Nurs. Rev. 2023, 70, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, I.; Bonsignore, M.; Hohenstein, S.; Bollmann, A.; Günther, R.; Kodde, C.; Englisch, M.; Ahmad-Nejad, P.; Schröder, A.; Glenz, C.; et al. Effect of gender, age and vaccine on reactogenicity and incapacity to work after COVID-19 vaccination: A survey among health care workers. BMC Infect. Dis. 2022, 22, 291. [Google Scholar] [CrossRef] [PubMed]
- Kuhlen, R.; Schmithausen, D.; Winklmair, C.; Schick, J.; Scriba, P. The Effects of the COVID-19 Pandemic and Lockdown on Routine Hospital Care for Other Illnesses. Dtsch. Arztebl. Int. 2020, 117, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, P.; Bollmann, A.; Hohenstein, S.; Glass, B.; Untch, M.; Reichardt, A.; Amrein, D.; Kuhlen, R. Decreased Incidence of Oncology Admissions in 75 Helios Hospitals in Germany during the COVID-19 Pandemic. Oncol. Res. Treat. 2021, 44, 71–75. [Google Scholar] [CrossRef]
- Haas, L.E.M.; Lange, D.W.d.; van Dijk, D.; van Delden, J.J.M. Should We Deny ICU Admission to the Elderly? Ethical Considerations in Times of COVID-19. 2020. Available online: https://link.springer.com/article/10.1186/s13054-020-03050-x (accessed on 4 May 2024).
- Salluh, J.I.F.; Soares, M.; Keegan, M.T. Understanding intensive care unit benchmarking. Intensive Care Med. 2017, 43, 1703–1707. [Google Scholar] [CrossRef]
- ERA Registry. ERA Registry Annual Report 2020; ERA Registry: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Mohr, N.M.; Wessman, B.T.; Bassin, B.; Elie-Turenne, M.-C.; Ellender, T.; Emlet, L.L.; Ginsberg, Z.M.; Gunnerson, K.M.; Jones, K.M.M.; Kram, B.P.; et al. Boarding of Critically Ill Patients in the Emergency Department. Crit. Care Med. 2020, 48, 1180–1187. [Google Scholar] [CrossRef]
- Pilcher, D.; Coatsworth, N.R.; Rosenow, M.; McClure, J. A national system for monitoring intensive care unit demand and capacity: The Critical Health Resources Information System (CHRIS). Med. J. Aust. 2021, 214, 297–298.e1. [Google Scholar] [CrossRef]
- Sauer, C.M.; Dam, T.A.; Celi, L.A.; Faltys, M.; La Hoz, M.A.A.d.; Adhikari, L.; Ziesemer, K.A.M.; Girbes, A.M.; Thoral, P.J.M.; Elbers, P.M. Systematic Review and Comparison of Publicly Available ICU Data Sets-A Decision Guide for Clinicians and Data Scientists. Crit. Care Med. 2022, 50, e581–e588. [Google Scholar] [CrossRef]
