Waist-to-Height Ratio, Waist Circumference, and Body Mass Index in Relation to Full Cardiometabolic Risk in an Adult Population from Medellin, Colombia
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Adiposity Markers—Independent Variables
2.3. Cardiometabolic Risk—Dependent Variables
2.4. Sociodemographic and Lifestyle Covariates
2.5. Data Analysis
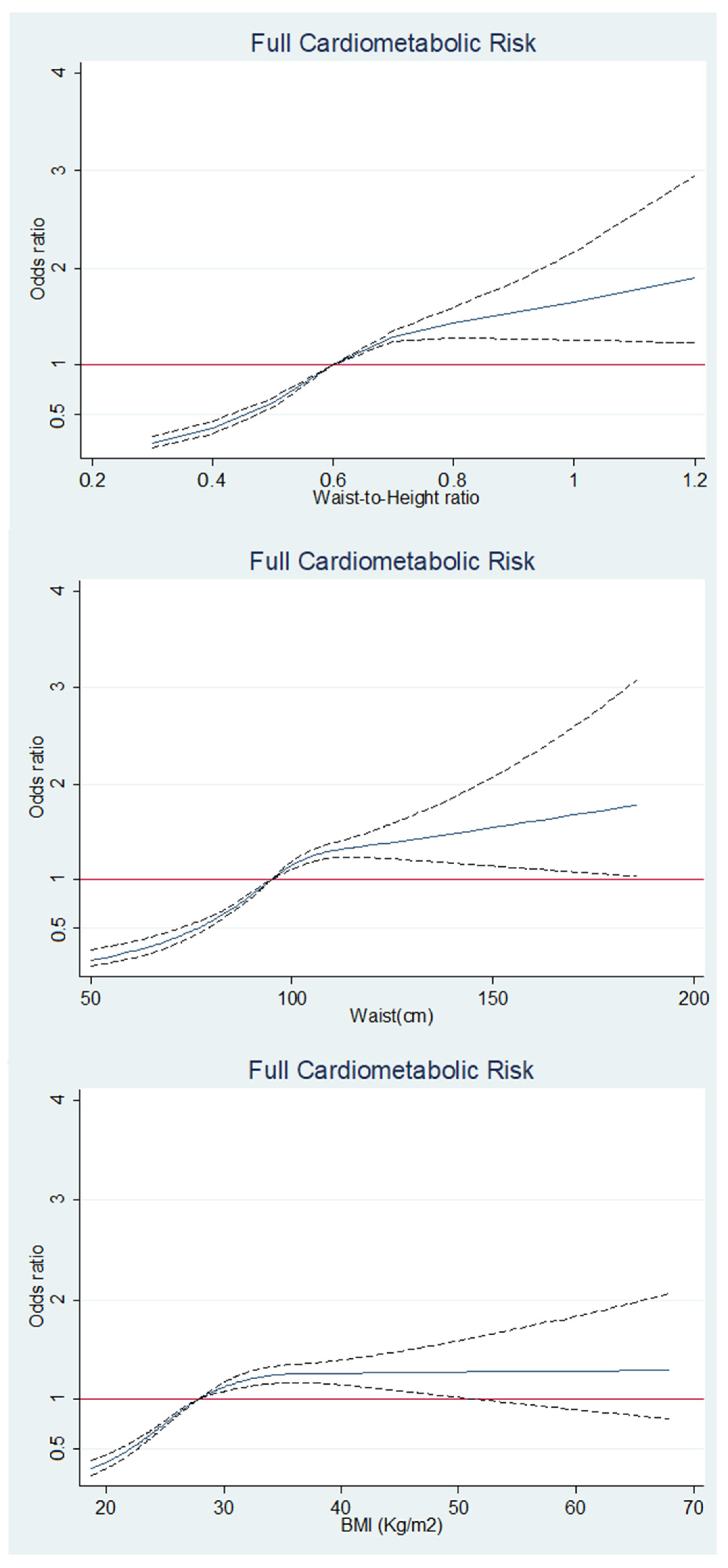
3. Results
4. Discussion
Weakness and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Zhang, L.; Wang, X.; Chen, Z.; Zheng, C.; Chen, L.; Zhou, H.; Cai, J.; Hu, Z.; Tian, Y.; et al. Cardiovascular disease and all-cause mortality associated with individual and combined cardiometabolic risk factors. BMC Public Health 2023, 23, 1725. [Google Scholar]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Qin, Y. Dyslipidemia and Cardiovascular Disease: Current Knowledge, Existing Challenges, and New Opportunities for Management Strategies. J. Clin. Med. 2023, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Nikolaou, M. From Cardiovascular-Kidney-Metabolic Syndrome to Cardiovascular-Renal-Hepatic-Metabolic Syndrome: Proposing an Expanded Framework. Biomolecules 2025, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Obesidad y Sobrepeso [Internet]. Available online: https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 2 September 2024).
