Intersections and Challenges in the Management of Acute Coronary Syndrome and Stroke: Pathophysiology, Treatment Dilemmas, and Integrated Prevention Strategies
Abstract
1. Introduction
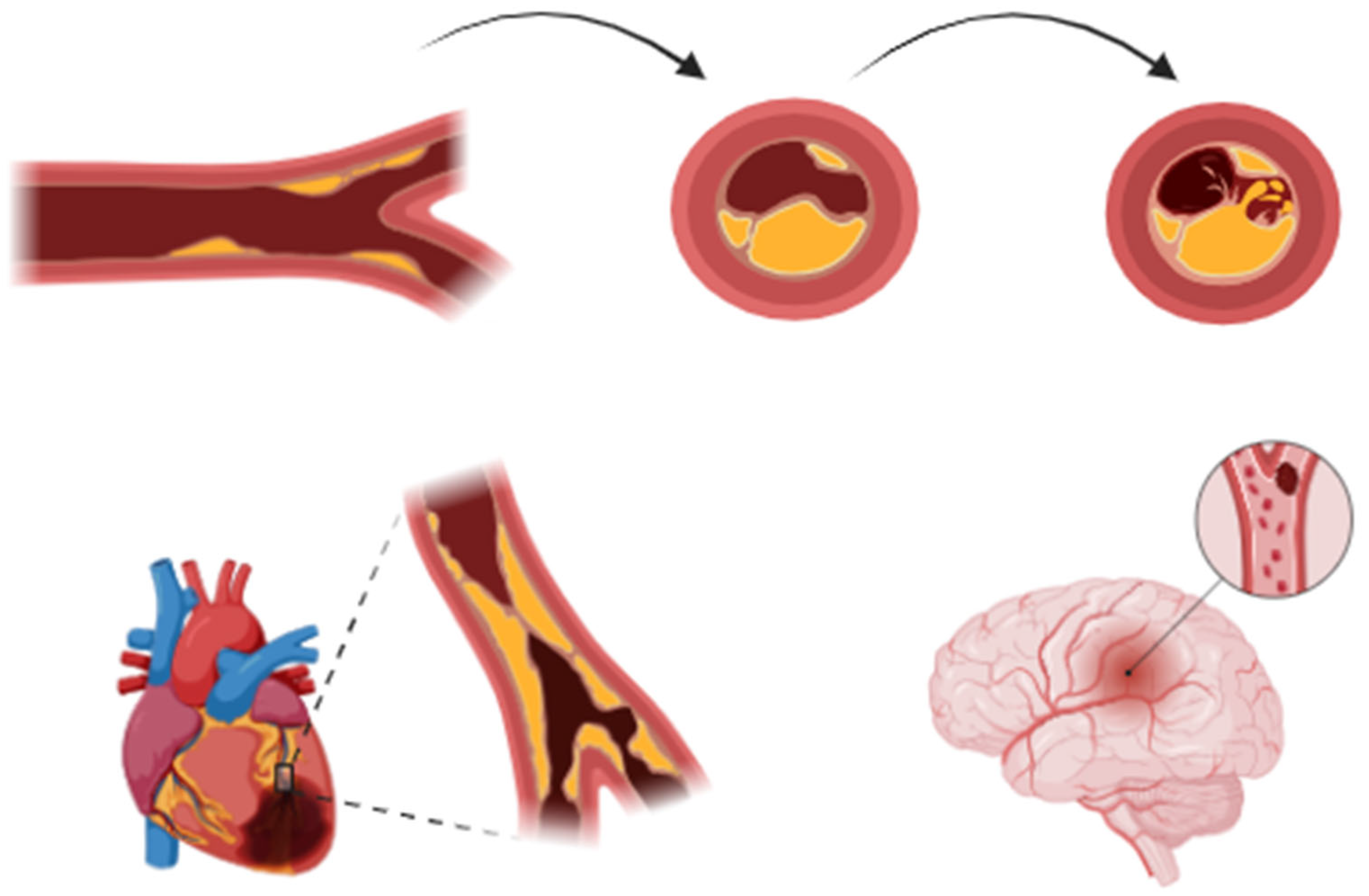
2. Pathophysiology of Acute Coronary Events and Stroke
2.1. Definition and Classification of Acute Myocardial Infarction
- STEMI (ST-Segment Elevation Myocardial Infarction): Defined by persistent ST-segment elevation on the ECG, typically indicating complete blockage of a coronary artery (Figure 2).
- NSTEMI (Non-ST Segment Elevation Myocardial Infarction): Occurs without persistent ST-segment elevation and is often associated with a partial blockage of a coronary artery.
2.2. Incidence and Risk of Stroke Following Acute Coronary Syndrome
- From the time of AMI hospitalization to one-month post-discharge, stroke incidence is estimated at 1.1–1.5% [15].
- A slight increase in ischemic stroke rates among STEMI patients (adjusted odds ratio, 1.10 [95% CI, 1.04–1.15]) over the study period.
- A notable decrease in ischemic stroke rates in NSTEMI patients (adjusted odds ratio, 0.47 [95% CI, 0.46–0.49]) (p < 0.001).
