What Are SAVR Indications in the TAVI Era?
Abstract
1. Introduction
2. Complex Anatomy Unsuitable for Tavi
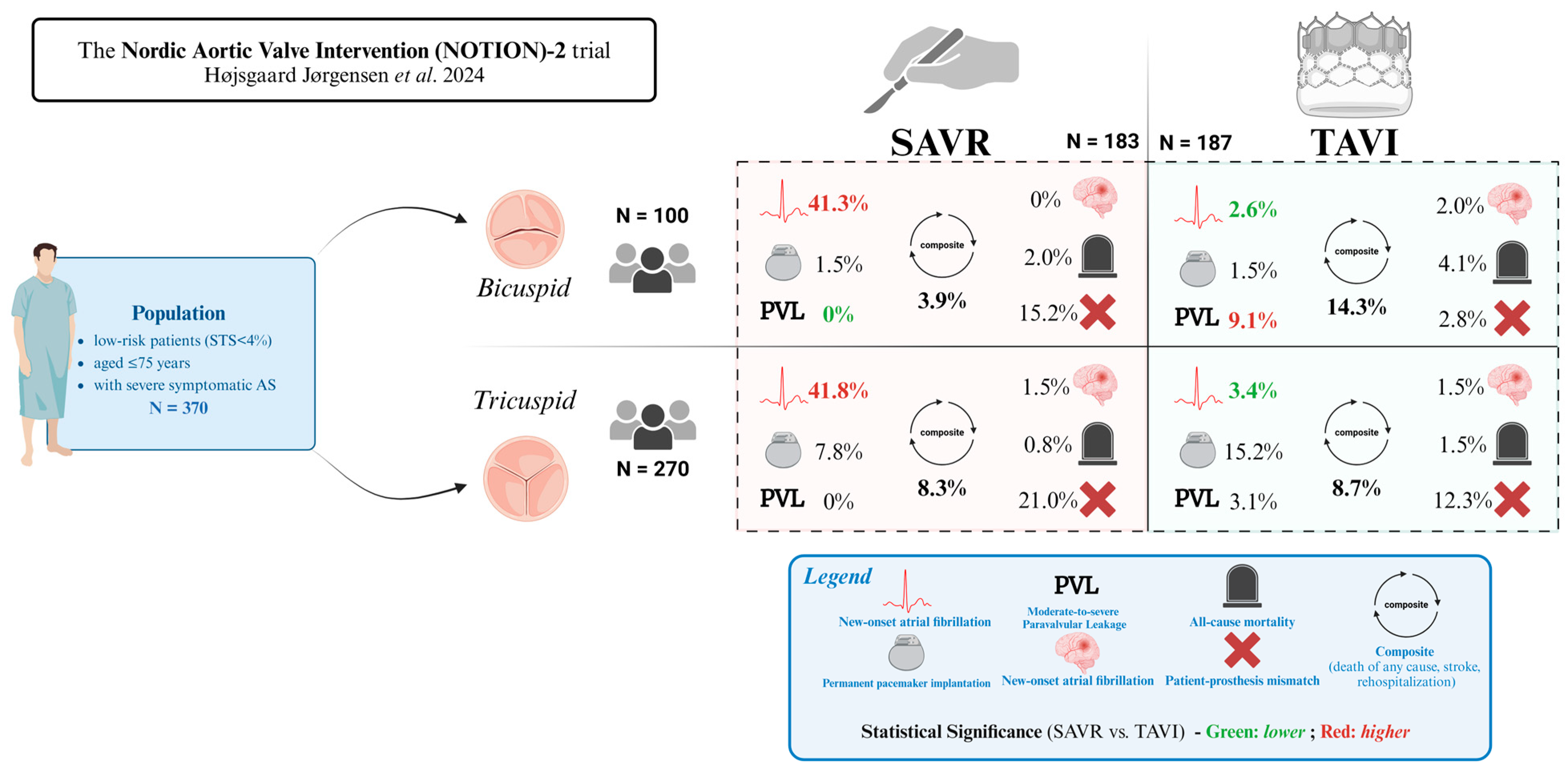
2.1. Bicuspid Aortic Valve
2.2. Aortic Regurgitation
2.3. Annular and Left Ventricular Outflow Tract (LOVT) Calcifications
2.4. Low Take-Off of Coronary Ostia and a Shallow Sinus of Valsalva
2.5. Small Aortic Annulus
3. Concomitant Disease
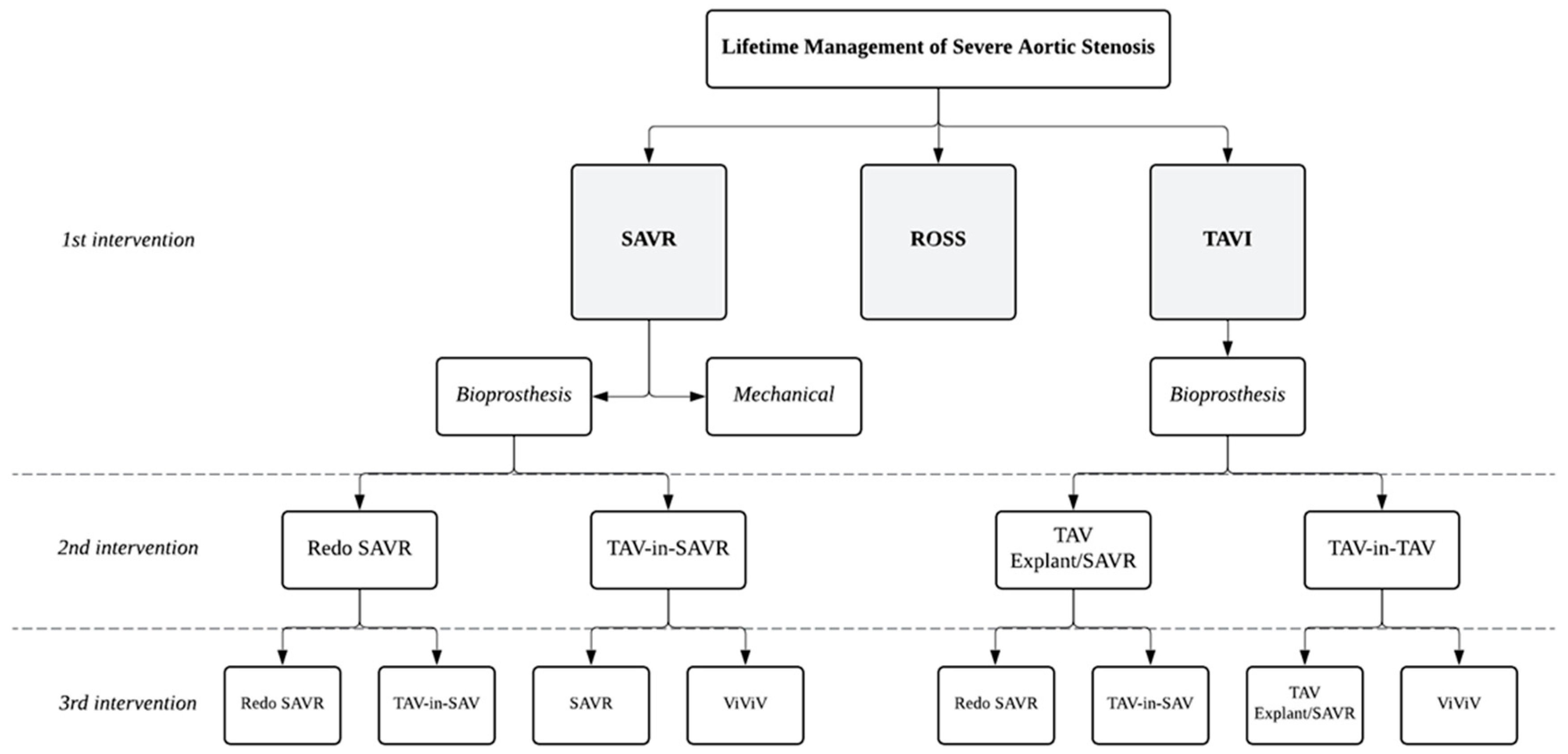
4. Young Patient and Lifetime Management
| NOTION [103] Thyregod et al. 2024 | PARTNER 3 [104] Mack et al. 2023 | Evolut Low Risk [12,105] Forrest et al. 2023 Popma/Reardon et al. 2019 | |
|---|---|---|---|
| Follow-up | 10 years | 5 years | 4 years |
| Number of patients | 145 (TAVI) 135 (SAVR) | 503 (TAVI) 497 (SAVR) | 725 (TAVI) 678 (SAVR) |
| Age | 79.2 years (TAVI) 79.0 years (SAVR) | 73.3 years (TAVI) 73.6 years (SAVR) | 74.1 years (TAVI) 73.6 years (SAVR) |
| Risk stratification | STS score <4%: 83% (TAVI) STS score <4%: 80% (SAVR) | STS score: 1.9% (TAVI) STS score: 1.9% (SAVR) EuroSCORE II: 1.5% (TAVI) EuroSCORE II: 1.5% (SAVR) | STS score: 1.9% (TAVI) STS score: 1.9% (SAVR) |
| Primary Outcome * | Composite All-cause mortality, stroke, or MI 65.5% vs. 65.5% (p = 0.90) | Composite All-cause mortality, stroke, or rehospitalization ** 22.8% vs. 27.2% (p = 0.07) | Composite All-cause mortality or disabling stroke 10.7% vs. 14.1% (p = 0.050) All-cause mortality, disabling stroke, or aortic valve rehospitalization 18.0% vs. 22.4% (p = 0.04) |
| All-Cause Mortality * | 62.7% vs. 64.0% (p = 0.80) | 10.0% vs. 8.2% (p = 0.35) | 9.0% vs. 12.1% (p = 0.07) |
| Stroke * | 9.7% vs. 16.4% (p = 0.10) | 5.8% vs. 6.4% (p = 0.60) | 2.9% vs. 3.8% (p = 0.32) |
| Valve Durability * | SVD Severe—1.5% vs. 10.0% (p = 0.02) Moderate-or-greater—15.4% vs. 20.8% (p > 0.05) BVF 9.7% vs. 13.8% (p = 0.4) Valve reintervention 4.3% vs. 2.2% (p > 0.05) | BVF 3.3% vs. 3.8% (p > 0.05) Valve reintervention 2.6% vs. 3.0% (p > 0.05) Valve thrombosis 2.5% vs. 0.2% (p < 0.05) | Valve reintervention 1.3% vs. 1.7% at 4 yrs (p = 0.63) |
| Other Outcomes * | Pacemaker 44.7% vs. 14.0% (p < 0.01) PVL Moderate/severe at 5 yrs—8.2% vs. 0% (p < 0.001) Endocarditis 7.2% vs. 7.4% (p = 1.0) | New-onset Afib 13.7% vs. 42.4% (p < 0.05) Major bleeding 10.2% vs. 14.8% (p < 0.05) Pacemaker 6.5% vs. 4.0% at 30 days (p > 0.05) Paravalvular leak Mild-or-greater—20.8% vs. 3.2% (p < 0.001) Moderate/severe—0.9% vs. 0% (p > 0.05) Rehospitalization ** 13.7% vs. 17.4% (p = 0.09) | Permanent pacemaker 24.6% vs. 9.9% (p < 0.001) Paravalvular leak No/trace—84.7% vs. 98.4% (p < 0.05) Moderate-or-greater—0.4% vs. 0.0% (p = 0.50) Valve endocarditis 0.9% vs. 2.2% (p = 0.06) |
5. Infective Endocarditis
6. Tavi Explant
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AS | Aortic Stenosis |
| AR | Aortic Regurgitation |
| MS | Mitral Stenosis |
| TR | Tricuspid Regurgitation |
| SAVR | Surgical Aortic Valve Replacement |
| TAVI | Transcatheter Aortic Valve Implantation |
| BAV | Bicuspid Aortic Valve |
| SAA | Small Aortic Annulus |
| THV | Transcatheter Heart Valve |
| LVOT | Left Ventricular Outflow Tract |
| PVL | Perivalvular Leakage |
| PPI | Permanent Pacemaker Implantation |
| VSRR | Valve-Sparing Aortic Root Replacement |
| AVC | Aortic Valve Calcification |
| CO | Coronary Obstruction |
| STJ | Sinotubular Junction |
| VTC | Virtual Transcatheter-to-Coronary |
| FEA | Finite Element Analysis |
| CEPD | Cerebral Embolic Protection Devices |
| PPM | Patient–Prosthesis Mismatch |
| EOA | Effective Orifice Areas |
| ViV | Valve-in-Valve |
| CAD | Coronary Artery Disease |
| CABG | Coronary Artery Bypass Grafting |
| AF | Atrial Fibrillation |
| NVE | Native Valve Endocarditis |
| PVE | Prosthetic Valve Infective Endocarditis |
| IE | Infective Endocarditis |
| SVD | Structural Valve Deterioration |
| BVD | Bioprosthetic Valve Dysfunction |
| BVF | Bioprosthetic Valve Failure |
| MI | Myocardial Infarction |
| STS | Society of Thoracic Surgeons |
| ESC | European Society of Cardiology |
| EACTS | European Association for Cardio-Thoracic Surgery |
| ACC | American College of Cardiology |
| AHA | American Heart Association |
References
- Mensah, G.A.; Roth, G.A.; Fuster, V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global Burden of Disease Study 2017 Nonrheumatic Valve Disease Collaborators. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Young, M.N.; Inglessis, I. Transcatheter aortic valve replacement: Outcomes, indications, complications, and innovations. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Jneid, H.; Chikwe, J.; Arnold, S.V.; Bonow, R.O.; Bradley, S.M.; Chen, E.P.; Diekemper, R.L.; Fugar, S.; Johnston, D.R.; Kumbhani, D.J.; et al. 2024 ACC/AHA clinical performance and quality measures for adults with valvular and structural heart disease: A report of the american heart association/american college of cardiology joint committee on performance Measures. Circ. Cardiovasc. Qual. Outcomes 2024, 17, e000129. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [PubMed]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Gleason, T.G.; Reardon, M.J.; Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Lee, J.S.; Kleiman, N.S.; Chetcuti, S.; Hermiller, J.B.; Heiser, J.; et al. CoreValve US Pivotal High Risk Trial Clinical Investigators. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2687–2696. [Google Scholar] [PubMed]
- Thyregod, H.G.H.; Ihlemann, N.; Jørgensen, T.H.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Five-Year Clinical and Echocardiographic Outcomes from the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation 2019, 139, 2714–2723. [Google Scholar] [PubMed]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [PubMed]