- GBD 2019 Risk Factor Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar]
- Zea, M.; Herrán, O. Meal Pattern in the Colombian Population: Results of the National Nutrition Survey. ENSIN, 2015. J. Nutr. Metab. 2022, 2022, 1–12. [Google Scholar]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar]
- Khan, I.; Chong, M.; Le, A.; Mohammadi-Shemirani, P.; Morton, R.; Brinza, C.; Kiflen, M.; Narula, S.; Akhabir, L.; Mao, S.; et al. Surrogate Adiposity Markers and Mortality. JAMA Netw. Open. 2023, 6, e2334836. [Google Scholar] [CrossRef]
- Hsieh, S.D.; Yoshinaga, H.; Muto, T. Waist-to-height ratio, a simple and practical index for assessing central fat distribution and metabolic risk in Japanese men and women. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 610–616. [Google Scholar]
- Ashwell, M.; Hsieh, S.D. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [PubMed]
- Koch, E.; Romero, T.; Manríquez, L.; Taylor, A.; Román, C.; Paredes, M. Razón cintura-estatura: Un mejor predictor antropométrico de riesgo cardiovascular y mortalidad en adultos chilenos: Nomograma diagnóstico utilizado en el Proyecto San Francisco. Rev. Chil. Cardiol. 2008, 27, 23–35. [Google Scholar]
- Corrêa, M.M.; Facchini, L.A.; Thumé, E.; Oliveira, E.R.A.; Tomasi, E. The ability of waist-to-height ratio to identify health risk. Rev. Saude Publica 2019, 53, 66. [Google Scholar] [PubMed]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Willumsen, J.F.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Ngoc, H.; Kriengsinyos, W.; Rojroongwasinkul, N.; Aekplakorn, W. Association of adiposity indices with hypertension in middle-aged and elderly Thai population: National Health Examination Survey 2009 (NHES-IV). J. Cardiovasc. Dev. Dis. 2019, 6, 13. [Google Scholar] [CrossRef]
- Liu, J.; Tse, L.A.; Liu, Z.; Rangarajan, S.; Hu, B.; Yin, L.; Leong, D.P.; Li, W.; PURE (Prospective Urban Rural Epidemiology) study in China. Predictive Values of Anthropometric Measurements for Cardiometabolic Risk Factors and Cardiovascular Diseases Among 44 048 Chinese. J. Am. Heart Assoc. 2019, 8, e010870. [Google Scholar]
- Rodríguez-Guerrero, E.; Romero-Saldaña, M.; Fernández-Carbonell, A.; Molina-Luque, R.; Molina-Recio, G. New Simplified Diagnostic Decision Trees for the Detention of Metabolic Syndrome in the Elderly. Int. J. Environ. Res. Public Health 2020, 17, 5191. [Google Scholar] [CrossRef]
- Alves, L.F.; Cruz, J.O.; da Costa Souza, A.L.; de Oliveira, C.C. Performance of adiposity indicators in predicting metabolic syndrome in older adults. Arch. Endocrinol. Metab. 2021, 65, 588–595. [Google Scholar]
- Marzban, M.; Farhadi, A.; Asadipooya, K.; Jaafari, Z.; Ghazbani, A.; Husseinzadeh, S.; Torkian, S.; Nabipour, I.; Ostovar, A.; Larijani, B.; et al. Evaluation of different anthropometric indices and association with metabolic syndrome in community-dwelling older adults: Bushehr Elderly Health (BEH) program. Obes. Med. 2021, 30, 100387. [Google Scholar]
