Vitamin D and Chronic Rhinosinusitis with Nasal Polyps: A Narrative Review and Perspectives
Abstract
1. Introduction
2. Vitamin D Physiology
- -
- Ergocalciférol (D2)/cholecalciferol (D3);
- -
- Calcidiol, also called 25(OH)D3, after a first hydroxylation by a 25-hydroxylase. There are several enzymes capable of carrying out this chemical reaction but the one with the most affinity for vitamin D is CYP2R1, which is mainly found in the liver [41]. 25-hydroxylase (CYP2R1) is an unregulated enzyme, only dependent on its substrate: cholecalciferol (D3) or ergocalciferol (D2). Its activity could nevertheless decrease with age or obesity [42,43,44];
- -
- -
- The catabolic enzyme, 24-hydroxylase (CYP24A1), is able to transform 25(OH)D3 into 24,25(OH)2D3 and 1,25(OH)2D3 into 1,24,25(OH)3D3, two forms considered inactive.
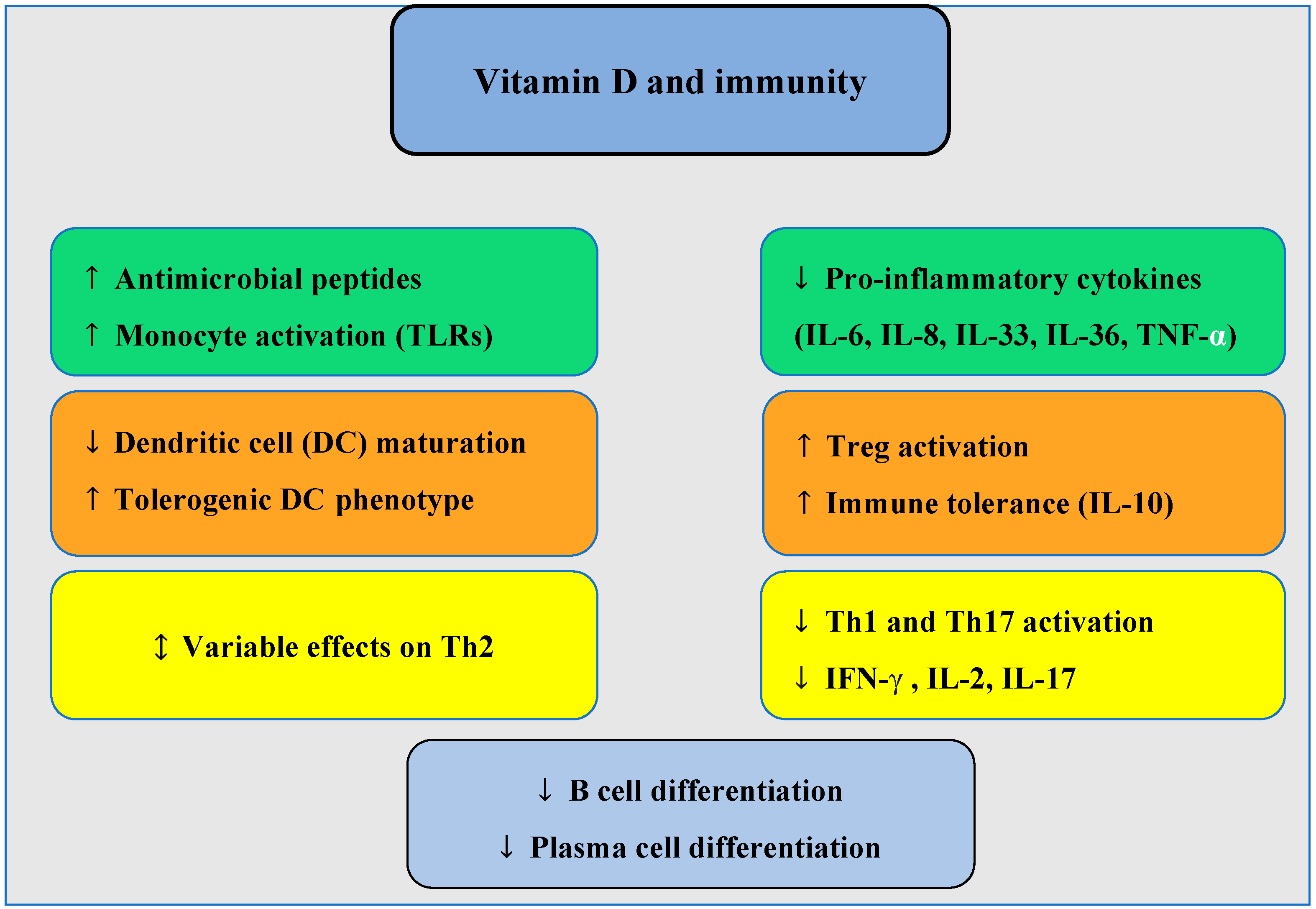
3. Potential Role of Vitamin D in the Pathogenesis of CRSwNP
4. Sinonasal Vitamin D Metabolism in CRSwNP
| Christensen et al. 2017 [77] | Schlosser et al. 2016 [80] | Tomawska et al. 2018 [78] | Xiao et al. 2024 [63] | Mulligan et al. 2014 [79] | |
|---|---|---|---|---|---|
| Number of patients | 32 total (10 CRSwNP, 8 CRSsNP, 13 controls) | 50 total (13 CRSwNP, 13 CRSsNP, 18 controls) | 166 total (55 CRSwNP, 52 CRSsNP, 59 controls) | 212 total (70 eosinophilic CRSwNP, 67 non-eosinophilic CRSwNP, 75 controls); sub-cohorts:
| 37 total (14 CRSwNP, 12 CRSsNP, 11 controls); sub-cohorts:
|
| 1-α-hydroxylase (CYP27B1) | Reduction in gene expression (RNA) but not statistically significant | Significant decrease in protein expression | No significant difference in protein and gene expression | Significant decrease in both RNA and protein levels | Significant decrease in RNA expression |
| Vitamin D receptor (VDR) | No difference in gene expression (RNA) | Not studied | Significantly lower protein levels; no change in gene expression | Significant decrease in both protein and RNA levels | No difference in gene expression |
| 25-hydroxylase (CYP2R1) | No difference in gene expression (RNA) | Not studied | Not studied | Not studied | No difference in gene expression (RNA) |
| 24-hydroxylase (CYP24A1) | Significant increase in RNA expression | Not studied | Not studied | Significant decrease in RNA expression | Not studied |
| Vitamin D levels | Positive significant correlation between serum 25(OH)D and VDR expression but not with CYP2R1, CYP27B1, and CYP24A1 | Serum 25(OH)D3 and sinonasal 1,25(OH)2D3 levels were significantly lower. No serum 1,25(OH)2D3 differences | No 25(OH)D3 serum difference | Serum and sinonasal tissue 25(OH)D3 levels were significantly lower | Serum 25(OH)D3, sinonasal tissue 25(OH)D3, and 1,25(OH)2D3 levels were significantly lower |
| Technique used | RT-PCR | Immunohistochemistry + flow cytometry | Immunohistochemistry with H-score quantification + DNA microarray technique | RT-PCR, Western Blot, immunohistochemistry, ELISA | RT-PCR, ELISA, immunohistochemistry + flow cytometry |