- Cosgriff, C.V.; Celi, L.A.; Stone, D.J. Critical Care, Critical Data. Biomed. Eng. Comput. Biol. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- O’Halloran, H.M.; Kwong, K.; Veldhoen, R.A.; Maslove, D.M. Characterizing the Patients, Hospitals, and Data Quality of the eICU Collaborative Research Database. Crit. Care Med. 2020, 48, 1737–1743. [Google Scholar] [CrossRef]
- Zimmerman, J.E.; Kramer, A.A. A model for identifying patients who may not need intensive care unit admission. J. Crit. Care 2010, 25, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Shapiro, M.F. Association Between Intensive Care Unit Utilization During Hospitalization and Costs, Use of Invasive Procedures, and Mortality. JAMA Intern. Med. 2016, 176, 1492–1499. [Google Scholar] [CrossRef]
- Yan, B.; Sun, W.; Wang, W.; Wu, J.; Wang, G.; Dou, Q. Prognostic significance of frailty in older patients with hip fracture: A systematic review and meta-analysis. Int. Orthop. 2022, 46, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tu, J.; She, Q.; Li, M.; Wang, K.; Zhao, W.; Huang, P.; Chen, B.; Wu, J. Prognostic significance of frailty in hospitalized elderly patients with community-acquired pneumonia: A retrospective cohort study. BMC Geriatr. 2023, 23, 308. [Google Scholar] [CrossRef] [PubMed]
- Dengler, J.; Gheewala, H.; Kraft, C.N.; Hegewald, A.A.; Dörre, R.; Heese, O.; Gerlach, R.; Rosahl, S.; Maier, B.; Burger, R.; et al. Changes in frailty among patients hospitalized for spine pathologies during the COVID-19 pandemic in Germany-a nationwide observational study. Eur. Spine J. 2024, 33, 19–30. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Morrison, L.; Costello, M.; Flannery, A.; Small, C.; O’Reilly, L.; Heffernan, L.; Mannion, E.; Waters, R.; O’keeffe, S. Frailty in an Adult Acute Hospital Population: Predictors, Prevalence, and Outcomes. Int. J. Environ. Res. Public Health 2024, 21, 273. [Google Scholar] [CrossRef]
- Boucher, E.L.; Gan, J.M.; Rothwell, P.M.; Shepperd, S.; Pendlebury, S.T. Prevalence and outcomes of frailty in unplanned hospital admissions: A systematic review and meta-analysis of hospital-wide and general (internal) medicine cohorts. eClinicalMedicine 2023, 59, 101947. [Google Scholar] [CrossRef]