- Sundt, T.M.; Jneid, H. Guideline update on indications for transcatheter aortic valve implantation based on the 2020 american college of cardiology/american heart association guidelines for management of valvular heart disease. JAMA Cardiol. 2021, 6, 1088–1089. [Google Scholar] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022, 43, 561–632. [Google Scholar] [PubMed]
- Williams, M.R.; Jilaihawi, H.; Makkar, R.; O’Neill, W.W.; Guyton, R.; Malaisrie, S.C.; Brown, D.L.; Blanke, P.; Leipsic, J.A.; Pibarot, P.; et al. The PARTNER 3 Bicuspid Registry for Transcatheter Aortic Valve Replacement in Low-Surgical-Risk Patients. JACC Cardiovasc. Interv. 2022, 15, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Attizzani, G.F.; Dallan, L.A.P.; Forrest, J.K.; Reardon, M.J.; Szeto, W.Y.; Liu, F.; Pelletier, M. Redo-transcatheter aortic valve replacement with the supra-annular, self-expandable Evolut platform: Insights from the Transcatheter valve Therapy Registry. Catheter. Cardiovasc. Interv. 2022, 99, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Ko, J.M. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005, 111, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Leon, M.B.; Makkar, R.R.; Tuzcu, E.M.; Svensson, L.G.; Kodali, S.; Webb, J.G.; Mack, M.J.; Douglas, P.S.; Thourani, V.H.; et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.K.F.; Delgado, V.; Bax, J.J. Bicuspid aortic valve: What to image in patients considered for transcatheter aortic valve replacement? Circ. Cardiovasc. Imaging 2017, 10, e005987. [Google Scholar] [CrossRef] [PubMed]
- Scharfschwerdt, M.; Meyer-Saraei, R.; Schmidtke, C.; Sievers, H.-H. Hemodynamics of the Edwards Sapien XT transcatheter heart valve in noncircular aortic annuli. J. Thorac. Cardiovasc. Surg. 2014, 148, 126–132. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conrotto, F.; D’Ascenzo, F.; Franchin, L.; Bruno, F.; Mamas, M.A.; Toutouzas, K.; Cuisset, T.; Leclercq, F.; Dumonteil, N.; Latib, A.; et al. Transcatheter Aortic Valve Implantation with or Without Predilation: A Meta-Analysis. J. Invasive Cardiol. 2022, 34, E104–E113. [Google Scholar] [CrossRef] [PubMed]
- Toutouzas, K.; Benetos, G.; Voudris, V.; Drakopoulou, M.; Stathogiannis, K.; Latsios, G.; Synetos, A.; Antonopoulos, A.; Kosmas, E.; Iakovou, I.; et al. Pre-Dilatation Versus No Pre-Dilatation for Implantation of a Self-Expanding Valve in All Comers Undergoing TAVR: The DIRECT Trial. JACC Cardiovasc. Interv. 2019, 12, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.A.; David, T.E. Management of the valve and ascending aorta in adults with bicuspid aortic valve disease. Semin. Thorac. Cardiovasc. Surg. 2005, 17, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.-Y.; Ali, W.B.; Feng, Y.; Hayashida, K.; Jilaihawi, H.; Latib, A.; Lee, M.K.-Y.; Leon, M.B.; Makkar, R.R.; Modine, T.; et al. Transcatheter aortic valve implantation in patients with bicuspid valve morphology: A roadmap towards standardization. Nat. Rev. Cardiol. 2023, 20, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Narayan, P. Bicuspid Aortic Valve dilemma: TAVI or SAVR? Insights from the NOTION-2 trial. Indian. J. Thorac. Cardiovasc. Surg. 2024, 40, 645–647. [Google Scholar] [CrossRef] [PubMed Central]
- Jørgensen, T.H.; Thyregod, H.G.H.; Savontaus, M.; Willemen, Y.; Bleie, Ø.; Tang, M.; Niemela, M.; Angerås, O.; Gudmundsdóttir, I.J.; Sartipy, U.; et al. Transcatheter aortic valve implantation in low-risk tricuspid or bicuspid aortic stenosis: The NOTION-2 trial. Eur. Heart. J. 2024, 37, 3804–3814. [Google Scholar] [PubMed]
- Delgado, V.; Peláez, E.D. Severe aortic regurgitation: The limits of TAVI. EuroIntervention 2024, 20, e1051–e1052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yousef, A.; MacDonald, Z.; Simard, T.; Russo, J.J.; Feder, J.; Froeschl, M.V.; Dick, A.; Glover, C.; Burwash, I.G.; Latib, A.; et al. Transcatheter Aortic Valve Implantation (TAVI) for Native Aortic Valve Regurgitation—A Systematic Review. Circ. J. 2018, 82, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Nombela-Franco, L.; Ribeiro, H.B.; Urena, M.; Allende, R.; Amat-Santos, I.; DeLarochellière, R.; Dumont, E.; Doyle, D.; DeLarochellière, H.; Laflamme, J.; et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: A comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J. Am. Coll. Cardiol. 2014, 63, 2643–2658. [Google Scholar] [PubMed]
- Cortés, C.; Amat-Santos, I.J.; Nombela-Franco, L.; Muñoz-Garcia, A.J.; Gutiérrez-Ibanes, E.; De La Torre Hernandez, J.M.; Córdoba-Soriano, J.G.; Jimenez-Quevedo, P.; Hernández-García, J.M.; Gonzalez-Mansilla, A.; et al. Mitral regurgitation after transcatheter aortic valve replacement: Prognosis, imaging predictors, and potential management. JACC Cardiovasc. Interv. 2016, 9, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Okuno, T.; Malebranche, D.; Lanz, J.; Praz, F.; Stortecky, S.; Windecker, S.; Pilgrim, T. Transcatheter aortic valve replacement in patients with multivalvular heart disease. JACC Cardiovasc. Interv. 2020, 13, 1503–1514. [Google Scholar] [PubMed]
- Ribeiro, H.B.; Doyle, D.; Urena, M.; Allende, R.; Amat-Santos, I.; Pasian, S.; Bilodeau, S.; Mohammadi, S.; Paradis, J.-M.; DeLarochellière, R.; et al. Transapical mitral implantation of a balloon-expandable valve in native mitral valve stenosis in a patient with previous transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2014, 7, e137–e139. [Google Scholar] [PubMed]