5. Vitamin D and Clinical CRSwNP Studies
6. Serum Vitamin D Levels
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CRSwNP | Chronic rhinosinusitis with nasal polyps |
| CRSsNP | Chronic rhinosinusitis without nasal polyps |
| IL | Interleukin |
| NSAID | Non-steroidal anti-inflammatory drug |
| Th | T helper |
| NARES | Non-allergic rhinitis with eosinophils |
| NARMA | Non-allergic rhinitis with mast cells |
| NARESMA | Non-allergic rhinitis with eosinophils and mast cells |
| IFN | Interferon gamma |
| CYP | Cytochrome P450 |
| TNF-α | Tumor Necrosis Factor-alpha |
| TGF-β1 | Transforming Growth Factor-beta 1 |
| PTH | Parathyroid hormone |
| FGF | Fibroblast Growth Factor |
| TLR | Toll-like receptors |
| VDR | Vitamin D receptor |
| PCR | Polymerase Chain Reaction |
| RNA | Ribonucleic Acid |
| IU | International Unit |
| CT scan | Computed tomography scan |
| SNOT-22 | Sinonasal Outcome Test-22 |
| BMI | Body Mass Index |
References
- Fokkens, W.J.; Lund, V.J.; Mullol, J. European Position Paper on Nasal Polyps. Rhinology 2007, 45 (Suppl. 20), 1–139. [Google Scholar]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2022, 11, 213–739, Erratum in: Int. Forum Allergy Rhinol. 2022, 12, 974. [Google Scholar] [CrossRef]
- Dietz de Loos, D.; Lourijsen, E.S.; Wildeman, M.A.M.; Freling, N.J.M.; Wolvers, M.D.J.; Reitsma, S.; Fokkens, W.J. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J. Allergy Clin. Immunol. 2019, 143, 1207–1214. [Google Scholar] [CrossRef]
- Sedaghat, A.R.; Kuan, E.C.; Scadding, G.K. Epidemiology of Chronic Rhinosinusitis: Prevalence and Risk Factors. J. Allergy Clin. Immunol. Pract. 2022, 10, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Akerlund, A.; Holmberg, K.; Bende, M. Prevalence of nasal polyps in adults: The Skövde population-based study. Ann. Otol. Rhinol. Laryngol. 2003, 112, 625–629. [Google Scholar] [CrossRef]
- Tomassen, P.; Vandeplas, G.; Van Zele, T.; Cardell, L.O.; Arebro, J.; Olze, H.; Förster-Ruhrmann, U.; Kowalski, M.L.; Olszewska-Ziąber, A.; Holtappels, G.; et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J. Allergy Clin. Immunol. 2016, 137, 1449–1456.e4. [Google Scholar] [CrossRef]
- Bachert, C.; Akdis, C.A. Phenotypes and Emerging Endotypes of Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2016, 4, 621–628. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef]
- Divekar, R.; Kita, H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 98–103. [Google Scholar] [CrossRef]
- Zhang, Y.; Gevaert, E.; Lou, H.; Wang, X.; Zhang, L.; Bachert, C.; Zhang, N. Chronic rhinosinusitis in Asia. J. Allergy Clin. Immunol. 2017, 140, 1230–1239. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Bo, M.; Holtappels, G.; Zheng, M.; Lou, H.; Wang, H.; Zhang, L.; Bachert, C. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J. Allergy Clin. Immunol. 