- Després, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Fried, S.K.; Lee, M.J.; Karastergiou, K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity 2015, 23, 1345–1352. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [PubMed]
- Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000, 106, 171–176. [Google Scholar] [CrossRef]
- Gast, K.B.; Smit, J.W.; Heijer, M.D.; Middeldorp, S.; Rippe, R.C.; le Cessie, S.; de Koning, E.J.; Jukema, J.; Rabelink, T.J.; de Roos, A.; et al. NEO study group. Abdominal adiposity largely explains associations between insulin resistance, hyperglycemia and subclinical atherosclerosis: The NEO study. Atherosclerosis 2013, 229, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Kissebah, A.H.; Krakower, G.R. Regional adiposity and morbidity. Physiol. Rev. 1994, 74, 761–811. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Ashwell, M.; Mayhew, L.; Richardson, J.; Rickayzen, B. Waist-to-height ratio is more predictive of years of life lost than body mass index. PLoS ONE 2014, 9, e103483. [Google Scholar] [CrossRef]
- Lee, C.M.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- Hara, M.; Saitou, E.; Iwata, F.; Okada, T.; Harada, K. Waist-to-height ratio is the best predictor of cardiovascular disease risk factors in Japanese schoolchildren. J. Atheroscler. Thromb. 2002, 9, 127–132. [Google Scholar] [PubMed]
- Savva, S.; Tornaritis, M.; Savva, M.; Kourides, Y.; Panagi, A.; Silikiotou, N.; Georgiou, C.; Kafatos, A. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1453–1458. [Google Scholar] [PubMed]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Chen, I.C.; Chang, Y.C.; Loke, S.S.; Wang, S.H.; Hsiao, K.Y. Waist-to-height ratio, waist circumference, and body mass index as indices of cardiometabolic risk among 36,642 Taiwanese adults. Eur. J. Nutr. 2013, 52, 57–65. [Google Scholar] [CrossRef]
| Age Group (Years) | n (%) |
|---|---|
| 18–39 | 720 (2.4) |
| 40–60 | 7743 (26.5) |
| >60 | 20,723 (71) |
| Sex Female n (%) | 21,410 (73.2) |
| Clinical, anthropometrical, and biochemical variables * | |
| BMI (Kg/mt2) | 27.9 (25–31.5) |
| Waist circumference (WC) cm | 95 (88–102) |
| Waist-to-Height Ratio (W-HtR) | 0.6 (0.6–0.7) |
| Systolic blood pressure mmHg | 125 (120–140) |
| Diastolic blood pressure mmHg | 80 (70–80) |
| Glucose mg/dL | 96 (90–105) |
| Triglycerides mg/dL | 150 (111–206) |
| HDL cholesterol mg/dL | 44 (37–52) |
| LDL cholesterol mg/dL | 110 (86–135) |
| Covariates | |
| Education level n (%) | |
| Illiterate | 4432 (15.1) |
| Elemental | 17,537 (59.9) |
| Secondary | 6850 (23.4) |
| Technical education | 263 (0.9) |
| Undergraduate | 125 (125) |
| Graduate | 29 (0.1) |
| Marital status n (%) | |
| Single | 11,566 (39.5) |
| Divorced | 1381 (4.5) |
| Free union | 3193 (10.9) |
| Married | 10,358 (35.4) |
| Widow/Widower | 2788 (9.5) |
| Ethnicity n (%) | |
| General population | 25,995 (88.9) |
| Afrodescendant | 725 (2.5) |
| Indígenous | 122 (0.4) |
| Palenquero | 96 (0.3) |
| Raizal | 2180 (7.4) |
| ROM | 118 (0.4) |
| Residential area n (%) | |
| Urban | 27,332 (93.5) |
| Rural | 1904 (6.5) |
| Alcohol consumption n (%) | |
| Yes | 1314 (4.5) |
| No | 27,922 (95.5) |
| Smoking n (%) | |