| Study | Population | Stroke Incidence | Key Findings |
|---|---|---|---|
| Hurskainen et al. (2022) [10] | Patients with ACS | 0.7–2% during hospitalization | 90% of strokes post-AMI are ischemic |
| Saczynski et al. (2008) [11] | Patients post-AMI | 1.1–1.5% at one month | Incidence of stroke peaks in the first 30 days post-MI |
| Ulvenstam et al. (2014) [16] | Post-AMI patients | 3% at six months | Stroke risk persists beyond hospitalization |
| Sundboll et al. (2016) [27] | AMI survivors | 8.6% at ten years | Long-term stroke risk remains substantial |
| Aggarwal et al. (2021) [26] | National Inpatient Sample (USA) 2000–2017 | 1.6% during hospitalization | STEMI patients had higher ischemic stroke rates |
| Hachet et al. (2014) [14] | Post-MI patients | 0.5–2.1% during hospitalization | In-hospital stroke incidence varies with MI severity and treatment strategies |
| Westerhout et al. (2006) [15] | NSTEMI patients | 0.7–2.1% within 30 days | Stroke risk in NSTEMI patients is influenced by treatment approaches and patient comorbidities |
2.3. Pathophysiological Mechanisms of Stroke in Acute Coronary Syndrome Patients
2.4. Atrial Fibrillation in the Setting of Acute Coronary Syndromes
2.5. Cardioembolic Mechanisms and Procedural Risk in Ischemic Stroke Following Myocardial Infarction
2.6. Procedural Risks in Stroke Development
3. Diagnosis and Biomarkers in Acute Coronary Syndrome and Stroke
3.1. Diagnostic Approach in Acute Coronary Syndrome
3.2. Diagnostic Approach in Stroke
3.3. Biomarkers in Stroke
3.4. Future Directions in Biomarkers and Diagnostics
4. Treatment and Acute Management of ACS and Stroke
4.1. Treatment of Acute Coronary Syndrome
4.2. Treatment of Acute Ischemic Stroke
4.3. Treatment of Intracranial Hemorrhage
4.4. Shared Strategies and Challenges
5. Secondary Prevention and Risk Management in Coexisting Stroke and Myocardial Infarction
5.1. Acute Management of Coexisting Stroke and Acute Myocardial Infarction
5.2. Chronic Management and Long-Term Pharmacotherapy
5.3. Lipid and Blood Pressure Management
5.4. Integrated Multidisciplinary Care
6. Discussion and Future Directions
6.1. Current Challenges in Management
6.2. Advances in Diagnosis and Risk Stratification
6.3. Future Directions in Treatment
6.4. Integrated Care Models
6.5. Bridging Knowledge Gaps
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEIs | angiotensin-converting enzyme inhibitors |
| ACS | acute coronary syndrome |
| AF | atrial fibrillation |
| AHA | American Heart Association |
| AI | artificial intelligence |
| AMI | acute myocardial infarction |
| ARBs | angiotensin receptor blockers |
| ASA | American Stroke Association |
| ASCVD | atherosclerotic cardiovascular disease |
| CABG | coronary artery bypass grafting |
| cTnI | cardiac troponin |
| CT | computed tomography |
| DALYs | disability-adjusted life years |
| DAPT | dual antiplatelet therapy |
| DOACs | direct oral anticoagulants |
| DWI | diffusion-weighted imaging |
| ECG | electrocardiogram |
| EVT | endovascular thrombectomy |
| GFAP | glial fibrillary acidic protein |
| hs-CRP | high-sensitivity C-reactive protein |
| hs-cTn | high-sensitivity troponin assays |
| IL-6 | interleukin-6 |
| IS | ischemic stroke |
| IVT | intravenous thrombolysis |
| LMWH | low molecular weight heparin |
| LVEF | left ventricular ejection fraction |
| LVT | left ventricular thrombus |
| LVO | large-vessel occlusion |
| MACE | major adverse cardiovascular events |
| MI | myocardial infarction |
| MRI | magnetic resonance imaging |
| NIS | National Inpatient Sample |
| NSTEMI | Non ST-Segment Elevation Myocardial Infarction |
| PCI | percutaneous coronary intervention |
| PCSK9 | Subtilisin/Kexin Type 9 |
| RR | risk ratio |
| S100B | S100 calcium-binding protein |
| SCAD | Spontaneous coronary artery dissection |
| STEMI | ST-Segment Elevation Myocardial Infarction |
| tPA | tissue plasminogen activator |
| TTE | transesophageal echocardiography |
| UA | unstable angina |
| UI | uncertainty interval |
| UFH | unfractionated heparin |
| URL | upper reference limit |
| WMA | wall motion abnormalities |
References
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar]
- Maffei, E.; Seitun, S.; Martini, C.; Palumbo, A.; Tarantini, G.; Berti, E.; Grilli, R.; Tedeschi, C.; Messalli, G.; Guaricci, A.; et al. CT coronary angiography and exercise ECG in a population with chest pain and low-to-intermediate pre-test likelihood of coronary artery disease. Heart 2010, 96, 1973–1979. [Google Scholar] [PubMed]
- Pontone, G.; Andreini, D.; Baggiano, A.; Bertella, E.; Mushtaq, S.; Conte, E.; Beltrama, V.; Guaricci, A.I.; Pepi, M. Functional relevance of coronary artery disease by cardiac magnetic resonance and cardiac computed tomography: Myocardial perfusion and fractional flow reserve. BioMed Res. Int. 2015, 2015, 297696. [Google Scholar]