2016, 138, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Hulse, K.E.; Stevens, W.W.; Tan, B.K.; Schleimer, R.P. Pathogenesis of nasal polyposis. Clin. Exp. Allergy 2015, 45, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, M.; Iannuzzi, L.; Tafuri, S.; Passalacqua, G.; Quaranta, N. Allergic and non-allergic rhinitis: Relationship with nasal polyposis, asthma and family history. Acta Otorhinolaryngol. Ital. 2014, 34, 36–41. [Google Scholar] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012, 50, 1–12. [Google Scholar] [CrossRef]
- Nasta, M.S.; Chatzinakis, V.A.; Georgalas, C.C. Updates on current evidence for biologics in chronic rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 18–24. [Google Scholar] [CrossRef]
- Kim, C.; Han, J.; Wu, T.; Bachert, C.; Fokkens, W.; Hellings, P.; Hopkins, C.; Lee, S.; Mullol, J.; Lee, J.T. Role of Biologics in Chronic Rhinosinusitis With Nasal Polyposis: State of the Art Review. Otolaryngol. Head Neck Surg. 2021, 164, 57–66. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Viskens, A.S.; Backer, V.; Conti, D.; De Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Bernal-Sprekelsen, M. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef]
- Papadopoulou, A.M.; Bakogiannis, N.; Skrapari, I.; Bakoyiannis, C. Anatomical Variations of the Sinonasal Area and Their Clinical Impact on Sinus Pathology: A Systematic Review. Int. Arch. Otorhinolaryngol. 2022, 26, e491–e498. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I. European Position Paper on Rhinosinusitis and Nasal Polyps. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Eloy, P.; Poirrier, A.L.; De Dorlodot, C.; Van Zele, T.; Watelet, J.B.; Bertrand, B. Actual concepts in rhinosinusitis: A review of clinical presentations, inflammatory pathways, cytokine profiles, remodeling, and management. Curr. Allergy Asthma Rep. 2011, 11, 146–162. [Google Scholar] [CrossRef]
- Ponikau, J.U.; Sherris, D.A.; Kern, E.B.; Homburger, H.A.; Frigas, E.; Gaffey, T.A.; Roberts, G.D. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin. Proc. 1999, 74, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Ponikau, J.U.; Sherris, D.A.; Weaver, A.; Kita, H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: A randomized, placebo-controlled, double-blind pilot trial. J. Allergy Clin. Immunol. 2005, 115, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ponikau, J.U.; Sherris, D.A.; Kita, H.; Kern, E.B. Intranasal antifungal treatment in 51 patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2002, 110, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Ponikau, J.U.; Sherris, D.A.; Kern, E.B.; Gaffey, T.A.; Kephart, G.; Kita, H. Detection of fungal organisms in eosinophilic mucin using a fluorescein-labeled chitin-specific binding protein. Otolaryngol. Head Neck Surg. 2002, 127, 377–383. [Google Scholar] [CrossRef]
- Ebbens, F.A.; Georgalas, C.; Rinia, A.B.; van Drunen, C.M.; Lund, V.J.; Fokkens, W.J. The fungal debate: Where do we stand today? Rhinology 2007, 45, 178–189. [Google Scholar]