| Yes | 3412 (11.7) |
| No | 25,824 (88.3) |
| Physical activity n (%) | |
| Yes | 8810 (30.1) |
| No | 20,426 (69.9) |
| n | (%) |
|---|---|
| Increased glycemia Component High fasting glucose (≥100 mg/dL) | 11,802 (37.9) |
| Dyslipidemia component | 18,294 (62.6) |
| High triglyceride level (≥150 mg/dL) | 14,493 (49.6) |
| Low HDL-C | 17,679 (60.5) |
| Increased LDL-C (>110 mg/dL) | 14,790 (50.6) |
| Diagnosis of dyslipidemia | 325 (1.2) |
| Increased Blood pressure component | 19,229 (65.7) |
| (SBP ≥ 130 or DBP ≥ 85 mg/dL) | 14,570 (49.9) |
| Diagnosis of hypertension | 9821 (35.6) |
| Number of Cardiometabolic risk components | |
| 0 | 2494 (8.5) |
| 1 | 9700 (33.2) |
| 2 | 12,221 (41.8) |
| 3 | 4821 (16.5) |
| Increased Glycemia Component | Increased Blood Pressure Component | Dyslipidemia Component | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Unadjusted | Adjusted for Model 1 * | Unadjusted | Adjusted for Model 1 * | Unadjusted | Adjusted for Model 1 * | |
| W-HtR | ||||||
| ≤0.5 | ||||||
| >0.5 (increased) | 1.74 (1.53–1.80) | 1.70 (1.51–1.92) | 1.20 (1.08–1.34) | 1.23 (1.10–1.38) | 1.78 (1.60–1.98) | 1.76 (1.58–1.96) |
| p value | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 |
| WC | ||||||
| Normal WC | ||||||
| Increased WC | 1.66 (1.53–1.80) | 1.73 (1.60–1.88) | 1.08 (1.006–1.16) | 1.18 (1.10–1.28) | 1.64 (1.52–1.76) | 1.58 (1.46–1.70) |
| p value | <0.001 | <0.001 | 0.033 | <0.001 | <0.001 | <0.001 |
| BMI | ||||||
| Normal weight | ||||||
| Overweight | 1.34 (1.26–1.42) | 1.39 (1.30–1.48) | 1.08 (1.02–1.15) | 1.13 (1.06–1.20) | 1.46 (1.38–1.55) | 1.43 (1.34–1.52) |
| p value | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 | <0.001 |
| Obesity | 1.73 (1.62–1.84) | 1.90 (1.78–2.03) | 1.18 (1.11–1.26) | 1.32 (1.24–1.41) | 1.47 (1.38–1.57) | 1.37 (1.28–1.46) |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| OR (95%CI) | p Value | OR (95%CI) | p Value | OR (95%CI) | p Value | OR (95%CI) | p Value | |
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for Model 1 * | Adjusted for Model 1 Plus WC | Adjusted for Model 1 Plus BMI | |||||
| W-HtR | ||||||||
| ≤0.5 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| >0.5 | 3.09 (2.49–3.83) | <0.001 | 3.04 (2.45–3.77) | <0.001 | 1.99 (1.59–2.50) | <0.001 | 2.48 (1.99–3.08) | <0.001 |
| Adjusted for model 1 plus W-HtR | Adjusted for model 1 plus BMI | |||||||
| WC | ||||||||
| Normal WC | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| Increased WC | 1.95 (1.73–2.20) | <0.001 | 2.04 (1.80–2.30) | <0.001 | 1.55 (1.36–1.77) | <0.001 | 1.70 (1.50–1.93) | <0.001 |
| Adjusted for model 1 plus WC | Adjusted for model 1 plus W-HtR | |||||||
| BMI | ||||||||
| Normal weight | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| Overweight | 1.57 (1.44–1.71) | <0.001 | 1.61 (1.48–1.76) | <0.001 | 1.42 (1.30–1.56) | <0.001 | 1.46 (1.34–1.60) | <0.001 |
| Obesity | 1.88 (1.72–2.05) | <0.001 | 2.01 (1.83–2.20) | <0.001 | 1.48 (1.32–1.65) | <0.001 | 1.59 (1.42–1.77) | <0.001 |
| Authors, Year, Ref. | Country | Design | n (% Male), Age | Exposure (s); Outcome | Results |