- Taramasso, M.; Benfari, G.; van der Bijl, P.; Alessandrini, H.; Attinger-Toller, A.; Biasco, L.; Lurz, P.; Braun, D.; Brochet, E.; Connelly, K.A.; et al. Transcatheter versus medical treatment of patients with symptomatic severe tricuspid regurgitation. J. Am. Coll. Cardiol. 2019, 74, 2998–3008. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Beurtheret, S.; Karam, N.; Resseguier, N.; Houel, R.; Modine, T.; Folliguet, T.; Chamandi, C.; Com, O.; Gelisse, R.; Bille, J.; et al. Femoral versus nonfemoral peripheral access for transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2019, 74, 2728–2739. [Google Scholar] [CrossRef] [PubMed]
- Elkasaby, M.H.; Khalefa, B.B.; Yassin, M.N.A.; Alabdallat, Y.J.; Atia, A.; Altobaishat, O.; Omar, I.; Hussein, A. Transcatheter aortic valve implantation versus surgical aortic valve replacement for pure aortic regurgitation: A systematic review and meta-analysis of 33,484 patients. BMC Cardiovasc. Disord. 2024, 24, 65. [Google Scholar] [PubMed Central]
- Takagi, H.; Hari, Y.; Kawai, N.; Ando, T. ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Meta-Analysis and Meta-Regression of Transcatheter Aortic Valve Implantation for Pure Native Aortic Regurgitation. Heart Lung Circ. 2020, 29, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, F.J.; Deutsch, M.-A.; Seiffert, M.; Yoon, S.-H.; Codner, P.; Wickramarachchi, U.; Latib, A.; Petronio, A.S.; Rodés-Cabau, J.; Taramasso, M.; et al. Safety and efficacy of transcatheter aortic valve replacement in the treatment of pure aortic regurgitation in native valves and failing surgical bioprostheses: Results from an international registry study. JACC Cardiovasc. Interv. 2017, 10, 1048–1056. [Google Scholar] [PubMed]
- Yoon, S.-H.; Schmidt, T.; Bleiziffer, S.; Schofer, N.; Fiorina, C.; Munoz-Garcia, A.J.; Yzeiraj, E.; Amat-Santos, I.J.; Tchetche, D.; Jung, C.; et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve Regurgitation. J. Am. Coll. Cardiol. 2017, 70, 2752–2763. [Google Scholar] [PubMed]
- David, T.E.; Feindel, C.M. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J. Thorac. Cardiovasc. Surg. 1992, 103, 617–621. [Google Scholar] [PubMed]
- Sharma, V.J.; Kangarajah, A.; Yang, A.; Kim, M.; Seevayanagam, S.; Matalanis, G. Valve-sparing aortic root replacement: Long-term variables significantly associated with mortality and morbidity. J. Thorac. Cardiovasc. Surg. 2025, 169, e68–e77. [Google Scholar] [CrossRef] [PubMed]
- Sá, M.P.; Jacquemyn, X.; Van den Eynde, J.; Chu, D.; Serna-Gallegos, D.; Coselli, J.S.; Sultan, I. Long-term outcomes of valve-sparing aortic root versus composite aortic valve graft replacement for aortic root aneurysm: Meta-analysis of reconstructed time-to-event data. Am. J. Surg. 2023, 226, 371–378. [Google Scholar] [PubMed]
- Pasic, M.; Unbehaun, A.; Buz, S.; Drews, T.; Hetzer, R. Annular rupture during transcatheter aortic valve replacement: Classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc. Interv. 2015, 8 Pt A, 1–9. [Google Scholar] [CrossRef] [PubMed]
- John, D.; Buellesfeld, L.; Yuecel, S.; Mueller, R.; Latsios, G.; Beucher, H.; Gerckens, U.; Grube, E. Correlation of Device landing zone calcification and acute procedural success in patients undergoing transcatheter aortic valve implantations with the self-expanding CoreValve prosthesis. JACC Cardiovasc. Interv. 2010, 3, 233–243. [Google Scholar] [PubMed]
- Langer, N.B.; Hamid, N.B.; Nazif, T.M.; Khalique, O.K.; Vahl, T.P.; White, J.; Terre, J.; Hastings, R.; Leung, D.; Hahn, R.T.; et al. Injuries to the aorta, aortic annulus, and left ventricle during transcatheter aortic valve replacement: Management and outcomes. Circ. Cardiovasc. Interv. 2017, 10, e004735. [Google Scholar] [PubMed]
- Barbanti, M.; Yang, T.-H.; Rodès Cabau, J.; Tamburino, C.; Wood, D.A.; Jilaihawi, H.; Blanke, P.; Makkar, R.R.; Latib, A.; Colombo, A.; et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013, 128, 244–253. [Google Scholar] [PubMed]
- Girdauskas, E.; Owais, T.; Fey, B.; Kuntze, F.; Lauer, B.; Borger, M.A.; Conradi, L.; Reichenspurner, H.; Kuntze, T. Subannular perforation of left ventricular outflow tract associated with transcatheter valve implantation: Pathophysiological background and clinical implications. Eur. J. Cardiothorac. Surg. 2017, 51, 91–96. [Google Scholar] [PubMed]
- Milhorini Pio, S.; Bax, J.; Delgado, V. How valvular calcification can affect the outcomes of transcatheter aortic valve implantation. Expert. Rev. Med. Devices 2020, 17, 773–784. [Google Scholar] [PubMed]
- Shi, J.; Li, W.; Zhang, T.; Han, C.; Wang, Z.; Pei, X.; Li, X.; Zhao, Z.; Wang, P.; Han, J.; et al. Quantity and location of aortic valve calcification predicts paravalvular leakage after transcatheter aortic valve replacement: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1170979. [Google Scholar] [PubMed Central]
- Gotzmann, M.; Korten, M.; Bojara, W.; Lindstaedt, M.; Rahlmann, P.; Mügge, A.; Ewers, A. Long-term outcome of patients with moderate and severe prosthetic aortic valve regurgitation after transcatheter aortic valve implantation. Am. J. Cardiol. 2012, 110, 1500–1506. [Google Scholar] [PubMed]
- Tastet, L.; Ali, M.; Pibarot, P.; Capoulade, R.; Øvrehus, K.A.; Arsenault, M.; Haujir, A.; Bédard, É.; Diederichsen, A.C.P.; Dahl, J.S.; et al. Grading of aortic valve calcification severity and risk stratification in aortic stenosis. J. Am. Heart Assoc. 2024, 13, e035605. [Google Scholar] [PubMed]
- Pawade, T.; Clavel, M.-A.; Tribouilloy, C.; Dreyfus, J.; Mathieu, T.; Tastet, L.; Renard, C.; Gun, M.; Jenkins, W.S.A.; Macron, L.; et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ. Cardiovasc. Imaging 2018, 11, e007146. [Google Scholar] [PubMed]
- Sá, M.P.; Jacquemyn, X.; Van den Eynde, J.; Tasoudis, P.; Erten, O.; Sicouri, S.; Macedo, F.Y.; Pasala, T.; Kaple, R.; Weymann, A.; et al. Impact of Paravalvular Leak on Outcomes After Transcatheter Aortic Valve Implantation: Meta-Analysis of Kaplan-Meier-derived Individual Patient Data. Struct. Heart. 2023, 7, 100118. [Google Scholar] [PubMed Central]