- Fokkens, W.J.; van Drunen, C.; Georgalas, C.; Ebbens, F. Role of fungi in pathogenesis of chronic rhinosinusitis: The hypothesis rejected. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 19–23. [Google Scholar] [CrossRef]
- Tyler, M.A.; Lam, K.; Marino, M.J.; Yao, W.C.; Schmale, I.; Citardi, M.J.; Luong, A.U. Revisiting the controversy: The role of fungi in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2021, 11, 1577–1587. [Google Scholar] [CrossRef]
- Zhang, N.; Gevaert, P.; Van Zele, T.; Perez-Novo, C.; Patou, J.; Holtappels, G.; van Cauwenberge, P.; Bachert, C. An update on the impact of Staphylococcus aureus enterotoxins in chronic sinusitis with nasal polyposis. Rhinology 2005, 43, 162–168. [Google Scholar]
- Vickery, T.W.; Ramakrishnan, V.R.; Suh, J.D. The Role of Staphylococcus aureus in Patients with Chronic Sinusitis and Nasal Polyposis. Curr. Allergy Asthma Rep. 2019, 19, 21. [Google Scholar] [CrossRef]
- Chegini, Z.; Didehdar, M.; Khoshbayan, A.; Karami, J.; Yousefimashouf, M.; Shariati, A. The role of Staphylococcus aureus enterotoxin B in chronic rhinosinusitis with nasal polyposis. Cell Commun. Signal. 2022, 20, 29. [Google Scholar] [CrossRef]
- Van Zele, T.; Gevaert, P.; Watelet, J.-B.; Claeys, G.; Holtappels, G.; Claeys, C.; van Cauwenberge, P.; Bachert, C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J. Allergy Clin. Immunol. 2004, 114, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Teufelberger, A.R.; Bröker, B.M.; Krysko, D.V.; Bachert, C.; Krysko, O. Staphylococcus aureus orchestrates type 2 airway diseases. Trends Mol. Med. 2019, 25, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Huvenne, W.; Hellings, P.W.; Bachert, C. Role of staphylococcal superantigens in airway disease. Int. Arch. Allergy Immunol. 2013, 161, 304–314. [Google Scholar] [CrossRef]
- Rossi, R.E.; Monasterolo, G. Prevalence of serum IgE antibodies to the Staphylococcus aureus enterotoxins (SAE, SEB, SEC, SED, TSST-1) in patients with persistent allergic rhinitis. Int. Arch. Allergy Immunol. 2004, 133, 261–266. [Google Scholar] [CrossRef]
- Niederfuhr, A.; Kirsche, H.; Deutschle, T.; Poppert, S.; Riechelmann, H.; Wellinghausen, N. Staphylococcus aureus in nasal lavage and biopsy of patients with chronic rhinosinusitis. Allergy 2008, 63, 1359–1367. [Google Scholar] [CrossRef]
- Bachert, C.; Gevaert, P.; Holtappels, G.; Johansson, S.G.; van Cauwenberg, P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J. Allergy Clin. Immunol. 2001, 107, 607–614. [Google Scholar] [CrossRef]
- Frank, D.N.; Feazel, L.M.; Bessesen, M.T.; Price, C.S.; Janoff, E.N.; Pace, N.R. The human nasal microbiota and Staphylococcus aureus carriage. PLoS ONE 2010, 5, e10598. [Google Scholar] [CrossRef]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Holick, M.F. Biological Effects of Sunlight, Ultraviolet Radiation, Visible Light, Infrared Radiation and Vitamin D for Health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar]