- Muscogiuri, G.; Van Assen, M.; Tesche, C.; De Cecco, C.N.; Chiesa, M.; Scafuri, S.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Guaricci, A.I.; et al. Artificial Intelligence in Coronary Computed Tomography Angiography: From Anatomy to Prognosis. BioMed Res. Int. 2020, 2020, 6649410. [Google Scholar]
- Maffei, E.; Seitun, S.; Martini, C.; Aldrovandi, A.; Cervellin, G.; Tedeschi, C.; Guaricci, A.; Messalli, G.; Catalano, O.; Cademartiri, F. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin. Radiol. Med. 2011, 116, 690–705. [Google Scholar]
- Muscogiuri, G.; Martini, C.; Gatti, M.; Dell’Aversana, S.; Ricci, F.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Bracciani, A.; Scafuri, S.; et al. Feasibility of late gadolinium enhancement (LGE) in ischemic cardiomyopathy using 2D-multisegment LGE combined with artificial intelligence reconstruction deep learning noise reduction algorithm. Int. J. Cardiol. 2021, 343, 164–170. [Google Scholar] [PubMed]
- Baggiano, A.; Fusini, L.; Del Torto, A.; Vivona, P.; Guglielmo, M.; Muscogiuri, G.; Soldi, M.; Martini, C.; Fraschini, E.; Rabbat, M.G.; et al. Sequential Strategy Including FFR(CT) Plus Stress-CTP Impacts on Management of Patients with Stable Chest Pain: The Stress-CTP RIPCORD Study. J. Clin. Med. 2020, 9, 2147. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar]
- Hurskainen, M.; Tynkkynen, J.; Eskola, M.; Hernesniemi, J. Incidence of stroke and mortality due to stroke after acute coronary syndrome. J. Stroke Cerebrovasc. Dis. 2022, 31, 106842. [Google Scholar]
- Saczynski, J.S.; Spencer, F.A.; Gore, J.M.; Gurwitz, J.H.; Yarzebski, J.; Lessard, D.; Goldberg, R.J. Twenty-year trends in the incidence of stroke complicating acute myocardial infarction: Worcester Heart Attack Study. Arch. Intern. Med. 2008, 168, 2104–2110. [Google Scholar] [PubMed]
- Li, L.; Murthy, S.B. Cardiovascular Events After Intracerebral Hemorrhage. Stroke 2022, 53, 2131–2141. [Google Scholar] [PubMed]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar]
- Hachet, O.; Guenancia, C.; Stamboul, K.; Daubail, B.; Richard, C.; Bejot, Y.; Yameogo, V.; Gudjoncik, A.; Cottin, Y.; Giroud, M.; et al. Frequency and predictors of stroke after acute myocardial infarction: Specific aspects of in-hospital and postdischarge events. Stroke 2014, 45, 3514–3520. [Google Scholar] [PubMed]
- Westerhout, C.M.; Hernandez, A.V.; Steyerberg, E.W.; Bueno, H.; White, H.; Theroux, P.; Moliterno, D.J.; Armstrong, P.W.; Califf, R.M.; Wallentin, L.C.; et al. Predictors of stroke within 30 days in patients with non-ST-segment elevation acute coronary syndromes. Eur. Heart J. 2006, 27, 2956–2961. [Google Scholar]
- Ulvenstam, A.; Kajermo, U.; Modica, A.; Jernberg, T.; Soderstrom, L.; Mooe, T. Incidence, trends, and predictors of ischemic stroke 1 year after an acute myocardial infarction. Stroke 2014, 45, 3263–3268. [Google Scholar]
- Pezzella, F.R.; Mangiardi, M.; Ferrante, M.; Fabiano, S.; Anticoli, S.; Pennacchi, F.G.; Urso, A.; De Luca, L.; Caso, V. Management of Oral Anticoagulation and Antiplatelet Therapy in Post-Myocardial Infarction Patients with Acute Ischemic Stroke with and without Atrial Fibrillation. J. Clin. Med. 2022, 11, 3894. [Google Scholar] [CrossRef]
- Wienbergen, H.; Schiele, R.; Gitt, A.K.; Schneider, S.; Heer, T.; Gottwik, M.; Gieseler, U.; Weber, M.A.; Muller, C.H.; Neubaur, J.; et al. Incidence, risk factors, and clinical outcome of stroke after acute myocardial infarction in clinical practice. MIR and MITRA Study Groups. Myocardial Infarction Registry. Maximal Individual Therapy in Acute Myocardial Infarction. Am. J. Cardiol. 2001, 87, 782–785. [Google Scholar]
- Witt, B.J.; Ballman, K.V.; Brown, R.D., Jr.; Meverden, R.A.; Jacobsen, S.J.; Roger, V.L. The incidence of stroke after myocardial infarction: A meta-analysis. Am. J. Med. 2006, 119, 354e1–354e9. [Google Scholar]
- Dutta, M.; Hanna, E.; Das, P.; Steinhubl, S.R. Incidence and prevention of ischemic stroke following myocardial infarction: Review of current literature. Cerebrovasc. Dis. 2006, 22, 331–339. [Google Scholar]
- Kumbhani, D.J.; Cannon, C.P.; Beavers, C.J.; Bhatt, D.L.; Cuker, A.; Gluckman, T.J.; Marine, J.E.; Mehran, R.; Messe, S.R.; Patel, N.S.; et al. 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients With Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention or With Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 629–658. [Google Scholar] [PubMed]
- Writing Committee, M.; Anderson, H.V.S.; Masri, S.C.; Abdallah, M.S.; Chang, A.M.; Cohen, M.G.; Elgendy, I.Y.; Gulati, M.; LaPoint, K.; Madan, N.; et al. 2022 ACC/AHA Key Data Elements and Definitions for Chest Pain and Acute Myocardial Infarction: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Data Standards. J. Am. Coll. Cardiol. 2022, 80, 1660–1700. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [PubMed]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar]
