Switching to Faricimab in Therapy-Resistant Macular Edema Due to Retinal Vein Occlusion: Initial Real-World Efficacy Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intravitreal Treatment
2.3. Pre-Treatment Evaluations
2.4. Data Management and Statistical Analysis
3. Results
3.1. Baseline Demographics
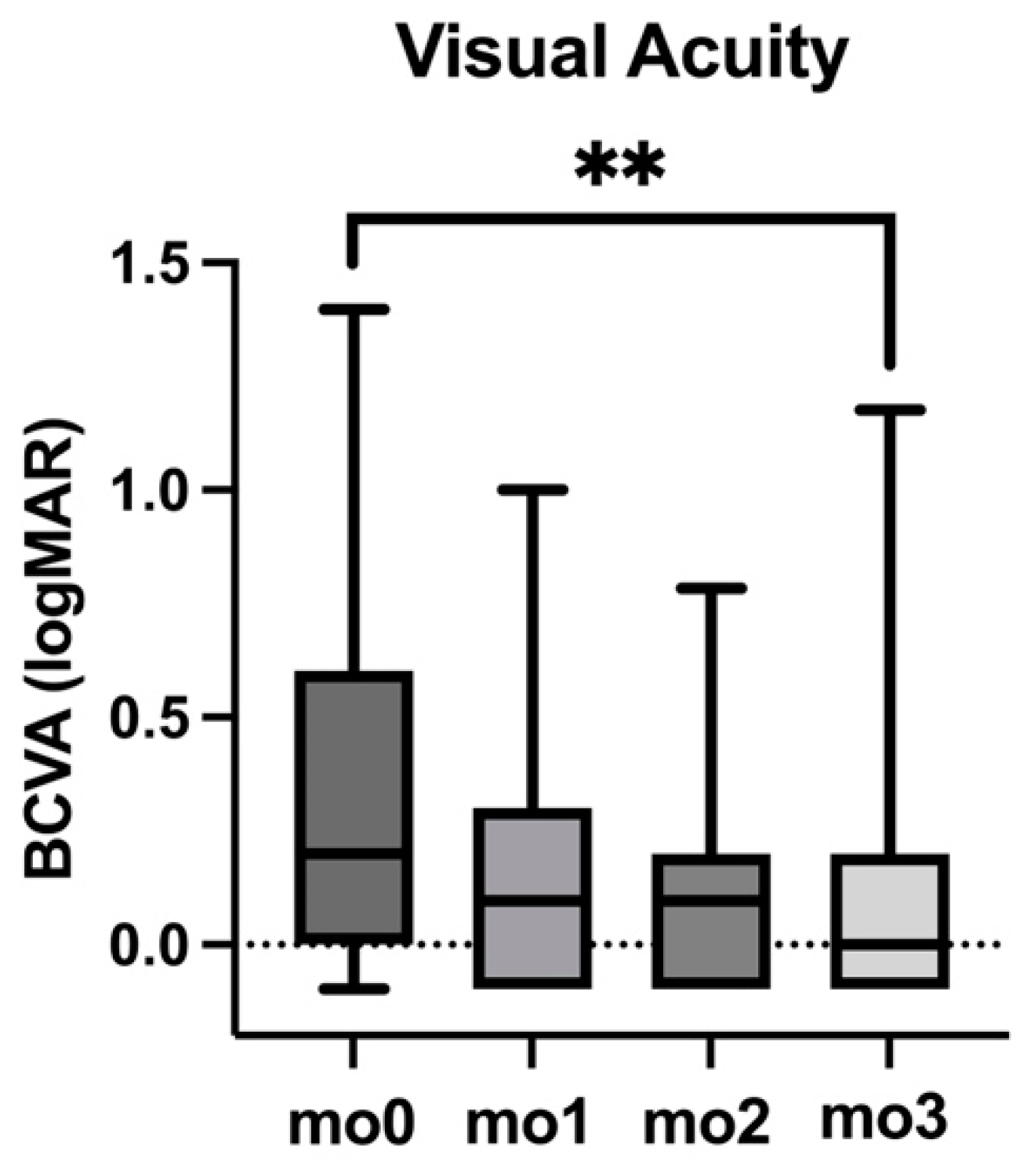
3.2. Visual Acuity (BCVA)
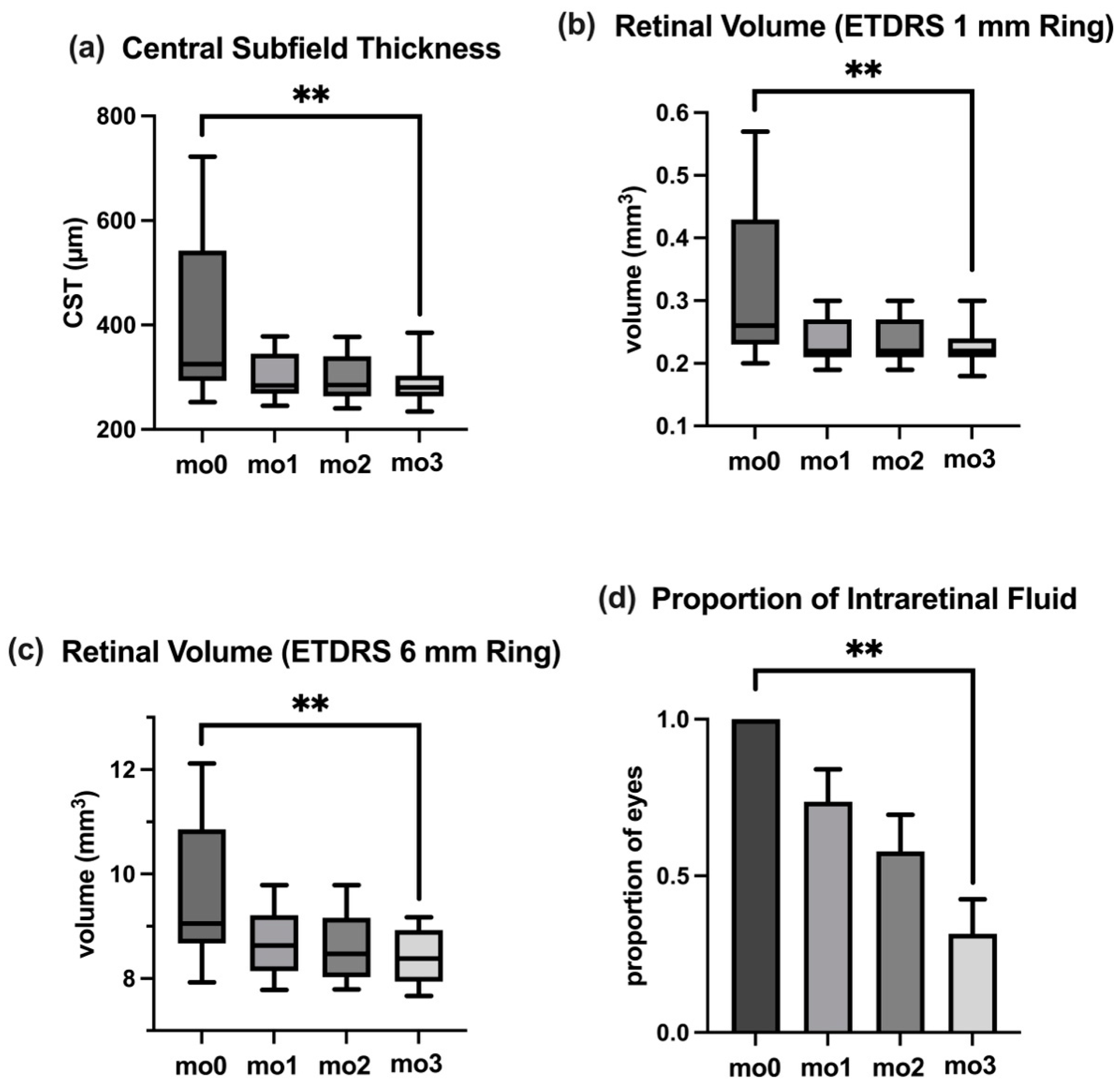
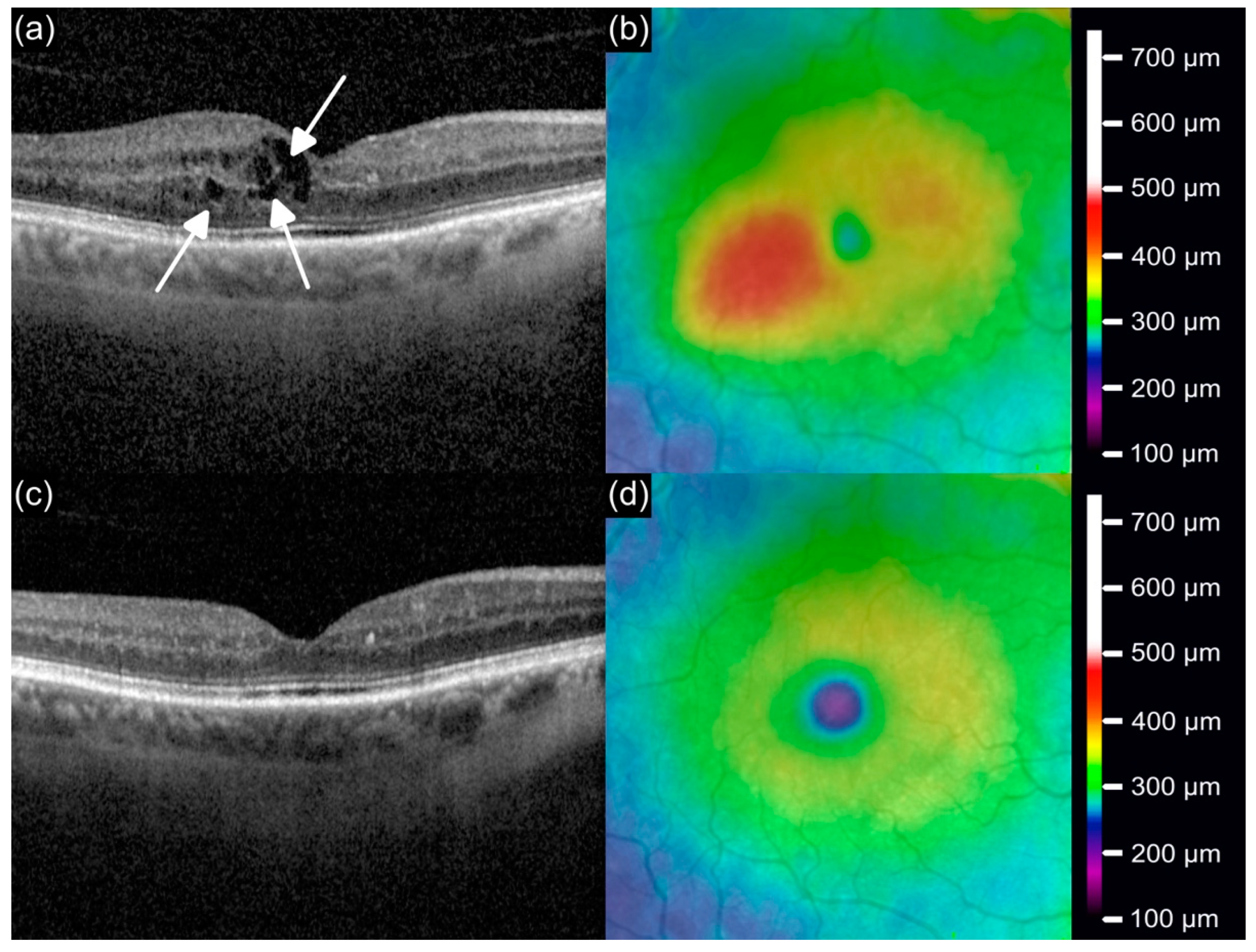
3.3. Central Subfield Thickness
3.4. Retinal Volume
3.5. Intraocular Pressure
3.6. Intraretinal Fluid
3.7. Correlation Analysis Between BCVA and Anatomical Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ME | Macular Edema |
| RVO | Retinal Vein Occlusion |
| anti-VEGF | Anti-vascular endothelial growth factor |
| Ang-2 | Angiopoietin-2 |
| nAMD | Neovascular age-related macular degeneration |
| BCVA | Best-corrected visual acuity |
| CST | Central subfield thickness |
| OCT | Optical coherence tomography |
| IRF | Intraretinal fluid |
| SEM | Standard error of the mean |
| IQR | Interquartile range |
| IOP | Intraocular Pressure |
References
- Wong, T.Y.; Scott, I.U. Clinical practice. Retinal-vein occlusion. N. Engl. J. Med. 2010, 363, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Laouri, M.; Chen, E.; Looman, M.; Gallagher, M. The burden of disease of retinal vein occlusion: Review of the literature. Eye 2011, 25, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Hafiz, G.; Shah, S.M.; Nguyen, Q.D.; Ying, H.; Do, D.V.; Quinlan, E.; Zimmer-Galler, I.; Haller, J.A.; Solomon, S.D.; et al. Ranibizumab for macular edema due to retinal vein occlusions: Implication of VEGF as a critical stimulator. Mol. Ther. 2008, 16, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Gerendas, B.S.; Midena, E.; Sivaprasad, S.; Tadayoni, R.; Wolf, S.; Loewenstein, A. Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2019, 242, 123–162. [Google Scholar] [CrossRef]