|---|---|---|---|---|---|
| Nguyen Ngoc et al., 2019, [17] | Thailand | Cross-sectionalsurvey | 15,842 (47.4%), 59.3 ± 13.2 years | W-HtR, WC, BMI; hypertension | Regardless of gender, the best method to distinguish performance in predict arterial hypertension was waist-to-height ratio (W-HtR) [AUC: 0.640 (0.631–0.649)] |
| Liu et al., 2019, [18] | China | Prospective cohort study | 4416 (41.2%), >65 years | W-HtR, WC, BMI; dyslipidemia, hypertension, hyperglycemia | Compared with other anthropometric indices, W-HtR had significantly higher areas under the curve (AUCs) for predicting dyslipidemia (AUCs: 0.646, sensitivity: 65%, specificity: 44%), hyperglycemia (AUCs: 0.595, sensitivity: 60%, specificity: 45%), and CVDs (AUCs: 0.619, sensitivity: 59%, specificity: 41%) |
| Rodríguez Guerrero et al., 2020, [19] | Spain | Cross-sectional study | 361 (46.8%), 73.2 ± 6.4 years | W-HtR, WC, BMI; metabolic syndrome | The W-HtR and the basal glucose had the best predictive capacity (S = 61.4%, SP = 89.2%, PPV = 81.5, validity index or VI = 77%) |
| Alves et al., 2021, [20] | Brazil | Cross-sectional study | 159 (49.7%), 70.9 ± 7.4 years | W-HtR, WC, BMI; metabolic syndrome | Lipid accumulation product (LAP) and W-HtR resulted in the largest AUC values (>0.80). In both sexes, the best indicators were LAP, WC, and WHtR. |
| Marzban et al., 2022, [21] | Iran | Prospective cohort study | 3000 (48.5%), 67.75 ± 7.1 years | W-HtR, WC, BMI; metabolic syndrome | The highest adjusted RRs for metabolic syndrome were observed for the following indices: W-HtR (RR = 15.24), Fat-to-muscle ratio (RR = 4.341), and Waist-to-hip ratio (RR = 3.14). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montoya Castillo, M.; Martínez Quiroz, W.d.J.; Suarez-Ortegón, M.F.; Higuita-Gutiérrez, L.F. Waist-to-Height Ratio, Waist Circumference, and Body Mass Index in Relation to Full Cardiometabolic Risk in an Adult Population from Medellin, Colombia. J. Clin. Med. 2025, 14, 2411. https://doi.org/10.3390/jcm14072411
Montoya Castillo M, Martínez Quiroz WdJ, Suarez-Ortegón MF, Higuita-Gutiérrez LF. Waist-to-Height Ratio, Waist Circumference, and Body Mass Index in Relation to Full Cardiometabolic Risk in an Adult Population from Medellin, Colombia. Journal of Clinical Medicine. 2025; 14(7):2411. https://doi.org/10.3390/jcm14072411
Chicago/Turabian StyleMontoya Castillo, Mariana, Wilson de Jesús Martínez Quiroz, Milton Fabian Suarez-Ortegón, and Luis Felipe Higuita-Gutiérrez. 2025. "Waist-to-Height Ratio, Waist Circumference, and Body Mass Index in Relation to Full Cardiometabolic Risk in an Adult Population from Medellin, Colombia" Journal of Clinical Medicine 14, no. 7: 2411. https://doi.org/10.3390/jcm14072411
APA StyleMontoya Castillo, M., Martínez Quiroz, W. d. J., Suarez-Ortegón, M. F., & Higuita-Gutiérrez, L. F. (2025). Waist-to-Height Ratio, Waist Circumference, and Body Mass Index in Relation to Full Cardiometabolic Risk in an Adult Population from Medellin, Colombia. Journal of Clinical Medicine, 14(7), 2411. https://doi.org/10.3390/jcm14072411