- Aggarwal, G.; Patlolla, S.H.; Aggarwal, S.; Cheungpasitporn, W.; Doshi, R.; Sundaragiri, P.R.; Rabinstein, A.A.; Jaffe, A.S.; Barsness, G.W.; Cohen, M.; et al. Temporal Trends, Predictors, and Outcomes of Acute Ischemic Stroke in Acute Myocardial Infarction in the United States. J. Am. Heart Assoc. 2021, 10, e017693. [Google Scholar]
- Sundboll, J.; Horvath-Puho, E.; Schmidt, M.; Pedersen, L.; Henderson, V.W.; Botker, H.E.; Sorensen, H.T. Long-Term Risk of Stroke in Myocardial Infarction Survivors: Thirty-Year Population-Based Cohort Study. Stroke 2016, 47, 1727–1733. [Google Scholar] [PubMed]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Leducq Transatlantic Network on, A. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar]
- Medina-Leyte, D.J.; Zepeda-Garcia, O.; Dominguez-Perez, M.; Gonzalez-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Chen, L.H.; Spagnolo-Allende, A.; Yang, D.; Qiao, Y.; Gutierrez, J. Epidemiology, Pathophysiology, and Imaging of Atherosclerotic Intracranial Disease. Stroke 2024, 55, 311–323. [Google Scholar] [PubMed]
- Elsheikh, S.; Hill, A.; Irving, G.; Lip, G.Y.H.; Abdul-Rahim, A.H. Atrial fibrillation and stroke: State-of-the-art and future directions. Curr. Probl. Cardiol. 2024, 49, 102181. [Google Scholar] [PubMed]
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867. [Google Scholar] [PubMed]
- Ntaios, G.; Baumgartner, H.; Doehner, W.; Donal, E.; Edvardsen, T.; Healey, J.S.; Iung, B.; Kamel, H.; Kasner, S.E.; Korompoki, E.; et al. Embolic strokes of undetermined source: A clinical consensus statement of the ESC Council on Stroke, the European Association of Cardiovascular Imaging and the European Heart Rhythm Association of the ESC. Eur. Heart J. 2024, 45, 1701–1715. [Google Scholar]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- Alem, M.M. Endothelial Dysfunction in Chronic Heart Failure: Assessment, Findings, Significance, and Potential Therapeutic Targets. Int. J. Mol. Sci. 2019, 20, 3198. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksass, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Back, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar]
- Botts, S.R.; Fish, J.E.; Howe, K.L. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment. Front. Pharmacol. 2021, 12, 787541. [Google Scholar]
- Napoli, G.; Pergola, V.; Basile, P.; De Feo, D.; Bertrandino, F.; Baggiano, A.; Mushtaq, S.; Fusini, L.; Fazzari, F.; Carrabba, N.; et al. Epicardial and Pericoronary Adipose Tissue, Coronary Inflammation, and Acute Coronary Syndromes. J. Clin. Med. 2023, 12, 7212. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pacheco, H.; Marquez, M.F.; Arias-Mendoza, A.; Alvarez-Sangabriel, A.; Eid-Lidt, G.; Gonzalez-Hermosillo, A.; Azar-Manzur, F.; Altamirano-Castillo, A.; Briseno-Cruz, J.L.; Garcia-Martinez, A.; et al. Clinical features and in-hospital mortality associated with different types of atrial fibrillation in patients with acute coronary syndrome with and without ST elevation. J. Cardiol. 2015, 66, 148–154. [Google Scholar]
- Krijthe, B.P.; Leening, M.J.; Heeringa, J.; Kors, J.A.; Hofman, A.; Franco, O.H.; Witteman, J.C.; Stricker, B.H. Unrecognized myocardial infarction and risk of atrial fibrillation: The Rotterdam Study. Int. J. Cardiol. 2013, 168, 1453–1457. [Google Scholar] [PubMed]
- Soliman, E.Z.; Safford, M.M.; Muntner, P.; Khodneva, Y.; Dawood, F.Z.; Zakai, N.A.; Thacker, E.L.; Judd, S.; Howard, V.J.; Howard, G.; et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern. Med. 2014, 174, 107–114. [Google Scholar]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [PubMed]
- Coscia, T.; Nestelberger, T.; Boeddinghaus, J.; Lopez-Ayala, P.; Koechlin, L.; Miro, O.; Keller, D.I.; Strebel, I.; Yufera Sanchez, A.; Okamura, B.; et al. Characteristics and Outcomes of Type 2 Myocardial Infarction. JAMA Cardiol. 2022, 7, 427–434. [Google Scholar]
- Park, D.Y.; Wang, P.; An, S.; Grimshaw, A.A.; Frampton, J.; Ohman, E.M.; Rao, S.V.; Nanna, M.G. Shortening the duration of dual antiplatelet therapy after percutaneous coronary intervention for acute coronary syndrome: A systematic review and meta-analysis. Am. Heart J. 2022, 251, 101–114. [Google Scholar]
- Gargiulo, G.; Goette, A.; Tijssen, J.; Eckardt, L.; Lewalter, T.; Vranckx, P.; Valgimigli, M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: A systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur. Heart J. 2019, 40, 3757–3767. [Google Scholar]
- Alexander, J.H.; Wojdyla, D.; Vora, A.N.; Thomas, L.; Granger, C.B.; Goodman, S.G.; Aronson, R.; Windecker, S.; Mehran, R.; Lopes, R.D. Risk/Benefit Tradeoff of Antithrombotic Therapy in Patients With Atrial Fibrillation Early and Late After an Acute Coronary Syndrome or Percutaneous Coronary Intervention: Insights From AUGUSTUS. Circulation 2020, 141, 1618–1627. [Google Scholar]