- Haller, J.A.; Bandello, F.; Belfort, R., Jr.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.H.; Jiao, J.; Li, X.Y.; et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology 2011, 118, 2453–2460. [Google Scholar] [CrossRef]
- Goncalves, M.B.; Alves, B.Q.; Moura, R.; Magalhaes, O., Jr.; Maia, A.; Belfort, R., Jr.; de Avila, M.P.; Zas, M.; Saravia, M.; Lousas, M.; et al. Intravitreal dexamethasone implant migration into the anterior chamber: A Multicenter Study From the Pan-American Collaborative Retina Study Group. Retina 2020, 40, 825–832. [Google Scholar] [CrossRef]
- Bhisitkul, R.B.; Blotner, S.; Steffen, V.; Haskova, Z. Clinical trial versus real-world outcomes with anti-VEGF therapy for central retinal vein occlusion. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1304. [Google Scholar]
- Jumper, J.M.; Dugel, P.U.; Chen, S.; Blinder, K.J.; Walt, J.G. Anti-VEGF treatment of macular edema associated with retinal vein occlusion: Patterns of use and effectiveness in clinical practice (ECHO study report 2). Clin. Ophthalmol. 2018, 12, 621–629. [Google Scholar] [CrossRef]
- Saharinen, P.; Eklund, L.; Alitalo, K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat. Rev. Drug Discov. 2017, 16, 635–661. [Google Scholar] [CrossRef]
- Regula, J.T.; Lundh von Leithner, P.; Foxton, R.; Barathi, V.A.; Cheung, C.M.; Bo Tun, S.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016, 8, 1265–1288. [Google Scholar] [CrossRef]