- Athappan, G.; Patvardhan, E.; Tuzcu, E.M.; Svensson, L.G.; Lemos, P.A.; Fraccaro, C.; Tarantini, G.; Sinning, J.-M.; Nickenig, G.; Capodanno, D.; et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: Meta-analysis and systematic review of literature. J. Am. Coll. Cardiol. 2013, 61, 1585–1595. [Google Scholar] [PubMed]
- Okuno, T.; Tomii, D.; Heg, D.; Lanz, J.; Praz, F.; Stortecky, S.; Reineke, D.; Windecker, S.; Pilgrim, T. Five-year outcomes of mild paravalvular regurgitation after transcatheter aortic valve implantation. EuroIntervention 2022, 18, 33–42. [Google Scholar] [PubMed Central]
- Vlastra, W.; van den Boogert, T.P.W.; Krommenhoek, T.; Bronzwaer, A.-S.G.T.; Mutsaerts, H.J.M.M.; Achterberg, H.C.; Bron, E.E.; Niessen, W.J.; Majoie, C.B.L.M.; Nederveen, A.J.; et al. Aortic valve calcification volumes and chronic brain infarctions in patients undergoing transcatheter aortic valve implantation. Int. J. Cardiovasc. Imaging 2019, 35, 2123–2133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vlastra, W.; Jimenez-Quevedo, P.; Tchétché, D.; Chandrasekhar, J.; de Brito, F.S.; Barbanti, M.; Kornowski, R.; Latib, A.; D’Onofrio, A.; Ribichini, F. Predictors, incidence, and outcomes of patients undergoing transfemoral transcatheter aortic valve implantation complicated by stroke. Circ. Cardiovasc. Interv. 2019, 12, e007546. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.; Hall, K.; Howard, J.P.; Ahmad, Y.; Gandhi, M.; Mahboobani, S.; Okafor, J.; Rahman, H.; Hadjiloizou, N.; Ruparelia, N.; et al. Aortic valve calcium score is associated with acute stroke in transcatheter aortic valve replacement patients. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100349. [Google Scholar] [CrossRef] [PubMed Central]
- Van Nieuwkerk, A.C.; Aarts, H.M.; Hemelrijk, K.I.; Urbano Carrillo, C.; Tchétché, D.; De Brito, F.S.; Barbanti, M.; Kornowski, R.; Latib, A.; D’onofrio, A.; et al. Cerebrovascular Events In Patients Undergoing Transfemoral Transcatheter Aortic Valve Implantation: A Pooled Patient-Level Study. J. Am. Heart Assoc. 2024, 13, E032901. [Google Scholar] [CrossRef] [PubMed Central]
- Collet, J.P.; Van Belle, E.; Thiele, H.; Berti, S.; Lhermusier, T.; Manigold, T.; Neumann, F.J.; Gilard, M.; Attias, D.; Beygui, F.; et al. Apixaban vs. standard of care after transcatheter aortic valve implantation: The ATLANTIS trial. Eur. Heart J. 2022, 43, 2783–2797. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, J.; Nijenhuis, V.J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 383, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.D.; Tijssen, J.G.P.; Wöhrle, J.; Søndergaard, L.; Gilard, M.; Möllmann, H.; Makkar, R.R.; Herrmann, H.C.; Giustino, G.; Baldus, S.; et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2019, 382, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Dhaliwal, A.S.; Sohal, S.; Gwon, Y.; Gupta, S.; Bhatia, K.; Dominguez, A.C.; Basman, C.; Tamis-Holland, J. Role of Cerebral Embolic Protection Devices in Patients Undergoing Transcatheter Aortic Valve Replacement: An Updated Meta-Analysis. J. Am. Heart Assoc. 2024, 13, e030587. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akinseye, O.A.; Jha, S.K.; Ibebuogu, U.N. Clinical outcomes of coronary occlusion following transcatheter aortic valve replacement: A systematic review. Cardiovasc. Revasc. Med. 2018, 19, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.B.; Nombela-Franco, L.; Urena, M.; Mok, M.; Pasian, S.; Doyle, D.; DeLarochellière, R.; Côté, M.; Laflamme, L.; DeLarochellière, H.; et al. Coronary obstruction following transcatheter aortic valve implantation: A systematic review. JACC Cardiovasc. Interv. 2013, 6, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Blanke, P.; Weir-McCall, J.R.; Achenbach, S.; Delgado, V.; Hausleiter, J.; Jilaihawi, H.; Marwan, M.; Nørgaard, B.L.; Piazza, N.; Schoenhagen, P.; et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (tavi)/transcatheter aortic valve replacement (TAVR): An expert consensus document of the society of cardiovascular computed tomography. JACC Cardiovasc. Imaging 2019, 12, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Dvir, D.; Leipsic, J.; Blanke, P.; Ribeiro, H.B.; Kornowski, R.; Pichard, A.; Rodés-Cabau, J.; Wood, D.A.; Stub, D.; Ben-Dor, I.; et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation: Preprocedural evaluation, device selection, protection, and treatment. Circ. Cardiovasc. Interv. 2015, 8, e002079. [Google Scholar] [CrossRef] [PubMed]
- Dowling, C.; Gooley, R.; McCormick, L.; Firoozi, S.; Brecker, S.J. Patient-specific Computer Simulation: An Emerging Technology for Guiding the Transcatheter Treatment of Patients with Bicuspid Aortic Valve. Interv. Cardiol. 2021, 16, e26. [Google Scholar] [CrossRef] [PubMed Central]
- Hokken, T.W.; Wienemann, H.; Dargan, J.; Ginkel, D.-J.; van Dowling, C.; Unbehaun, A.; Bosmans, J.; Bader-Wolfe, A.; Gooley, R.; Swaans, M.; et al. Clinical value of CT-derived simulations of transcatheter-aortic-valve-implantation in challenging anatomies the PRECISE-TAVI trial. Catheter. Cardiovasc. Interv. 2023, 102, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Eng, M.H.; Paone, G.; Leshnower, B.G.; Reisman, M.; Satler, L.; Waksman, R.; et al. The BASILICA trial: Prospective multicenter investigation of intentional leaflet laceration to prevent TAVR coronary obstruction. JACC Cardiovasc. Interv. 2019, 12, 1240–1252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, J.M.; Dvir, D.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Aldea, G.; Reisman, M.; Mackensen, G.B.; Eng, M.H.K.; Paone, G.; et al. Transcatheter Laceration of Aortic Leaflets to Prevent Coronary Obstruction During Transcatheter Aortic Valve Replacement: Concept to First-in-Human. JACC Cardiovasc. Interv. 2018, 11, 677–689. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shemin, R.J. Percutaneous valve intervention: A surgeon’s perspective. Circulation 2006, 113, 774–775. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hayek, A.; Prieur, C.; Dürrleman, N.; Chatelain, Q.; Ibrahim, R.; Asgar, A.; Modine, T.; Ben Ali, W. Clinical considerations and challenges in TAV-in-TAV procedures. Front. Cardiovasc. Med. 2024, 11, 1334871. [Google Scholar] [CrossRef] [PubMed Central]