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115. [Google Scholar]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar]
- Yasuda, S.; Kaikita, K.; Akao, M.; Ako, J.; Matoba, T.; Nakamura, M.; Miyauchi, K.; Hagiwara, N.; Kimura, K.; Hirayama, A.; et al. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease. N. Engl. J. Med. 2019, 381, 1103–1113. [Google Scholar]
- Leary, M.C.; Caplan, L.R. Cardioembolic stroke: An update on etiology, diagnosis and management. Ann. Indian. Acad. Neurol. 2008, 11, S52–S63. [Google Scholar] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [PubMed]
- Boivin-Proulx, L.A.; Ieroncig, F.; Demers, S.P.; Nozza, A.; Soltani, M.; Ghersi, I.; Verreault-Julien, L.; Alansari, Y.; Massie, C.; Simard, P.; et al. Contemporary incidence and predictors of left ventricular thrombus in patients with anterior acute myocardial infarction. Clin. Res. Cardiol. 2023, 112, 558–565. [Google Scholar]
- Camaj, A.; Fuster, V.; Giustino, G.; Bienstock, S.W.; Sternheim, D.; Mehran, R.; Dangas, G.D.; Kini, A.; Sharma, S.K.; Halperin, J.; et al. Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1010–1022. [Google Scholar] [PubMed]
- Di Odoardo, L.A.F.; Bianco, M.; Gil, I.J.N.; Motolese, I.G.; Chinaglia, A.; Vicenzi, M.; Carugo, S.; Stefanini, G.G.; Cerrato, E. Left Ventricular Thrombus Management After Acute Myocardial Infarction in Clinical Practice: Results from LEVITATION Survey and Narrative Review. Cardiovasc. Drugs Ther. 2024, 38, 483–492. [Google Scholar]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar]
- Kamran, S.; Akhtar, N.; Singh, R.; Imam, Y.; Haroon, K.H.; Amir, N.; Hussain, S.; Al Jerdi, S.; Ojha, L.; Own, A.; et al. Association of Major Adverse Cardiovascular Events in Patients With Stroke and Cardiac Wall Motion Abnormalities. J. Am. Heart Assoc. 2021, 10, e020888. [Google Scholar]
- Schafer, A.; Flierl, U.; Bauersachs, J. Anticoagulants for stroke prevention in heart failure with reduced ejection fraction. Clin. Res. Cardiol. 2022, 111, 1–13. [Google Scholar]
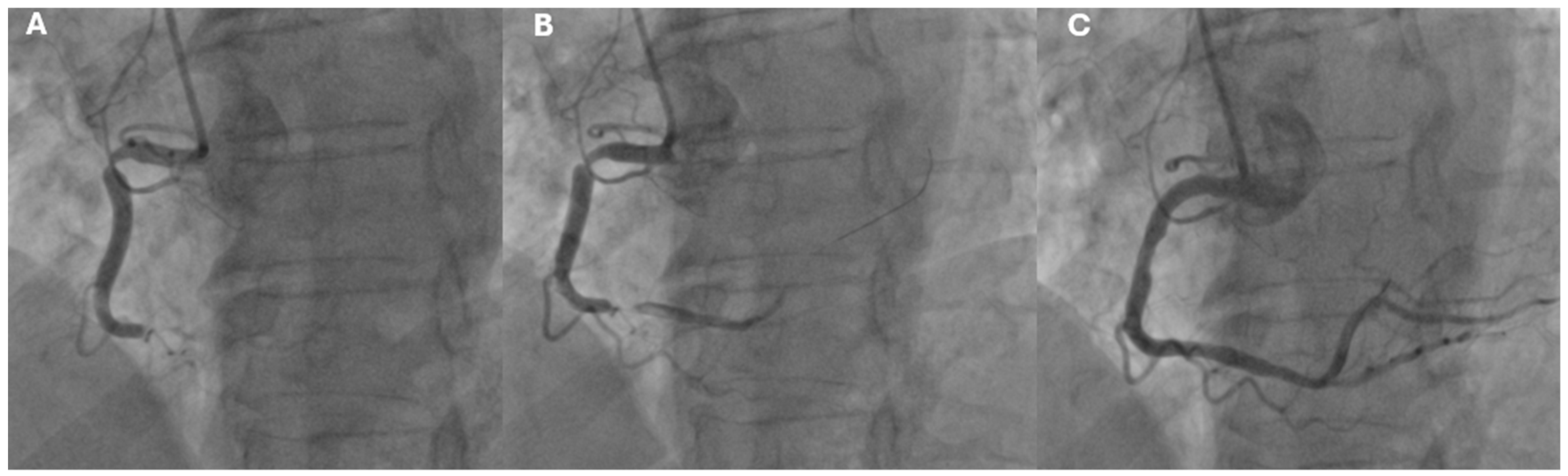
- Alkhouli, M.; Alqahtani, F.; Tarabishy, A.; Sandhu, G.; Rihal, C.S. Incidence, Predictors, and Outcomes of Acute Ischemic Stroke Following Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1497–1506. [Google Scholar]
- Dukkipati, S.; O’Neill, W.W.; Harjai, K.J.; Sanders, W.P.; Deo, D.; Boura, J.A.; Bartholomew, B.A.; Yerkey, M.W.; Sadeghi, H.M.; Kahn, J.K. Characteristics of cerebrovascular accidents after percutaneous coronary interventions. J. Am. Coll. Cardiol. 2004, 43, 1161–1167. [Google Scholar]
- Filsoufi, F.; Rahmanian, P.B.; Castillo, J.G.; Bronster, D.; Adams, D.H. Incidence, topography, predictors and long-term survival after stroke in patients undergoing coronary artery bypass grafting. Ann. Thorac. Surg. 2008, 85, 862–870. [Google Scholar] [PubMed]
- Murakami, T.; Sakakura, K.; Jinnouchi, H.; Taniguchi, Y.; Tsukui, T.; Watanabe, Y.; Yamamoto, K.; Seguchi, M.; Wada, H.; Fujita, H. Acute Ischemic Stroke and Transient Ischemic Attack in ST-Segment Elevation Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention. J. Clin. Med. 2023, 12, 840. [Google Scholar] [CrossRef]
- Tarakji, K.G.; Sabik, J.F., 3rd; Bhudia, S.K.; Batizy, L.H.; Blackstone, E.H. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA 2011, 305, 381–390. [Google Scholar] [PubMed]
- Chapman, A.R.; Adamson, P.D.; Shah, A.S.V.; Anand, A.; Strachan, F.E.; Ferry, A.V.; Lee, K.K.; Berry, C.; Findlay, I.; Cruikshank, A.; et al. High-Sensitivity Cardiac Troponin and the Universal Definition of Myocardial Infarction. Circulation 2020, 141, 161–171. [Google Scholar] [PubMed]
- Carrabba, N.; Amico, M.A.; Guaricci, A.I.; Carella, M.C.; Maestrini, V.; Monosilio, S.; Pedrotti, P.; Ricci, F.; Monti, L.; Figliozzi, S.; et al. CMR Mapping: The 4th-Era Revolution in Cardiac Imaging. J. Clin. Med. 2024, 13, 337. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Tuttolomondo, D.; Guaricci, A.I.; Di Giannuario, G. Pulse-Cancellation Echocardiography for Clinical Evaluation of Myocardial Scar Burden. Curr. Cardiol. Rep. 2021, 23, 100. [Google Scholar]
- Neglia, D.; Liga, R.; Gimelli, A.; Podlesnikar, T.; Cvijic, M.; Pontone, G.; Miglioranza, M.H.; Guaricci, A.I.; Seitun, S.; Clemente, A.; et al. Use of cardiac imaging in chronic coronary syndromes: The EURECA Imaging registry. Eur. Heart J. 2023, 44, 142–158. [Google Scholar]