- Joussen, A.M.; Ricci, F.; Paris, L.P.; Korn, C.; Quezada-Ruiz, C.; Zarbin, M. Angiopoietin/Tie2 signalling and its role in retinal and choroidal vascular diseases: A review of preclinical data. Eye 2021, 35, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Hattenbach, L.O.; Abreu, F.; Arrisi, P.; Basu, K.; Danzig, C.J.; Guymer, R.; Haskova, Z.; Heier, J.S.; Kotecha, A.; Liu, Y.; et al. BALATON and COMINO: Phase III Randomized Clinical Trials of Faricimab for Retinal Vein Occlusion: Study Design and Rationale. Ophthalmol. Sci. 2023, 3, 100302. [Google Scholar] [CrossRef] [PubMed]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Tadayoni, R.; Paris, L.P.; Danzig, C.J.; Abreu, F.; Khanani, A.M.; Brittain, C.; Lai, T.Y.Y.; Haskova, Z.; Sakamoto, T.; Kotecha, A.; et al. Efficacy and Safety of Faricimab for Macular Edema due to Retinal Vein Occlusion: 24-Week Results from the BALATON and COMINO Trials. Ophthalmology 2024, 131, 950–960. [Google Scholar] [CrossRef]
- Vaz-Pereira, S.; Marques, I.P.; Matias, J.; Mira, F.; Ribeiro, L.; Flores, R. Real-world outcomes of anti-VEGF treatment for retinal vein occlusion in Portugal. Eur. J. Ophthalmol. 2017, 27, 756–761. [Google Scholar]
- Kal, M.; Winiarczyk, M.; Gluszek, S.; Mackiewicz, J. Choroidal thickness in lamellar macular holes. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 653–659. [Google Scholar] [CrossRef]
- Kinyoun, J.; Barton, F.; Fisher, M.; Hubbard, L.; Aiello, L.; Ferris, F., 3rd. Detection of diabetic macular edema. Ophthalmoscopy versus photography--Early Treatment Diabetic Retinopathy Study Report Number 5. The ETDRS Research Group. Ophthalmology 1989, 96, 746–750. [Google Scholar] [CrossRef]