- Ribeiro, H.B.; Rodés-Cabau, J.; Blanke, P.; Leipsic, J.; Kwan Park, J.; Bapat, V.; Makkar, R.; Simonato, M.; Barbanti, M.; Schofer, J.; et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: Insights from the VIVID registry. Eur. Heart J. 2018, 39, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Mauri, V.; Kim, W.K.; Abumayyaleh, M.; Walther, T.; Moellmann, H.; Schaefer, U.; Conradi, L.; Hengstenberg, C.; Hilker, M.; Wahlers, T.; et al. Short-Term Outcome and Hemodynamic Performance of Next-Generation Self-Expanding Versus Balloon-Expandable Transcatheter Aortic Valves in Patients With Small Aortic Annulus: A Multicenter Propensity-Matched Comparison. Circ. Cardiovasc. Interv. 2017, 10, e005013. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.C.; Abdel-Wahab, M.; Attizzani, G.F.; Batchelor, W.; Bleiziffer, S.; Verdoliva, S.; Chang, Y.; Gada, H.; Gillam, L.; Guerrero, M.; et al. Rationale and design of the SMall Annuli Randomized to Evolut or SAPIEN Trial (SMART Trial). Am. Heart J. 2022, 243, 92–102. [Google Scholar] [PubMed]
- Herrmann, H.C.; Mehran, R.; Blackman, D.J.; Bailey, S.; Möllmann, H.; Abdel-Wahab, M.; Ben Ali, W.; Mahoney, P.D.; Ruge, H.; Wood, D.A.; et al. Self-Expanding or Balloon-Expandable TAVR in Patients with a Small Aortic Annulus. N. Engl. J. Med. 2024, 390, 1959–1971. [Google Scholar] [PubMed]
- Pibarot, P.; Dumesnil, J.G. Prosthesis-patient mismatch: Definition, clinical impact, and prevention. Heart. 2006, 92, 1022–1029. [Google Scholar] [PubMed Central]
- Fallon, J.M.; DeSimone, J.P.; Brennan, J.M.; O’Brien, S.; Thibault, D.P.; DiScipio, A.W.; Pibarot, P.; Jacobs, J.P.; Malenka, D.J. The Incidence and Consequence of Prosthesis-Patient Mismatch After Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 106, 14–22. [Google Scholar] [PubMed]
- Freitas-Ferraz, A.B.; Tirado-Conte, G.; Dagenais, F.; Ruel, M.; Al-Atassi, T.; Dumont, E.; Mohammadi, S.; Bernier, M.; Pibarot, P.; Rodés-Cabau, J. Aortic stenosis and small aortic annulus. Circulation 2019, 139, 2685–2702. [Google Scholar] [PubMed]
- Puri, R.; Byrne, J.; Muller, R.; Baumbach, H.; Eltchaninoff, H.; Redwood, S.; Cheema, A.; Dubois, C.; Ihlberg, L.; Wijeysundera, H.C.; et al. Transcatheter aortic valve implantation in patients with small aortic annuli using a 20 mm balloon-expanding valve. Heart 2017, 103, 148–153. [Google Scholar] [PubMed]
- Rodés-Cabau, J.; Pibarot, P.; Suri, R.M.; Kodali, S.; Thourani, V.H.; Szeto, W.Y.; Svensson, L.G.; Dumont, E.; Xu, K.; Hahn, R.T.; et al. Impact of aortic annulus size on valve hemodynamics and clinical outcomes after transcatheter and surgical aortic valve replacement: Insights from the PARTNER Trial. Circ. Cardiovasc. Interv. 2014, 7, 701–711. [Google Scholar] [PubMed]
- Rodés-Cabau, J.; Ribeiro, H.B.; Mohammadi, S.; Serra, V.; Al-Atassi, T.; Iñiguez, A.; Vilalta, V.; Nombela-Franco, L.; Sáez de Ibarra Sánchez, J.I.; Auffret, V.; et al. VIVA (Transcatheter Aortic Valve Replacement Versus Surgical Aortic Valve Replacement for Treating Elderly Patients with Severe Aortic Stenosis and Small Aortic Annuli) Trial Investigators. Transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis and small aortic annulus: A randomized clinical trial. Circulation 2024, 149, 644–655. [Google Scholar] [PubMed]
- Ayyad, M.; Jabri, A.; Khalefa, B.B.; Al-Abdouh, A.; Madanat, L.; Albandak, M.; Alhuneafat, L.; Sukhon, F.; Shahrori, Z.; Mourid, M.R.; et al. Efficacy and safety of TAVR versus SAVR in patients with small aortic annuli: A systematic review and meta-analysis. Int. J. Cardiol. 2024, 411, 132243. [Google Scholar] [PubMed]
- Coutinho, G.F.; Correia, P.M.; Paupério, G.; de Oliveira, F.; Antunes, M.J. Aortic root enlargement does not increase the surgical risk and short-term patient outcome? Eur. J. Cardiothorac. Surg. 2011, 40, 441–447. [Google Scholar] [PubMed][Green Version]
- Tanaka, D.; Vervoort, D.; Mazine, A.; Elfaki, L.; Chung, J.C.Y.; Friedrich, J.O.; Ouzounian, M. Early and mid-term outcomes of aortic annular enlargement: A systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2024, 13, 187–205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Witberg, G.; Regev, E.; Chen, S.; Assali, A.; Barbash, I.M.; Planer, D.; Vaknin-Assa, H.; Guetta, V.; Vukasinovic, V.; Orvin, K.; et al. The prognostic effects of coronary disease severity and completeness of revascularization on mortality in patients undergoing transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2017, 10, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Sankaramangalam, K.; Banerjee, K.; Kandregula, K.; Mohananey, D.; Parashar, A.; Jones, B.M.; Jobanputra, Y.; Mick, S.; Krishnaswamy, A.; Svensson, L.G.; et al. Impact of Coronary Artery Disease on 30-Day and 1-Year Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: A Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e006092. [Google Scholar] [CrossRef] [PubMed Central]