- Ysea-Hill, O.; Gomez, C.J.; Mansour, N.; Wahab, K.; Hoang, M.; Labrada, M.; Ruiz, J.G. The association of a frailty index from laboratory tests and vital signs with clinical outcomes in hospitalized older adults. J. Am. Geriatr. Soc. 2022, 70, 3163–3175. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Webb, S.A.R.; Delaney, A.; George, C.; Pilcher, D.; Hart, G.K.; Bellomo, R. Very old patients admitted to intensive care in Australia and New Zealand: A multi-centre cohort analysis. Crit. Care. 2009, 13, R45. [Google Scholar] [CrossRef]
- Fuchs, L.; Novack, V.; McLennan, S.; Celi, L.A.; Baumfeld, Y.; Park, S.; Howell, M.D.; Talmor, D.S. Trends in severity of illness on ICU admission and mortality among the elderly. PLoS ONE 2014, 9, e93234. [Google Scholar] [CrossRef]
- Frezza, E.E.; Squillario, D.M.; Smith, T.J. The ethical challenge and the futile treatment in the older population admitted to the intensive care unit. Am. J. Med. Qual. 1998, 13, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Covino, M.; Petruzziello, C.; Onder, G.; Migneco, A.; Simeoni, B.; Franceschi, F.; Ojetti, V. A 12-year retrospective analysis of differences between elderly and oldest old patients referred to the emergency department of a large tertiary hospital. Maturitas 2019, 120, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Shlyafer, S.I. The hospital medical care support of individuals older than able-bodied age in The Russian Federation. Probl. Sotsial’noi Gig. Zdr. Istor. Meditsiny 2021, 29, 238–244. [Google Scholar] [CrossRef]
- Upparakadiyala, R.; Singapati, S.; Sarkar, M.K.; Swathi, U. Clinical Profile and Factors Affecting Outcomes in Elderly Patients Admitted to the Medical Intensive Care Unit of a Tertiary Care Hospital. Cureus 2022, 14, e22136. [Google Scholar] [CrossRef]
- Bundesministerium der Justiz, Bundesamt für Justiz. Übermittlung von Leistungsdaten von Krankenhäusers und Rehabilitationseinrichtungen: SGB V §301; Bundesministerium der Justiz, Bundesamt für Justiz: Hamburg, Germany, 2023. [Google Scholar]
- Kaier, K.; Heidenreich, A.; Jäckel, M.; Oettinger, V.; Maier, A.; Hilgendorf, I.; Breitbart, P.; Hartikainen, T.; Keller, T.; Westermann, D.; et al. Reweighting and validation of the hospital frailty risk score using electronic health records in Germany: A retrospective observational study. BMC Geriatr. 2024, 24, 517. [Google Scholar] [CrossRef]
- Jung, C.; Bruno, R.R.; Wernly, B.; Wolff, G.; Beil, M.; Kelm, M. Frailty as a Prognostic Indicator in Intensive Care. Dtsch. Ärzteblatt Int. 2020, 117, 668–673. [Google Scholar] [CrossRef]