- Gaibazzi, N.; Porter, T.; Lorenzoni, V.; Pontone, G.; De Santis, D.; De Rosa, A.; Guaricci, A.I. Effect of Coronary Revascularization on the Prognostic Value of Stress Myocardial Contrast Wall Motion and Perfusion Imaging. J. Am. Heart Assoc. 2017, 6, e006202. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Chiarello, G.; Gherbesi, E.; Fusini, L.; Soldato, N.; Siena, P.; Ursi, R.; Ruggieri, R.; Guglielmo, M.; Muscogiuri, G.; et al. Coronary-specific quantification of myocardial deformation by strain echocardiography may disclose the culprit vessel in patients with non-ST-segment elevation acute coronary syndrome. Eur. Heart J. Open 2022, 2, oeac010. [Google Scholar] [PubMed]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Soldi, M.; Fazzari, F.; Berzovini, C.; et al. Quantitative vs. qualitative evaluation of static stress computed tomography perfusion to detect haemodynamically significant coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Joyce, T.; Lorenzoni, V.; Guaricci, A.I.; Pavon, A.G.; Fusini, L.; Andreini, D.; Rabbat, M.G.; Aquaro, G.D.; Abete, R.; et al. AI Cardiac MRI Scar Analysis Aids Prediction of Major Arrhythmic Events in the Multicenter DERIVATE Registry. Radiology 2023, 307, e222239. [Google Scholar]
- Pontone, G.; Guaricci, A.I.; Fusini, L.; Baggiano, A.; Guglielmo, M.; Muscogiuri, G.; Volpe, A.; Abete, R.; Aquaro, G.; Barison, A.; et al. Cardiac Magnetic Resonance for Prophylactic Implantable-Cardioverter Defibrillator Therapy in Ischemic Cardiomyopathy: The DERIVATE-ICM International Registry. JACC Cardiovasc. Imaging 2023, 16, 1387–1400. [Google Scholar]
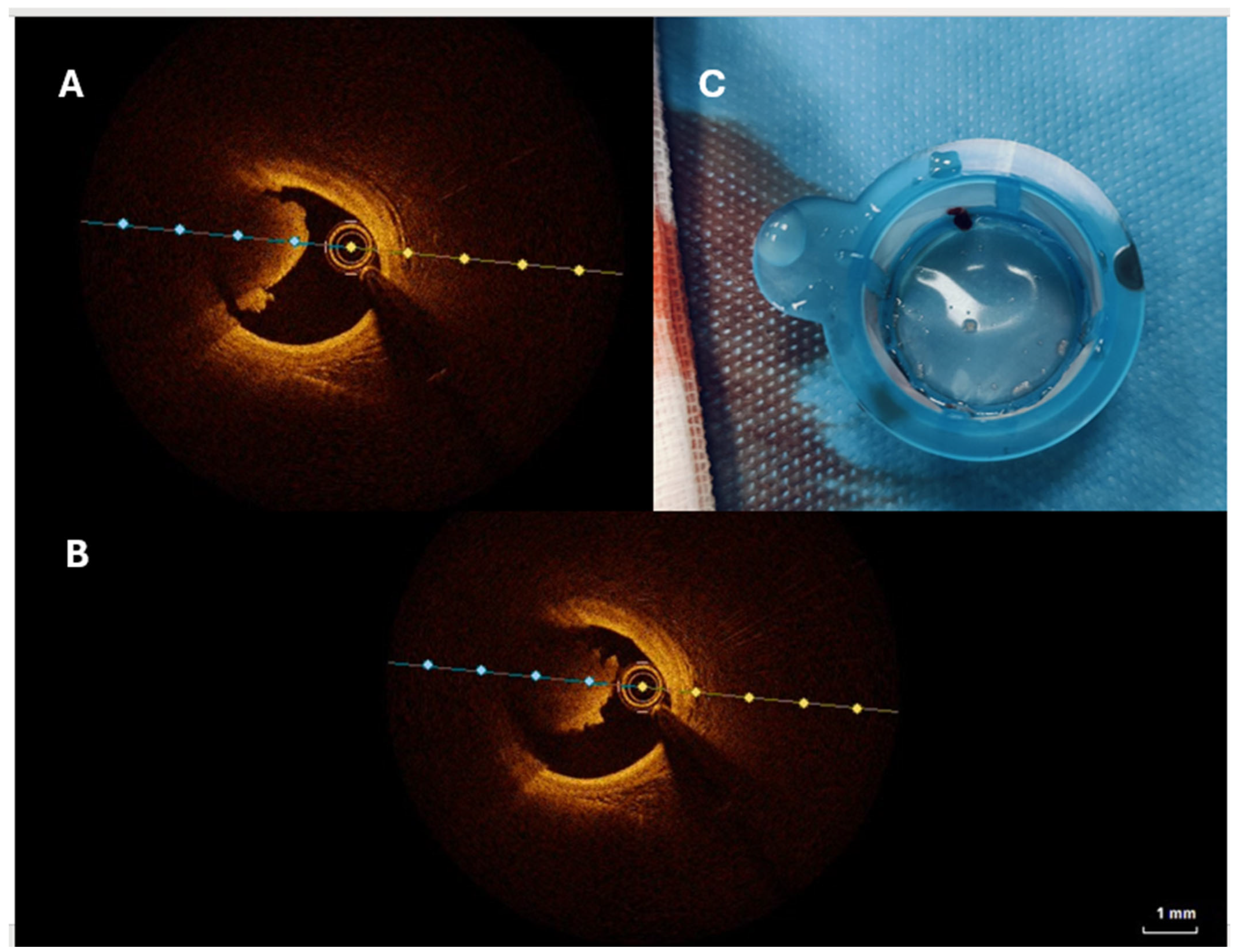
- Nafee, T.; Shah, A.; Forsberg, M.; Zheng, J.; Ou, J. State-of-art review: Intravascular imaging in percutaneous coronary interventions. Cardiol. Plus 2023, 8, 227–246. [Google Scholar] [PubMed]
- Buonpane, A.; Trimarchi, G.; Ciardetti, M.; Coceani, M.A.; Alagna, G.; Benedetti, G.; Berti, S.; Ando, G.; Burzotta, F.; De Caterina, A.R. Optical Coherence Tomography in Myocardial Infarction Management: Enhancing Precision in Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 5791. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Gold, H.K.; Yuan, J.; Narula, J.; Finn, A.V.; Virmani, R. The thin-cap fibroatheroma: A type of vulnerable plaque: The major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 2001, 16, 285–292. [Google Scholar]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [PubMed]
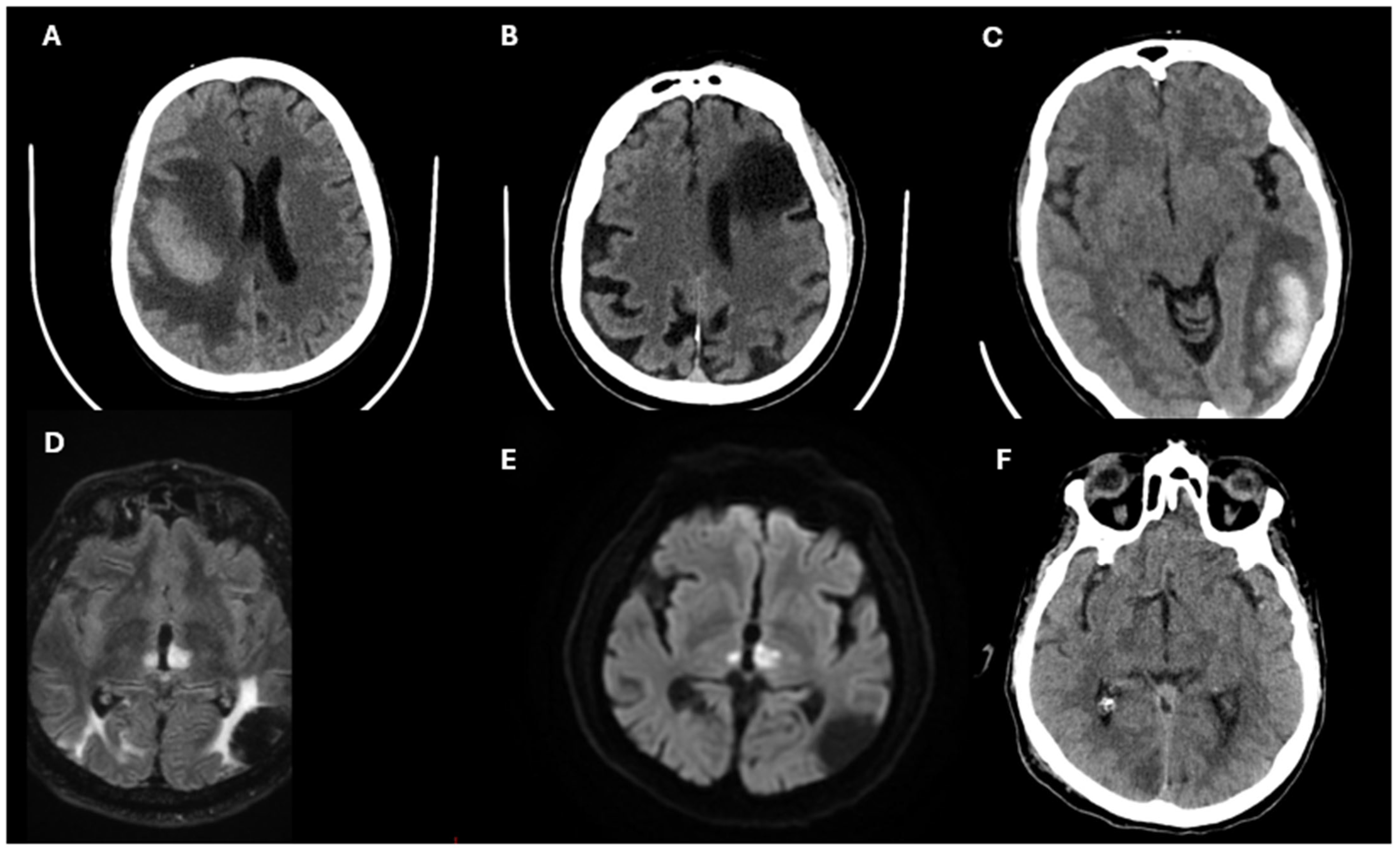
- Jadhav, A.P.; Desai, S.M.; Liebeskind, D.S.; Wechsler, L.R. Neuroimaging of Acute Stroke. Neurol. Clin. 2020, 38, 185–199. [Google Scholar]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar]
- Fonseca, A.C.; Ferro, J.M.; Almeida, A.G. Cardiovascular magnetic resonance imaging and its role in the investigation of stroke: An update. J. Neurol. 2021, 268, 2597–2604. [Google Scholar] [PubMed]
- Kamel, H.; Healey, J.S. Cardioembolic Stroke. Circ. Res. 2017, 120, 514–526. [Google Scholar] [PubMed]
- Barakzie, A.; Jansen, A.J.G.; Ten Cate, H.; de Maat, M.P.M. Coagulation biomarkers for ischemic stroke. Res. Pract. Thromb. Haemost. 2023, 7, 100160. [Google Scholar]
- Luo, Y.; Dong, W.; Yuan, L.; Zhu, Y.A.; Zhang, D.D.; Ni, H.; Zhu, W. The Role of Thrombo-inflammation in Ischemic Stroke: Focus on the Manipulation and Clinical Application. Mol. Neurobiol. 2025, 62, 2362–2375. [Google Scholar]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [PubMed]
- Bustamante, A.; Penalba, A.; Orset, C.; Azurmendi, L.; Llombart, V.; Simats, A.; Pecharroman, E.; Ventura, O.; Ribo, M.; Vivien, D.; et al. Blood Biomarkers to Differentiate Ischemic and Hemorrhagic Strokes. Neurology 2021, 96, e1928–e1939. [Google Scholar]
- McCabe, J.J.; Walsh, C.; Gorey, S.; Harris, K.; Hervella, P.; Iglesias-Rey, R.; Jern, C.; Li, L.; Miyamoto, N.; Montaner, J.; et al. C-Reactive Protein, Interleukin-6, and Vascular Recurrence After Stroke: An Individual Participant Data Meta-Analysis. Stroke 2023, 54, 1289–1299. [Google Scholar]
- Filipovic, M.G.; Luedi, M.M. Cardiovascular Biomarkers: Current Status and Future Directions. Cells 2023, 12, 2647. [Google Scholar] [CrossRef]
- Koska, I.O.; Selver, A. Artificial Intelligence in Stroke Imaging: A Comprehensive Review. Eurasian J. Med. 2023, 55, 91–97. [Google Scholar]
- Chaturvedi, S.; De Marchis, G.M. Inflammatory Biomarkers and Stroke Subtype: An Important New Frontier. Neurology 2024, 102, e208098. [Google Scholar]
- Johansen, M.C.; von Rennenberg, R.; Nolte, C.H.; Jensen, M.; Bustamante, A.; Katan, M. Role of Cardiac Biomarkers in Stroke and Cognitive Impairment. Stroke 2024, 55, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M.; et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef]
- Authors/Task Force, M.; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 2022, 24, 4–131. [Google Scholar]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, T.; Payne, C.D.; Winters, K.J.; Darstein, C.; Brandt, J.T.; Jakubowski, J.A.; Naganuma, H.; Siegbahn, A.; Wallentin, L. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur. Heart J. 2006, 27, 1166–1173. [Google Scholar] [CrossRef]