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; Del Val, D.; Muntané-Carol, G.; Mohammadi, S.; Paradis, J.-M.; Rodés-Cabau, J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Patrick, W.L.; Chen, Z.; Han, J.J.; Smood, B.; Rao, A.; Khurshan, F.; Yarlagadda, S.; Iyengar, A.; Kelly, J.J.; Grimm, J.C.; et al. Patients with Atrial Fibrillation Benefit from SAVR with Surgical Ablation Compared to TAVR Alone. Cardiol. Ther. 2022, 11, 283–296. [Google Scholar] [CrossRef] [PubMed Central]
- Toggweiler, S.; Boone, R.H.; Rodés-Cabau, J.; Humphries, K.H.; Lee, M.; Nombela-Franco, L.; Bagur, R.; Willson, A.B.; Binder, R.K.; Gurvitch, R.; et al. Transcatheter aortic valve replacement: Outcomes of patients with moderate or severe mitral regurgitation. J. Am. Coll. Cardiol. 2012, 59, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Löw, K.; Steffen, J.; Theiss, H.; Orban, M.; Rizas, K.D.; Haum, M.; Doldi, P.M.; Stolz, L.; Gmeiner, J.; Hagl, C.; et al. CTA-determined tricuspid annular dilatation is associated with persistence of tricuspid regurgitation after transcatheter aortic valve replacement. Clin. Res. Cardiol. 2023, 112, 645–655. [Google Scholar] [CrossRef] [PubMed Central]
- Bäz, L.; Möbius-Winkler, S.; Diab, M.; Kräplin, T.; Westphal, J.G.; Ibrahim, K.; Schulze, P.C.; Franz, M. Prognostic relevance of mitral and tricuspid regurgitation after transcatheter aortic valve implantation: Impact of follow-up time point for decision-making. Front. Cardiovasc. Med. 2023, 10, 990373. [Google Scholar] [CrossRef] [PubMed Central]
- Nickenig, G.; Weber, M.; Lurz, P.; von Bardeleben, R.S.; Sitges, M.; Sorajja, P.; Hausleiter, J.; Denti, P.; Trochu, J.-N.; Näbauer, M.; et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet 2019, 394, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Zaid, S.; Kamioka, N.; Terre, J.; Miyasaka, M.; Hirji, S.A.; Hensey, M.; Geloo, N.; Petrossian, G.; Robinson, N.; et al. Mid-Term Outcomes of Transcatheter Aortic Valve Replacement in Extremely Large Annuli with Edwards SAPIEN 3 Valve. JACC Cardiovasc. Interv. 2020, 13, 210–216. [Google Scholar] [CrossRef] [PubMed]
- D’Errigo, P.; Barbanti, M.; Santini, F.; Grossi, C.; Ranucci, M.; Onorati, F.; Covello, R.D.; Rosato, S.; Tamburino, C.; Santoro, G.; et al. Results of the OBSERVANT study: Clinical characteristics and short-term outcome of the enrolled population treated with transcatheter versus surgical aortic valve implantation. G. Ital. Cardiol. 2014, 15, 177–184. [Google Scholar] [PubMed]
- Giordana, F.; Bruno, F.; Conrotto, F.; Saglietto, A.; D’Ascenzo, F.; Grosso Marra, W.; Dvir, D.; Webb, J.; D’Onofrio, A.; Camboni, D.; et al. Incidence, predictors and outcomes of valve-in-valve TAVI: A systematic review and meta-analysis. Int. J. Cardiol. 2020, 316, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.H.; Jørgensen, T.H.; Ihlemann, N.; Steinbrüchel, D.A.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; De Backer, O.; Olsen, P.S.; Søndergaard, L. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur. Heart J. 2024, 45, 1116–1124. [Google Scholar] [CrossRef] [PubMed Central]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; Tchétché, D.; Huang, J.; et al. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J. Am. Coll. Cardiol. 2023, 82, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.R.; Soltesz, E.G.; Vakil, N.; Rajeswaran, J.; Roselli, E.E.; Sabik, J.F.; Smedira, N.G.; Svensson, L.G.; Lytle, B.W.; Blackstone, E.H. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann. Thorac. Surg. 2015, 99, 1239–1247. [Google Scholar] [CrossRef] [PubMed Central]
- Ternacle, J.; Hecht, S.; Eltchaninoff, H.; Salaun, E.; Clavel, M.-A.; Côté, N.; Pibarot, P. Durability of transcatheter aortic valve implantation. EuroIntervention 2024, 20, e845–e864. [Google Scholar] [CrossRef] [PubMed Central]
- Salaun, E.; Mahjoub, H.; Girerd, N.; Dagenais, F.; Voisine, P.; Mohammadi, S.; Yanagawa, B.; Kalavrouziotis, D.; Juni, P.; Verma, S.; et al. Rate, timing, correlates, and outcomes of hemodynamic valve deterioration after bioprosthetic surgical aortic valve replacement. Circulation 2018, 138, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.H.; Thyregod, H.G.H.; Ihlemann, N.; Nissen, H.; Petursson, P.; Kjeldsen, B.J.; Steinbrüchel, D.A.; Olsen, P.S.; Søndergaard, L. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur. Heart J. 2021, 42, 2912–2919. [Google Scholar] [CrossRef] [PubMed Central]
- Fatima, B.; Mohananey, D.; Khan, F.W.; Jobanputra, Y.; Tummala, R.; Banerjee, K.; Krishnaswamy, A.; Mick, S.; Tuzcu, E.M.; Blackstone, E.; et al. Durability data for bioprosthetic surgical aortic valve: A systematic review. JAMA Cardiol. 2019, 4, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Tang, G.H.L.; Sangiorgi, G.; Pedicino, D.; Enriquez-Sarano, M.; Maisano, F.; Taramasso, M. Lifetime Management of Aortic Stenosis: Transcatheter Versus Surgical Treatment for Young and Low-Risk Patients. Circ. Cardiovasc. Interv. 2022, 15, 915–927. [Google Scholar] [PubMed]
- Hammermeister, K.; Sethi, G.K.; Henderson, W.G.; Grover, F.L.; Oprian, C.; Rahimtoola, S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: Final report of the Veterans Affairs randomized trial. J. Am. Coll. Cardiol. 2000, 36, 1152–1158. [Google Scholar] [PubMed]
- Oxenham, H.; Bloomfield, P.; Wheatley, D.J.; Lee, R.J.; Cunningham, J.; Prescott, R.J.; Miller, H.C. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart 2003, 89, 715–721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Glaser, N.; Jackson, V.; Holzmann, M.J.; Franco-Cereceda, A.; Sartipy, U. Aortic valve replacement with mechanical vs. biological prostheses in patients aged 50–69 years. Eur. Heart J. 2016, 37, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Tasoudis, P.T.; Varvoglis, D.N.; Vitkos, E.; Mylonas, K.S.; Sá, M.P.; Ikonomidis, J.S.; Caranasos, T.G.; Athanasiou, T. Mechanical versus bioprosthetic valve for aortic valve replacement: Systematic review and meta-analysis of reconstructed individual participant data. Eur. J. Cardiothorac. Surg. 2022, 62, Ezac268. [Google Scholar] [PubMed]