| Nr. | Abbreviation | Definition and Its Criteria | Reason for Choice | Total Number of All Cases per Definition (n) and Proportion of All Cases (%) |
|---|---|---|---|---|
| 1 | CodeBased ICU | Based on the ICU definition of the German Initiative for Quality in Medicine
|
| 281,537 (4.5%) |
| 2 | BedBased ICU | Based on the bed classification system of the German Helios Hospital Group. It includes a combination of the Hospital Section Intensive Care Unit and Intermediate Care Unit combined
|
| 457,717 (7.4%) |
| All Cases n (%) | Cases per Definition n (%) | Cases without COVID-19 n (%) | Only COVID-19 Cases n (%) | ||||
|---|---|---|---|---|---|---|---|
| Definition | None | CodeBased ICU | BedBased ICU | CodeBased ICU | BedBased ICU | CodeBased ICU | BedBased ICU |
| Year/Total n Cases | 6,204,093 | 281,537 | 457,717 | 273,140 | 453,553 | 8397 | 4164 |
| 2016 | 1,068,610 (17,2%) | 57,414 (20%) | 88,317 (19%) | 57,414 (21%) | 88,317 (19%) | 0 (0%) | 0 (0%) |
| 2017 | 1,076,906 (17,4%) | 54,319 (19%) | 91,902 (20%) | 54,319 (20%) | 91,902 (20%) | 0 (0%) | 0 (0%) |
| 2018 | 1,071,445 (17,3%) | 50,244 (18%) | 81,822 (18%) | 50,244 (18%) | 81,822 (18%) | 0 (0%) | 0 (0%) |
| 2019 | 1,073,693 (17,3%) | 40,429 (14%) | 76,413 (17%) | 40,429 (15%) | 76,413 (17%) | 0 (0%) | 0 (0%) |
| 2020 | 963,883 (15,5%) | 40,807 (14%) | 63,345 (14%) | 38,019 (14%) | 61,801 (14%) | 2788 (33%) | 1544 (37%) |
| 2021 | 949,556 (15,3%) | 38,324 (14%) | 55,918 (12%) | 32,715 (12%) | 53,298 (12%) | 5609 (67%) | 2620 (63%) |
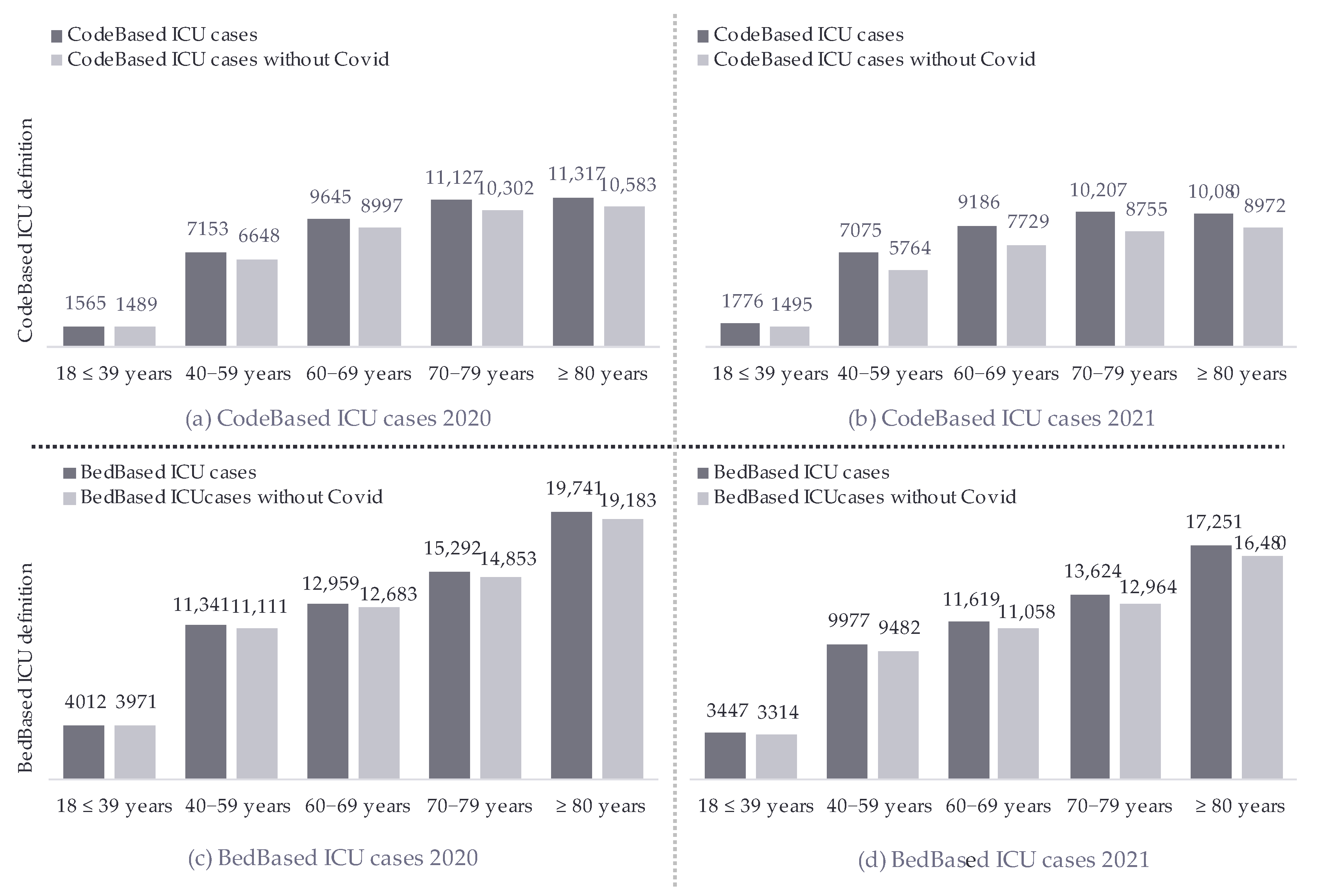
| CodeBased ICU: All Cases—Includes the Impact of COVID-19 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Groups/Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend (a) | Trend (n) | p Value |
| 18–39 years | 2416 (4.2%) | 2238 (4.1%) | 2028 (4.0%) | 1721 (4.3%) | 1565 (3.8%) | 1776 (4.6%) | → | 1.01 | 0.12 |
| 40−59 years | 10,733 (19%) | 9745 (18%) | 9015 (18%) | 7166 (18%) | 7153 (18%) | 7075 (18%) | ↓ | 0.99 | 0.025 |
| 60−69 years | 11,882 (21%) | 11,694 (22%) | 11,121 (22%) | 9412 (23%) | 9645 (24%) | 9186 (24%) | ↑ | 1.04 | <0.001 |
| 70−79 years | 17,770 (31%) | 16,551 (30%) | 14,850 (30%) | 11,355 (28%) | 11,127 (27%) | 10,207 (27%) | ↓ | 0.96 | <0.001 |
| ≥80 years | 14,613 (25%) | 14,091 (26%) | 13,230 (26%) | 10,775 (27%) | 11,317 (28%) | 10,080 (26%) | ↑ | 1.02 | <0.001 |