- Rehak, M.; Tilgner, E.; Franke, A.; Rauscher, F.G.; Brosteanu, O.; Wiedemann, P. Early peripheral laser photocoagulation of nonperfused retina improves vision in patients with central retinal vein occlusion (Results of a proof of concept study). Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 745–752. [Google Scholar] [CrossRef]
- Michl, M.; Liu, X.; Kaider, A.; Sadeghipour, A.; Gerendas, B.S.; Schmidt-Erfurth, U. The impact of structural optical coherence tomography changes on visual function in retinal vein occlusion. Acta Ophthalmol. 2021, 99, 418–426. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Hafiz, G.; Mir, T.A.; Scott, A.W.; Solomon, S.; Zimmer-Galler, I.; Sodhi, A.; Duh, E.; Ying, H.; Wenick, A.; et al. Scatter Photocoagulation Does Not Reduce Macular Edema or Treatment Burden in Patients with Retinal Vein Occlusion: The RELATE Trial. Ophthalmology 2015, 122, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H. Mechanisms of vision loss in eyes with macular edema associated with retinal vein occlusion. Jpn. J. Ophthalmol. 2018, 62, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Callizo, J.; Ziemssen, F.; Bertelmann, T.; Feltgen, N.; Vogeler, J.; Koch, M.; Eter, N.; Liakopoulos, S.; Schmitz-Valckenberg, S.; Spital, G. Real-World Data: Ranibizumab Treatment For Retinal Vein Occlusion In The OCEAN Study. Clin. Ophthalmol. 2019, 13, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.U.; VanVeldhuisen, P.C.; Ip, M.S.; Blodi, B.A.; Oden, N.L.; Altaweel, M.; Berinstein, D.M.; Group, S.I. Comparison of Monthly vs Treat-and-Extend Regimens for Individuals With Macular Edema Who Respond Well to Anti-Vascular Endothelial Growth Factor Medications: Secondary Outcomes From the SCORE2 Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 337–345. [Google Scholar] [CrossRef]