- Wallentin, L.; Varenhorst, C.; James, S.; Erlinge, D.; Braun, O.O.; Jakubowski, J.A.; Sugidachi, A.; Winters, K.J.; Siegbahn, A. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur. Heart J. 2008, 29, 21–30. [Google Scholar] [CrossRef]
- Fox, K.A.; Mehta, S.R.; Peters, R.; Zhao, F.; Lakkis, N.; Gersh, B.J.; Yusuf, S. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: The Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004, 110, 1202–1208. [Google Scholar] [CrossRef]
- Kuliczkowski, W.; Witkowski, A.; Polonski, L.; Watala, C.; Filipiak, K.; Budaj, A.; Golanski, J.; Sitkiewicz, D.; Pregowski, J.; Gorski, J.; et al. Interindividual variability in the response to oral antiplatelet drugs: A position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur. Heart J. 2009, 30, 426–435. [Google Scholar]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K.; Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar]
- Kim, B.K.; Hong, S.J.; Cho, Y.H.; Yun, K.H.; Kim, Y.H.; Suh, Y.; Cho, J.Y.; Her, A.Y.; Cho, S.; Jeon, D.W.; et al. Effect of Ticagrelor Monotherapy vs Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome: The TICO Randomized Clinical Trial. JAMA 2020, 323, 2407–2416. [Google Scholar] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahi Salman, R.; Greenberg, S.M. Antiplatelet Agent Use After Stroke due to Intracerebral Hemorrhage. Stroke 2023, 54, 3173–3181. [Google Scholar] [CrossRef]
- Shulga, O.; Bornstein, N. Antiplatelets in secondary stroke prevention. Front. Neurol. 2011, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Schrag, M.; Kirshner, H. Management of Intracerebral Hemorrhage: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1819–1831. [Google Scholar] [CrossRef]
- Christensen, H.; Cordonnier, C.; Korv, J.; Lal, A.; Ovesen, C.; Purrucker, J.C.; Toni, D.; Steiner, T. European Stroke Organisation Guideline on Reversal of Oral Anticoagulants in Acute Intracerebral Haemorrhage. Eur. Stroke J. 2019, 4, 294–306. [Google Scholar] [CrossRef]
- Rodriguez, F.; Harrington, R.A. Management of Antithrombotic Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2021, 384, 452–460. [Google Scholar] [CrossRef]
- Castrichini, M.; Luzum, J.A.; Pereira, N. Pharmacogenetics of Antiplatelet Therapy. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 211–229. [Google Scholar] [CrossRef]
- Ghozy, S.; Reda, A.; Varney, J.; Elhawary, A.S.; Shah, J.; Murry, K.; Sobeeh, M.G.; Nayak, S.S.; Azzam, A.Y.; Brinjikji, W.; et al. Neuroprotection in Acute Ischemic Stroke: A Battle Against the Biology of Nature. Front. Neurol. 2022, 13, 870141. [Google Scholar] [CrossRef]
- Bozyel, S.; Simsek, E.; Kocyigit Burunkaya, D.; Guler, A.; Korkmaz, Y.; Seker, M.; Erturk, M.; Keser, N. Artificial Intelligence-Based Clinical Decision Support Systems in Cardiovascular Diseases. Anatol. J. Cardiol. 2024, 28, 74–86. [Google Scholar]
- Sobolewski, P.; Sledzinska-Dzwigal, M.; Szczuchniak, W.; Hatalska-Zerebiec, R.; Grzesik, M.; Sobota, A. The efficacy and safety of intravenous thrombolysis with alteplase in the treatment of ischaemic stroke in a rural hospital. Neurol. Neurochir. Pol. 2013, 47, 310–318. [Google Scholar] [PubMed][Green Version]
- Matsuzawa, Y.; Kimura, K.; Yasuda, S.; Kaikita, K.; Akao, M.; Ako, J.; Matoba, T.; Nakamura, M.; Miyauchi, K.; Hagiwara, N.; et al. Antithrombotic Therapy for Atrial Fibrillation and Coronary Artery Disease in Patients With Prior Atherothrombotic Disease: A Post Hoc Analysis of the AFIRE Trial. J. Am. Heart Assoc. 2021, 10, e020907. [Google Scholar]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar]
- Lip, G.Y.H.; Gue, Y.; Zhang, J.; Chao, T.F.; Calkins, H.; Potpara, T. Stroke prevention in atrial fibrillation. Trends Cardiovasc. Med. 2022, 32, 501–510. [Google Scholar]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar]
- Counseller, Q.; Aboelkassem, Y. Recent technologies in cardiac imaging. Front. Med. Technol. 2022, 4, 984492. [Google Scholar]
- Liu, F.; Yao, Y.; Zhu, B.; Yu, Y.; Ren, R.; Hu, Y. The novel imaging methods in diagnosis and assessment of cerebrovascular diseases: An overview. Front. Med. 2024, 11, 1269742. [Google Scholar]
- Montellano, F.A.; Ungethum, K.; Ramiro, L.; Nacu, A.; Hellwig, S.; Fluri, F.; Whiteley, W.N.; Bustamante, A.; Montaner, J.; Heuschmann, P.U. Role of Blood-Based Biomarkers in Ischemic Stroke Prognosis: A Systematic Review. Stroke 2021, 52, 543–551. [Google Scholar] [PubMed]
- Tsay, D.; Patterson, C. From Machine Learning to Artificial Intelligence Applications in Cardiac Care. Circulation 2018, 138, 2569–2575. [Google Scholar]
- Vandenberk, B.; Chew, D.S.; Prasana, D.; Gupta, S.; Exner, D.V. Successes and challenges of artificial intelligence in cardiology. Front. Digit. Health 2023, 5, 1201392. [Google Scholar]