| Mean Age | 69.3 (14.4) | 69.5 (14.3) | 69.5 (14.2) | 69.4 (14.2) | 69.5 (14.1) | 68.7 (14.4) | ↓ | −0.06 | <0.001 |
| Total Cases | 57,414 | 54,319 | 50,244 | 40,429 | 40,807 | 38,324 | ↓ | x | x |
| BedBased ICU: All Cases—Includes The Impact of COVID-19 | |||||||||
| Age Groups/Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend (a) | Trend (n) | p value |
| 18–39 years | 5588 (6.3%) | 5844 (6.4%) | 5314 (6.5%) | 4874 (6.4%) | 4012 (6.3%) | 3447 (6.2%) | → | 1.00 | 0.3 |
| 40−59 years | 16,695 (19%) | 16,920 (18%) | 14,810 (18%) | 13,752 (18%) | 11,341 (18%) | 9977 (18%) | ↓ | 0.99 | <0.001 |
| 60−69 years | 16,520 (19%) | 17,428 (19%) | 15,888 (19%) | 15,340 (20%) | 12,959 (20%) | 11,619 (21%) | ↑ | 1.03 | <0.001 |
| 70−79 years | 24,984 (28%) | 25,425 (28%) | 21,856 (27%) | 19,502 (26%) | 15,292 (24%) | 13,624 (24%) | ↓ | 0.95 | <0.001 |
| ≥80 years | 24,530 (28%) | 26,285 (29%) | 23,954 (29%) | 22,945 (30%) | 19,741 (31%) | 17,251 (31%) | ↑ | 1.03 | <0.001 |
| Mean Age | 68.7 (16.0) | 68.9 (16.1) | 69.0 (16.1) | 69.0 (16.0) | 69.1 (16.0) | 69.1 (15.9) | ↑ | 0.07 | <0.001 |
| Total Cases | 88,317 | 91,902 | 81,822 | 76,413 | 63,345 | 55,918 | ↓ | x | x |
| Sex/Female Proportion | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| CodeBased | All cases | 23,740 (41%) | 22,375 (41%) | 20,794 (41%) | 16,509 (41%) | 16,422 (40%) | 15,232 (40%) | ↓ | <0.001 |
| Cases without COVID-19 | 23,740 (41%) | 22,375 (41%) | 20,794 (41%) | 16,509 (41%) | 15,433 (41%) | 13,202 (40%) | ↓ | <0.001 | |
| BedBased | All cases | 40,039 (45%) | 41,584 (45%) | 36,917 (45%) | 34,180 (45%) | 28,185 (44%) | 24,986 (45%) | ↓ | <0.001 |
| Cases without COVID-19 | 40,039 (45%) | 41,584 (45%) | 36,917 (45%) | 34,180 (45%) | 27,541 (45%) | 23,948 (45%) | ↓ | 0.002 | |
| Elixhauser Comorbidity Index (ECI) | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend | p value | |
| CodeBased | All cases | 17.1 (12.9) | 16.9 (12.8) | 16.9 (12.6) | 17.7 (12.8) | 17.9 (13.0) | 18.0 (12.9) | ↑ | <0.001 |
| ECI < 0 | 4506 (7.8%) | 4104 (7.6%) | 3619 (7.2%) | 2730 (6.8%) | 2862 (7.0%) | 2600 (6.8%) | ↓ | <0.001 | |
| ECI 0 | 2512 (4.4%) | 2448 (4.5%) | 2157 (4.3%) | 1541 (3.8%) | 1558 (3.8%) | 1470 (3.8%) | ↓ | <0.001 | |
| ECI 1−4 | 3278 (5.7%) | 3206 (5.9%) | 2976 (5.9%) | 2194 (5.4%) | 2034 (5.0%) | 1811 (4.7%) | ↓ | <0.001 | |
| ECI ≥ 5 | 47,118 (82%) | 44,561 (82%) | 41,492 (83%) | 33,964 (84%) | 34,353 (84%) | 32,443 (85%) | ↑ | <0.001 | |