- Khodor, A.; Choi, S.; Nanda, T.; Caranfa, J.T.; Ruiz-Lozano, R.E.; Desai, S.H.; Liang, M.; Baumal, C.R.; Reed, D.C.; Cleary, T.S.; et al. Visual and Anatomic Responses in Patients With Neovascular Age-Related Macular Degeneration and a Suboptimal Response to Anti-VEGF Therapy Switched to Faricimab. J. Vitreoretin. Dis. 2024, 8, 643–650. [Google Scholar] [CrossRef]
| Number of patients | 19 |
| Number of eyes | 19 |
| Mean age (years) | 63.0 ± 14.2 |
| Gender | |
| Male | 12 |
| Female | 7 |
| Mean intravitreal injections prior to switch to Faricimab | |
| Total (n) | 35.1 ± 27.2 |
| Total Ranibizumab | 16.8 ± 22.7 |
| Total Aflibercept | 17.2 ± 25.9 |
| Total dexamethasone implants (OzurdexTM) | 1.1 ± 3.3 |
| Mean injections per year prior to switch to Faricimab | 9.6 ± 3.0 |
| Last injection prior to switch to Faricimab | |
| Ranibizumab | 8 |
| Aflibercept | 9 |
| Dexamethasone implant (OzurdexTM) | 2 |
| Retinal laser coagulation | 10 |
| BCVA (logMAR) | IOP (mmHg) | CST (µm) | Volume: ETDRS 1 mm (mm3) | Volume: ETDRS 6 mm (mm3) | IRF (Proportion of Eyes %) | |
|---|---|---|---|---|---|---|
| mo0 | 0.20 | 16 | 325 | 0.26 | 9.05 | 100% |
| mo1 | 0.10 | 16 | 284 | 0.22 | 8.63 | 74% |
| mo2 | 0.10 | 16 | 278 | 0.22 | 8.47 | 58% |
| mo3 | 0.00 | 16 | 280 | 0.22 | 8.38 | 32% |
| IQR (mo0) | 0.60 | 3 | 249 | 0.20 | 2.19 | SEM: 0% |
| IQR (mo1) | 0.40 | 7 | 77 | 0.06 | 1.07 | SEM: 10% |
| IQR (mo2) | 0.30 | 6 | 79 | 0.06 | 1.13 | SEM: 12% |
| IQR (mo3) | 0.30 | 6 | 40 | 0.03 | 0.99 | SEM: 11% |
| p (mo0–mo3) | <0.01 | 0.91 | <0.01 | <0.01 | <0.01 | <0.01 |
| BCVA (logMAR) | CST | Vol (ETDRS 1 mm Ring) | Vol (ETDRS 6 mm Ring) |
|---|---|---|---|
| r | 0.58 | 0.57 | 0.49 |
| p-value (correlation) | <0.01 | <0.01 | 0.02 |