- Warraich, N.; Sá, M.P.; Jacquemyn, X.; Ahmad, D.; Serna-Gallegos, D.; Sultan, I. Long-Term Outcomes of Mechanical Versus Bioprosthetic Aortic Valve Replacement in Patients Aged Under 50 Years: Meta-Analysis of Reconstructed Time-to-Event Data. Am. J. Cardiol. 2024, 227, 11–17. [Google Scholar] [PubMed]
- Leviner, D.B.; Witberg, G.; Levi, A.; Landes, U.; Schwartz, N.; Shiran, A.; Kornowski, R.; Sharoni, E. Mechanical vs. Bioprosthetic Aortic Valve Replacement in Patients Younger Than 70 Years of Age: A Hazard Ratio Meta-analysis. Can. J. Cardiol. 2022, 38, 355–364. [Google Scholar] [PubMed]
- Vankayalapati, D.K.; Segun-Omosehin, O.; El Ghazal, N.; Suresh Daniel, R.; El Haddad, J.; Mansour, R.; Yap, N.; Miangul, S.; Nakanishi, H.; Than, C.A. Long-Term Outcomes of Mechanical Versus Bioprosthetic Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e52550. [Google Scholar] [PubMed Central]
- Dib, N.; Ben Ali, W.; Ducruet, T.; Trudeau, O.; Bernier, P.-L.; Poirier, N.; Khairy, P. The ross procedure in children and infants: A systematic review with pooled analyses. CJC Pediatr. Congenit. Heart Dis. 2024, 3, 117–124. [Google Scholar] [PubMed Central]
- Sá, M.P.; Van den Eynde, J.; Jacquemin, X.; Tasoudis, P.; Erten, O.; McDonald, C.; Weymann, A.; Ruhparwar, A.; Clavel, M.-A.; Pibarot, P.; et al. Long-Term Outcomes of Ross Procedure versus Mechanical Aortic Valve Replacement: Meta-Analysis of Reconstructed Time-To-Event Data. Trends Cardiovasc. Med. 2024, 34, 29–36. [Google Scholar] [PubMed]
- Yokoyama, Y.; Kuno, T.; Toyoda, N.; Fujisaki, T.; Takagi, H.; Itagaki, S.; Ibrahim, M.; Ouzounian, M.; El-Hamamsy, I.; Fukuhara, S. Ross Procedure Versus Mechanical Versus Bioprosthetic Aortic Valve Replacement: A Network Meta-Analysis. J. Am. Heart Assoc. 2023, 12, e8066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sá, M.P.B.O.; Van den Eynde, J.; Simonato, M.; Cavalcanti, L.R.P.; Doulamis, I.P.; Weixler, V.; Kampaktsis, P.N.; Gallo, M.; Laforgia, P.L.; Zhigalov, K.; et al. Valve-in-Valve Transcatheter Aortic Valve Replacement Versus Redo Surgical Aortic Valve Replacement: An Updated Meta-Analysis. JACC Cardiovasc. Interv. 2021, 14, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Raby, J.; Jewell, P.D.; Brennan, P.F.; Banning, A.P.; Byrne, J.; Kharbanda, R.K.; MacCarthy, P.A.; Thornhill, M.H.; Sandoe, J.A.T.; et al. Risk of infective endocarditis after surgical and transcatheter aortic valve replacement. Heart 2022, 108, 639–647. [Google Scholar] [PubMed]
- Del Val, D.; Panagides, V.; Mestres, C.A.; Miró, J.M.; Rodés-Cabau, J. Infective Endocarditis After Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2023, 81, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Strange, J.E.; Østergaard, L.; Køber, L.; Bundgaard, H.; Iversen, K.; Voldstedlund, M.; Gislason, G.H.; Olesen, J.B.; Fosbøl, E.L. Patient characteristics, microbiology, and mortality of infective endocarditis after transcatheter aortic valve implantation. Clin. Infect. Dis. 2023, 77, 1617–1625. [Google Scholar] [PubMed Central]
- Lanz, J.; Reardon, M.J.; Pilgrim, T.; Stortecky, S.; Deeb, G.M.; Chetcuti, S.; Yakubov, S.J.; Gleason, T.G.; Huang, J.; Windecker, S. Incidence and outcomes of infective endocarditis after transcatheter or surgical aortic valve replacement. J. Am. Heart Assoc. 2021, 10, e020368. [Google Scholar] [PubMed Central]
- Bapat, V.N.; Zaid, S.; Fukuhara, S.; Saha, S.; Vitanova, K.; Kiefer, P.; Squiers, J.J.; Voisine, P.; Pirelli, L.; von Ballmoos, M.W.; et al. EXPLANT-TAVR Investigators. Surgical Explantation After TAVR Failure: Mid-Term Outcomes From the EXPLANT-TAVR International Registry. JACC Cardiovasc. Interv. 2021, 14, 1978–1991. [Google Scholar] [PubMed]
- Fukuhara, S.; Brescia, A.A.; Shiomi, S.; Rosati, C.M.; Yang, B.; Kim, K.M.; Deeb, G.M. Surgical explantation of transcatheter aortic bioprostheses: Results and clinical implications. J. Thorac. Cardiovasc. Surg. 2021, 162, 539–547.e1. [Google Scholar] [CrossRef] [PubMed Central]
- Tang, G.H.L.; Zaid, S.; Kleiman, N.S.; Goel, S.S.; Fukuhara, S.; Marin-Cuartas, M.; Kiefer, P.; Abdel-Wahab, M.; De Backer, O.; Søndergaard, L.; et al. Explant vs. Redo-TAVR After Transcatheter Valve Failure: Mid-Term Outcomes From the EXPLANTORREDO-TAVR International Registry. JACC Cardiovasc. Interv. 2023, 16, 927–941. [Google Scholar] [PubMed]
- Percy, E.D.; Harloff, M.T.; Hirji, S.; McGurk, S.; Yazdchi, F.; Newell, P.; Malarczyk, A.; Sabe, A.; Landes, U.; Webb, J.; et al. Nationally representative repeat transcatheter aortic valve replacement outcomes: Report from the centers for medicare and medicaid services. JACC Cardiovasc. Interv. 2021, 14, 1717–1726. [Google Scholar] [PubMed]
- Dvir, D.; Webb, J.G.; Bleiziffer, S.; Pasic, M.; Waksman, R.; Kodali, S.; Barbanti, M.; Latib, A.; Schaefer, U.; Rodés-Cabau, J.; et al. Valve-in-Valve International Data Registry Investigators. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014, 312, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Tuzcu, E.M.; Kapadia, S.R.; Vemulapalli, S.; Carroll, J.D.; Holmes, D.R.; Mack, M.J.; Thourani, V.H.; Grover, F.L.; Brennan, J.M.; Suri, R.M.; et al. Transcatheter aortic valve replacement of failed surgically implanted bioprostheses: The STS/ACC registry. J. Am. Coll. Cardiol. 2018, 72, 370–382. [Google Scholar] [PubMed]
- Webb, J.G.; Mack, M.J.; White, J.M.; Dvir, D.; Blanke, P.; Herrmann, H.C.; Leipsic, J.; Kodali, S.K.; Makkar, R.; Miller, D.C.; et al. Transcatheter Aortic Valve Implantation Within Degenerated Aortic Surgical Bioprostheses: PARTNER 2 Valve-in-Valve Registry. J. Am. Coll. Cardiol. 2017, 69, 2253–2262. [Google Scholar] [PubMed]