| Cases without COVID-19 | 17.1 (12.9) | 16.9 (12.8) | 16.9 (12.6) | 17.7 (12.8) | 18.0 (13.0) | 18.3 (13.0) | ↑ | <0.001 | |
| BedBased | All cases | 12.2 (12.5) | 12.1 (12.3) | 11.9 (12.2) | 11.8 (12.2) | 12.2 (12.3) | 12.3 (12.4) | → | 0.3 |
| ECI < 0 | 14,280 (16%) | 14,311 (16%) | 12,607 (15%) | 12,182 (16%) | 9734 (15%) | 8269 (15%) | ↓ | <0.001 | |
| ECI 0 | 7700 (8.7%) | 8235 (9.0%) | 7390 (9.0%) | 6957 (9.1%) | 5808 (9.2%) | 5189 (9.3%) | ↑ | <0.001 | |
| ECI 1−4 | 6573 (7.4%) | 6813 (7.4%) | 6318 (7.7%) | 6010 (7.9%) | 4721 (7.5%) | 4121 (7.4%) | → | 0.7 | |
| ECI ≥ 5 | 59,764 (68%) | 62,543 (68%) | 55,507 (68%) | 51,264 (67%) | 43,082 (68%) | 38,339 (69%) | → | 0.066 | |
| Cases without COVID-19 | 12.2 (12.5) | 12.1 (12.3) | 11.9 (12.2) | 11.8 (12.2) | 12.0 (12.3) | 12.2 (12.4) | → | 0.10 | |
| Hospital Frailty Risk Score (HFR) | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend | p value | |
| CodeBased | All cases | 9.5 (7.4) | 9.7 (7.5) | 9.9 (7.4) | 10.4 (7.3) | 10.6 (7.4) | 10.7 (7.3) | ↑ | <0.001 |
| HFR < 5 | 19,084 (33%) | 17,465 (32%) | 15,380 (31%) | 10,846 (27%) | 10,526 (26%) | 9488 (25%) | ↓ | <0.001 | |
| HFR 5−15 | 25,715 (45%) | 24,536 (45%) | 23,165 (46%) | 19,493 (48%) | 19,998 (49%) | 19,120 (50%) | ↑ | <0.001 | |
| HFR > 15 | 12,615 (22%) | 12,318 (23%) | 11,699 (23%) | 10,090 (25%) | 10,283 (25%) | 9716 (25%) | ↑ | <0.001 | |
| Cases without COVID-19 | 9.5 (7.4) | 9.7 (7.5) | 9.9 (7.4) | 10.4 (7.3) | 10.5 (7.4) | 10.6 (7.3) | ↑ | <0.001 | |
| BedBased | All cases | 7.4 (7.2) | 7.5 (7.2) | 7.5 (7.2) | 7.5 (7.1) | 7.5 (7.2) | 7.4 (7.1) | → | 0.12 |
| HFR < 5 | 42,716 (48%) | 44,167 (48%) | 38,893 (48%) | 36,238 (47%) | 29,870 (47%) | 26,726 (48%) | ↓ | <0.001 | |
| HFR 5−15 | 31,953 (36%) | 33,458 (36%) | 30,223 (37%) | 28,250 (37%) | 23,601 (37%) | 20,748 (37%) | ↑ | <0.001 | |
| HFR > 15 | 13,648 (15%) | 14,277 (16%) | 12,706 (16%) | 11,925 (16%) | 9874 (16%) | 8444 (15%) | → | 0.3 | |
| Cases without COVID-19 | 7.4 (7.2) | 7.5 (7.2) | 7.5 (7.2) | 7.5 (7.1) | 7.4 (7.2) | 7.2 (7.0) | ↓ | 0.001 | |
| Mechanical ventilation (MV) | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend | p value | |
| CodeBased | All cases | 24,754 (43%) | 24,320 (45%) | 23,810 (47%) | 22,939 (57%) | 23,284 (57%) | 23,444 (61%) | ↑ | <0.001 |