| slope | 0.0019 logMAR/µm | 2.4180 logMAR/mm3 | 0.1616 logMAR/mm3 |
| standard error (slope) | 0.0004 logMAR/µm | 0.5345 logMAR/mm3 | 0.0402 logMAR/mm3 |
| Y-intercept | −0.43 logMAR | −0.43 logMAR | −1.22 logMAR |
| standard error (Y-intercept) | 0.19 logMAR | 0.19 logMAR | 0.40 logMAR |
| R2 | 0.54 | 0.55 | 0.49 |
| p-value (regression) | <0.01 | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafner, M.; Herold, T.R.; Kufner, A.; Asani, B.; Anschütz, A.; Eckardt, F.; Priglinger, S.G.; Schiefelbein, J. Switching to Faricimab in Therapy-Resistant Macular Edema Due to Retinal Vein Occlusion: Initial Real-World Efficacy Outcomes. J. Clin. Med. 2025, 14, 2454. https://doi.org/10.3390/jcm14072454
Hafner M, Herold TR, Kufner A, Asani B, Anschütz A, Eckardt F, Priglinger SG, Schiefelbein J. Switching to Faricimab in Therapy-Resistant Macular Edema Due to Retinal Vein Occlusion: Initial Real-World Efficacy Outcomes. Journal of Clinical Medicine. 2025; 14(7):2454. https://doi.org/10.3390/jcm14072454
Chicago/Turabian StyleHafner, Michael, Tina R. Herold, Alexander Kufner, Ben Asani, Andreas Anschütz, Franziska Eckardt, Siegfried G. Priglinger, and Johannes Schiefelbein. 2025. "Switching to Faricimab in Therapy-Resistant Macular Edema Due to Retinal Vein Occlusion: Initial Real-World Efficacy Outcomes" Journal of Clinical Medicine 14, no. 7: 2454. https://doi.org/10.3390/jcm14072454
APA StyleHafner, M., Herold, T. R., Kufner, A., Asani, B., Anschütz, A., Eckardt, F., Priglinger, S. G., & Schiefelbein, J. (2025). Switching to Faricimab in Therapy-Resistant Macular Edema Due to Retinal Vein Occlusion: Initial Real-World Efficacy Outcomes. Journal of Clinical Medicine, 14(7), 2454. https://doi.org/10.3390/jcm14072454