- Almarzooq, Z.I.; Al-Roub, N.M.; Kinlay, S. Antithrombotic treatment following percutaneous coronary intervention in patients with high bleeding risk. Curr. Opin. Cardiol. 2023, 38, 515–520. [Google Scholar]
- Nolte, C.H. Factor XI inhibitors—Rising stars in anti-thrombotic therapy? J. Neurol. Sci. 2024, 464, 123157. [Google Scholar]
- Han, X.; Qin, Y.; Mei, C.; Jiao, F.; Khademolqorani, S.; Nooshin Banitaba, S. Current trends and future perspectives of stroke management through integrating health care team and nanodrug delivery strategy. Front. Cell Neurosci. 2023, 17, 1266660. [Google Scholar] [CrossRef]
- Tromp, J.; Jindal, D.; Redfern, J.; Bhatt, A.; Severin, T.; Banerjee, A.; Ge, J.; Itchhaporia, D.; Jaarsma, T.; Lanas, F.; et al. World Heart Federation Roadmap for Digital Health in Cardiology. Glob. Heart 2022, 17, 61. [Google Scholar]
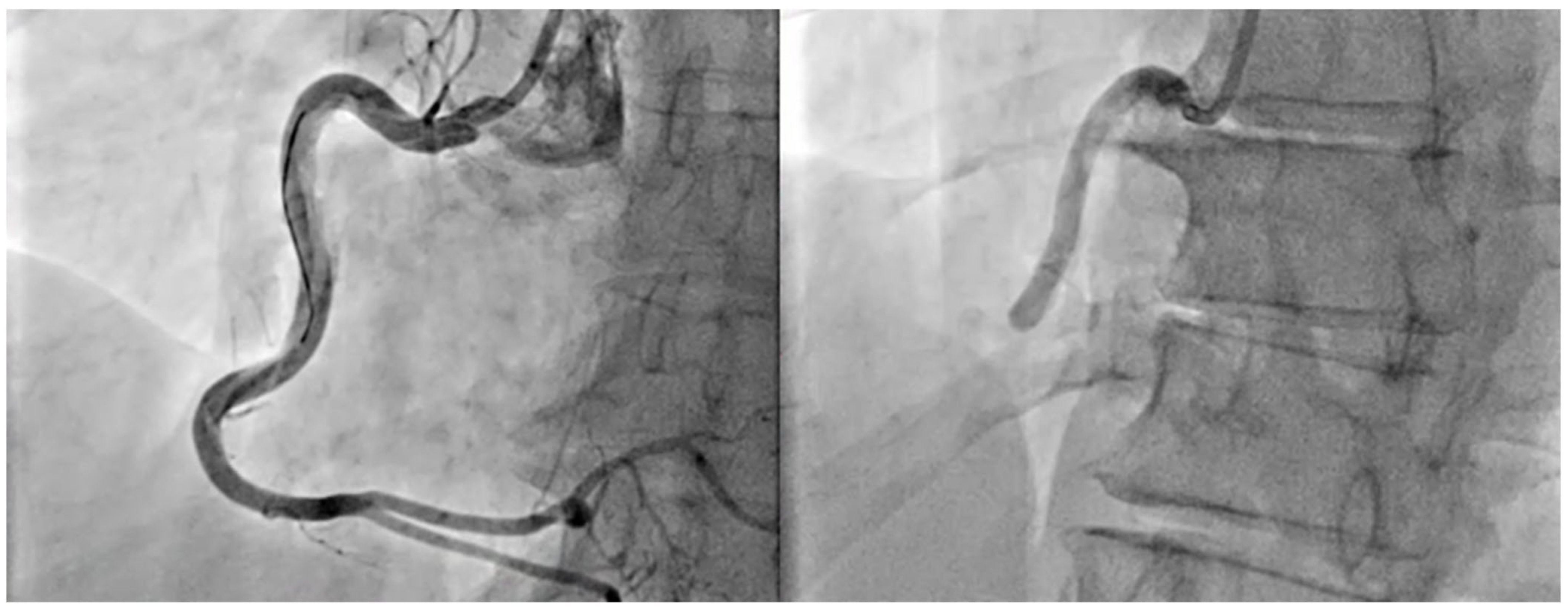
| Aspect | Acute Coronary Syndrome (ACS) | Stroke | Shared Considerations |
|---|---|---|---|
| Primary pathophysiology | Atherosclerotic plaque rupture leading to thrombotic occlusion of coronary arteries | Ischemic stroke: Thrombosis or embolism; Hemorrhagic stroke: Rupture of intracranial vessels | Atherosclerosis, inflammation, endothelial dysfunction, thrombosis |
| Common risk factors | Hypertension, diabetes, smoking, dyslipidemia, atrial fibrillation (AF) | Hypertension, diabetes, smoking, AF, carotid atherosclerosis | Shared cardiovascular risk factors require integrated prevention strategies |
| Acute management | - Primary PCI for STEMI < 90 min - DAPT (aspirin + P2Y12 inhibitor) - Anticoagulation (UFH/LMWH) in selected cases - Beta-blockers, ACE inhibitors, statins | - IV thrombolysis (tPA) if eligible - Mechanical thrombectomy for large vessel occlusion - Antiplatelet therapy after 24 h (aspirin ± clopidogrel) - Blood pressure control | Timing of antithrombotic therapy must be balanced against bleeding risks |
| Antithrombotic therapy | - DAPT for ACS (aspirin + ticagrelor/prasugrel/clopidogrel) - Triple therapy (DAPT + anticoagulation) in AF with ACS | - Antiplatelets for non-cardioembolic stroke (aspirin, clopidogrel) - Anticoagulation for cardioembolic stroke (DOAC/warfarin) | Stroke prevention in AF patients with prior ACS requires tailored therapy to balance ischemic vs. bleeding risk |
| Procedural risks | Stroke risk from PCI/CABG due to embolization | Hemorrhagic transformation risk with reperfusion therapies (tPA, thrombectomy) | Individualized risk assessment for interventions in patients with dual pathology |
| Long-term prevention | - Lifestyle modification - Statins (high-intensity for ASCVD)/Ezetimibe/Bempedoic Acid/PCSK9 inhibitors - ACE inhibitors/ARBs for blood pressure control - Smoking cessation | - Antiplatelets (aspirin ± clopidogrel) - Blood pressure control (target depends on stroke type) - Lipid management with statins/Ezetimibe/Bempedoic Acid/PCSK9 inhibitors | Comprehensive cardiovascular prevention strategies are required for patients with prior ACS or stroke |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carella, M.C.; Carulli, E.; Loizzi, F.; Quarta, S.; Freda, A.; Basile, P.; Amati, F.; Dicorato, M.M.; Latorre, M.D.; Naccarati, M.L.; et al. Intersections and Challenges in the Management of Acute Coronary Syndrome and Stroke: Pathophysiology, Treatment Dilemmas, and Integrated Prevention Strategies. J. Clin. Med. 2025, 14, 2354. https://doi.org/10.3390/jcm14072354
Carella MC, Carulli E, Loizzi F, Quarta S, Freda A, Basile P, Amati F, Dicorato MM, Latorre MD, Naccarati ML, et al. Intersections and Challenges in the Management of Acute Coronary Syndrome and Stroke: Pathophysiology, Treatment Dilemmas, and Integrated Prevention Strategies. Journal of Clinical Medicine. 2025; 14(7):2354. https://doi.org/10.3390/jcm14072354
Chicago/Turabian StyleCarella, Maria Cristina, Eugenio Carulli, Francesco Loizzi, Simona Quarta, Alessandra Freda, Paolo Basile, Fabio Amati, Marco Maria Dicorato, Michele Davide Latorre, Maria Ludovica Naccarati, and et al. 2025. "Intersections and Challenges in the Management of Acute Coronary Syndrome and Stroke: Pathophysiology, Treatment Dilemmas, and Integrated Prevention Strategies" Journal of Clinical Medicine 14, no. 7: 2354. https://doi.org/10.3390/jcm14072354
APA StyleCarella, M. C., Carulli, E., Loizzi, F., Quarta, S., Freda, A., Basile, P., Amati, F., Dicorato, M. M., Latorre, M. D., Naccarati, M. L., Lenoci, C. D., Cicco, S., Pontone, G., Forleo, C., Guaricci, A. I., Ciccone, M. M., & Santobuono, V. E. (2025). Intersections and Challenges in the Management of Acute Coronary Syndrome and Stroke: Pathophysiology, Treatment Dilemmas, and Integrated Prevention Strategies. Journal of Clinical Medicine, 14(7), 2354. https://doi.org/10.3390/jcm14072354