| Cases without COVID-19 | 24,754 (43%) | 24,320 (45%) | 23,810 (47%) | 22,939 (57%) | 20,999 (55%) | 18,563 (57%) | ↑ | <0.001 | |
| BedBased | All cases | 10,990 (12%) | 12,257 (13%) | 11,309 (14%) | 10,342 (14%) | 8827 (14%) | 8126 (15%) | ↑ | <0.001 |
| Cases without COVID-19 | 10,990 (12%) | 12,257 (13%) | 11,309 (14%) | 10,342 (14%) | 8058 (13%) | 6618 (12%) | → | 0.8 | |
| In-hospital mortality | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend | p value | |
| CodeBased | All cases | 9802 (20%) | 9594 (21%) | 9321 (22%) | 8461 (25%) | 9110 (26%) | 9648 (30%) | ↑ | <0.001 |
| Cases without COVID-19 | 9802 (20%) | 9594 (21%) | 9321 (22%) | 8461 (25%) | 7953 (25%) | 7390 (27%) | ↑ | <0.001 | |
| BedBased | All cases | 6824 (8.5%) | 7371 (8.9%) | 6600 (9.0%) | 5851 (8.5%) | 5343 (9.3%) | 4889 (9.8%) | ↑ | <0.001 |
| Cases without COVID-19 | 6824 (8.5%) | 7371 (8.9%) | 6600 (9.0%) | 5851 (8.5%) | 4867 (8.7%) | 4145 (8.7%) | → | 0.8 | |
| In-hospital mortality of MV patients | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend | p value | |
| CodeBased | All cases | 7975 (39%) | 7943 (40%) | 7813 (40%) | 7337 (39%) | 7867 (41%) | 8458 (44%) | ↑ | <0.001 |
| Cases without COVID-19 | 7975 (39%) | 7943 (40%) | 7813 (40%) | 7337 (39%) | 6801 (40%) | 6324 (42%) | ↑ | 0.007 | |
| BedBased | All cases | 3513 (38%) | 3787 (37%) | 3310 (35%) | 2908 (34%) | 2636 (36%) | 2527 (38%) | ↓ | 0.018 |
| Cases without COVID-19 | 3513 (38%) | 3787 (37%) | 3310 (35%) | 2908 (34%) | 2300 (34%) | 1929 (35%) | ↓ | <0.001 | |
| Length of stay in hospital (LOSh) | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend | p value | |
| CodeBased | All cases | 15.6 (15.6); 11.0 [6.0–20.0] | 15.4 (15.8); 11.0 [6.0–20.0] | 15.1 (15.9); 11.0 [6.0–19.0] | 16.0 (16.7); 11.0 [6.0–20.0] | 15.5 (16.3); 11.0 [6.0–20.0] | 15.9 (16.4); 11.0 [6.0–20.0] | ↓ | <0.001 |
| Cases without COVID-19 | 15.6 (15.6); 11.0 [6.0–20.0] | 15.4 (15.8); 11.0 [6.0–20.0] | 15.1 (15.9); 11.0 [6.0–19.0] | 16.0 (16.7); 11.0 [6.0–20.0] | 15.0 (15.8); 11.0 [6.0–19.0] | 15.2 (16.2); 11.0 [6.0–19.0] | ↓ | <0.001 | |
| BedBased | All cases | 11.1 (11.2); 8.0 [4.0–14.0] | 11.1 (11.1); 8.0 [4.0–14.0] | 10.8 (11.5); 8.0 [4.0–14.0] | 10.9 (11.7); 7.0 [4.0–14.0] | 10.3 (11.3); 7.0 [4.0–13.0] | 10.5 (11.6); 7.0 [4.0–13.0] | ↓ | <0.001 |
| Cases without COVID-19 | 11.1 (11.2); 8.0 [4.0–14.0] | 11.1 (11.1); 8.0 [4.0–14.0] | 10.8 (11.5); 8.0 [4.0–14.0] | 10.9 (11.7); 7.0 [4.0–14.0] | 10.0 (10.9); 7.0 [4.0–13.0] | 10.1 (11.1); 7.0 [4.0–13.0] | ↓ | <0.001 | |