- Deeb, G.M.; Chetcuti, S.J.; Reardon, M.J.; Patel, H.J.; Grossman, P.M.; Schreiber, T.; Forrest, J.K.; Bajwa, T.K.; O’Hair, D.P.; Petrossian, G.; et al. 1-Year Results in Patients Undergoing Transcatheter Aortic Valve Replacement with Failed Surgical Bioprostheses. JACC Cardiovasc. Interv. 2017, 10, 1034–1044. [Google Scholar] [PubMed]
- Mahmoud, A.N.; Gad, M.M.; Elgendy, I.Y.; Mahmoud, A.A.; Taha, Y.; Elgendy, A.Y.; Ahuja, K.R.; Saad, A.M.; Simonato, M.; McCabe, J.M.; et al. Systematic review and meta-analysis of valve-in-valve transcatheter aortic valve replacement in patients with failed bioprosthetic aortic valves. EuroIntervention 2020, 16, 539–548. [Google Scholar] [PubMed]
- Allen, K.B.; Chhatriwalla, A.K.; Cohen, D.J.; Saxon, J.T.; Aggarwal, S.; Hart, A.; Baron, S.; Davis, J.R.; Pak, A.F.; Dvir, D.; et al. Bioprosthetic Valve Fracture to Facilitate Transcatheter Valve-in-Valve Implantation. Ann. Thorac. Surg. 2017, 104, 1501–1508. [Google Scholar] [PubMed]
- Rocha, R.V.; Manlhiot, C.; Feindel, C.M.; Yau, T.M.; Mueller, B.; David, T.E.; Ouzounian, M. Surgical enlargement of the aortic root does not increase the operative risk of aortic valve replacement. Circulation 2018, 137, 1585–1594. [Google Scholar] [PubMed]
- Tang, G.H.L.; Zaid, S.; Gupta, E.; Ahmad, H.; Khan, A.; Kovacic, J.C.; Lansman, S.L.; Dangas, G.D.; Sharma, S.K.; Kini, A. Feasibility of repeat tavr after SAPIEN 3 TAVR: A novel classification scheme and pilot angiographic study. JACC Cardiovasc. Interv. 2019, 12, 1290–1292. [Google Scholar] [PubMed]
- Damlin, A.; Meduri, C.; Manouras, A.; Verouhis, D.; Linder, R.; Rück, A.; Settergren, M. BASILICA Procedure Prior to Valve-in-Valve TAVR in a Supra-Annular TAV Prosthesis. JACC Case Rep. 2023, 11, 101777. [Google Scholar] [PubMed Central]
- Khan, J.M.; Bruce, C.G.; Babaliaros, V.C.; Greenbaum, A.B.; Rogers, T.; Lederman, R.J. TAVR Roulette: Caution Regarding BASILICA Laceration for TAVR-in-TAVR. JACC Cardiovasc. Interv. 2020, 13, 787–789. [Google Scholar] [CrossRef] [PubMed Central]
- Pirelli, L.; Basman, C.L.; Brinster, D.R.; Wang, D.; Patel, N.; Scheinerman, S.J.; Kliger, C.A. Surgical resection of prosthetic valve leaflets under direct vision (SURPLUS) for redo TAVR. JACC Cardiovasc. Interv. 2021, 14, 1036–1037. [Google Scholar] [PubMed]
- Huded, C.P.; Tuzcu, E.M.; Krishnaswamy, A.; Mick, S.L.; Kleiman, N.S.; Svensson, L.G.; Carroll, J.; Thourani, V.H.; Kirtane, A.J.; Manandhar, P.; et al. Association between transcatheter aortic valve replacement and early postprocedural stroke. JAMA 2019, 321, 2306–2315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Macherey, S.; Meertens, M.; Mauri, V.; Frerker, C.; Adam, M.; Baldus, S.; Schmidt, T. Meta-Analysis of Stroke and Mortality Rates in Patients Undergoing Valve-in-Valve Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2021, 10, e019512. [Google Scholar] [PubMed] [PubMed Central]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [PubMed]

| Patient Population | Preferred Treatment Strategy | Key Clinical Trial Evidence | Guideline Recommendations *,‡ |
|---|---|---|---|
| High-Risk or Inoperable Severe AS (STS >8%) | TAVI preferred (transfemoral); SAVR not an option |
| ACC/AHA: TAVI (Class I) ESC/EACTS: TAVI if ≥75 years or STS >8% (Class I) |
| Intermediate-Risk Severe AS (STS 4–8%) | TAVI or SAVR; Heart Team decision based on anatomy and comorbidities |
| ACC/AHA & ESC/EACTS: Shared decision-making by Heart Team |
| Low-Risk Severe AS (STS <4%) | SAVR (<65 years); TAVI for older patients |
| ACC/AHA: <65—SAVR (Class I) 65–80—SAVR/TAVI (Class I) >80—TAVI (Class I)/SAVR (Class II) ESC/EACTS: SAVR (Class I) |
| Bicuspid Aortic Valve (BAV) | SAVR preferred for young/low-risk or with aortopathy; TAVI selectively (older/high-risk) |
| ACC/AHA & ESC/EACTS: SAVR preferred in low-risk BAV or those with aortopathy; TAVI selectively |
| Severe Aortic Regurgitation (AR) | SAVR preferred; TAVI if surgery is prohibitive |
| ACC/AHA & ESC/EACTS: SAVR in symptomatic AR or reduced LVEF (Class I) |
| Small Aortic Annulus (SAA) | TAVI (supra-annular valves) or SAVR with annular enlargement |
| ACC/AHA: consider surgical annulus-enlarging procedures. ESC/EACTS: consider aortic valve-sparing root replacement for younger patients with an enlarged aortic root and normal cusp motion |
| Young Patients with AS (≤65 years) | SAVR preferred (mechanical valve or Ross procedure) |
| ACC/AHA & ESC/EACTS: SAVR preferred in patients <65 or with >20-year life expectancy |
| Concomitant Surgical Needs | SAVR with combined procedures; TAVI only high-risk |
| ACC/AHA & ESC/EACTS: Class I for SAVR in AS with multivessel CAD (CABG required), severe mitral/tricuspid disease, or aortopathy >4.5–5.0 cm. |
| Infective Endocarditis | SAVR preferred |
| ACC/AHA & ESC: Class I for SAVR in infective endocarditis; TAVI not recommended |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davalan, W.; Ben Ali, W.; Mrad, S.; Noly, P.-E. What Are SAVR Indications in the TAVI Era? J. Clin. Med. 2025, 14, 2357. https://doi.org/10.3390/jcm14072357
Davalan W, Ben Ali W, Mrad S, Noly P-E. What Are SAVR Indications in the TAVI Era? Journal of Clinical Medicine. 2025; 14(7):2357. https://doi.org/10.3390/jcm14072357
Chicago/Turabian StyleDavalan, William, Walid Ben Ali, Sebastián Mrad, and Pierre-Emmanuel Noly. 2025. "What Are SAVR Indications in the TAVI Era?" Journal of Clinical Medicine 14, no. 7: 2357. https://doi.org/10.3390/jcm14072357
APA StyleDavalan, W., Ben Ali, W., Mrad, S., & Noly, P.-E. (2025). What Are SAVR Indications in the TAVI Era? Journal of Clinical Medicine, 14(7), 2357. https://doi.org/10.3390/jcm14072357