| Length of stay in ICU (LOSi) | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Trend | p value | |
| CodeBased | All cases | 7.0 (10.3); 4.0 [2.0–7.0] | 7.0 (10.8); 4.0 [2.0–7.0] | 6.8 (10.3); 3.0 [2.0–7.0] | 7.6 (10.8); 4.0 [2.0–8.0] | 7.8 (11.1); 4.0 [2.0–9.0] | 8.3 (11.7); 4.0 [2.0–10.0] | ↑ | <0.001 |
| Cases without COVID-19 | 7.0 (10.3); 4.0 [2.0–7.0] | 7.0 (10.8); 4.0 [2.0–7.0] | 6.8 (10.3); 3.0 [2.0–7.0] | 7.6 (10.8); 4.0 [2.0–8.0] | 7.4 (10.7); 4.0 [2.0–8.0] | 7.5 (11.2); 4.0 [2.0–8.0] | ↑ | <0.001 | |
| BedBased | All cases | 3.5 (6.1); 2.0 [1.0–3.0] | 3.5 (6.2); 2.0 [1.0–3.0] | 3.4 (6.4); 2.0 [1.0–3.0] | 3.5 (6.5); 2.0 [1.0–3.0] | 3.5 (6.6); 2.0 [1.0–3.0] | 3.6 (7.0); 2.0 [1.0–3.0] | ↓ | 0.018 |
| Cases without COVID-19 | 3.5 (6.1); 2.0 [1.0–3.0] | 3.5 (6.2); 2.0 [1.0–3.0] | 3.4 (6.4); 2.0 [1.0–3.0] | 3.5 (6.5); 2.0 [1.0–3.0] | 3.4 (6.2); 2.0 [1.0–3.0] | 3.3 (6.5); 2.0 [1.0–3.0] | ↓ | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, K.; Hohenstein, S.; Brederlau, J.; Hirsch, J.; Groesdonk, H.V.; Bollmann, A.; Kuhlen, R. A Systematic Comparison of Age, Comorbidity and Frailty of Two Defined ICU Populations in the German Helios Hospital Group from 2016–2021. J. Clin. Med. 2025, 14, 2332. https://doi.org/10.3390/jcm14072332
Hoffmann K, Hohenstein S, Brederlau J, Hirsch J, Groesdonk HV, Bollmann A, Kuhlen R. A Systematic Comparison of Age, Comorbidity and Frailty of Two Defined ICU Populations in the German Helios Hospital Group from 2016–2021. Journal of Clinical Medicine. 2025; 14(7):2332. https://doi.org/10.3390/jcm14072332
Chicago/Turabian StyleHoffmann, Kristina, Sven Hohenstein, Jörg Brederlau, Jan Hirsch, Heinrich V. Groesdonk, Andreas Bollmann, and Ralf Kuhlen. 2025. "A Systematic Comparison of Age, Comorbidity and Frailty of Two Defined ICU Populations in the German Helios Hospital Group from 2016–2021" Journal of Clinical Medicine 14, no. 7: 2332. https://doi.org/10.3390/jcm14072332
APA StyleHoffmann, K., Hohenstein, S., Brederlau, J., Hirsch, J., Groesdonk, H. V., Bollmann, A., & Kuhlen, R. (2025). A Systematic Comparison of Age, Comorbidity and Frailty of Two Defined ICU Populations in the German Helios Hospital Group from 2016–2021. Journal of Clinical Medicine, 14(7), 2332. https://doi.org/10.3390/jcm14072332