- Schuster, I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim. Biophys. Acta 2011, 1814, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Roizen, J.D.; Casella, A.; Lai, M.; Long, C.; Tara, Z.; Caplan, I.; O’Lear, L.; Levine, M.A. Decreased Serum 25-Hydroxyvitamin D in Aging Male Mice Is Associated With Reduced Hepatic Cyp2r1 Abundance. Endocrinology 2018, 159, 3083–3089. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef] [PubMed]
- Roizen, J.D.; Long, C.; Casella, A.; O’Lear, L.; Caplan, I.; Lai, M.; Sasson, I.; Singh, R.; Makowski, A.J.; Simmons, R.; et al. Obesity Decreases Hepatic 25-Hydroxylase Activity Causing Low Serum 25-Hydroxyvitamin D. J. Bone Miner. Res. 2019, 34, 1068–1073. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Rosen, C.J. Clinical practice. Vitamin D insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: Case report and review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef]
- Menéndez, S.G.; Manucha, W. Vitamin D as a Modulator of Neuroinflammation: Implications for Brain Health. Curr. Pharm. Des. 2024, 30, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Meng, X. Vitamin D and neurodegenerative diseases. Heliyon 2023, 9, e12877. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81. [Google Scholar] [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Thompson, B.; Waterhouse, M.; English, D.R.; McLeod, D.S.; Armstrong, B.K.; Baxter, C.; Duarte Romero, B.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; et al. Vitamin D supplementation and major cardiovascular events: D-Health randomised controlled trial. BMJ 2023, 381, e075230. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef]
- Ruiter, B.; Patil, S.U.; Shreffler, W.G. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin. Exp. Allergy 2015, 45, 1214–1225. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Kerley, C.P.; Elnazir, B.; Faul, J.; Cormican, L. Vitamin D as an adjunctive therapy in asthma. Part 1: A review of potential mechanisms. Pulm. Pharmacol. Ther. 2015, 32, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, P.E.; Chen, Y.H.; Woszczek, G.; Matthew, N.C.; Chevretton, E.; Gupta, A.; Saglani, S.; Bush, A.; Corrigan, C.; Cousins, D.J.; et al. Vitamin D enhances production of soluble ST2, inhibiting the action of IL-33. J. Allergy Clin. Immunol. 2015, 135, 824–827.e3. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, H.; Song, J.; Qin, Z.Y.; Pan, L.; Liao, B.; Deng, Y.K.; Ma, J.; Liu, J.X.; Hu, J.; et al. Impaired local Vitamin D3 metabolism contributes to IL-36g overproduction in epithelial cells in chronic rhinosinusitis with nasal polyps. Rhinology 2024, 62, 236–249. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Akbar, N.A.; Zacharek, M.A. Vitamin D: Immunomodulation of asthma, allergic rhinitis, and chronic rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 224–228. [Google Scholar] [CrossRef]
- Kanhere, M.; He, J.; Chassaing, B.; Alvarez, J.A.; Ivie, E.A.; Hao, L.; Hanfelt, J.; Gewirtz, A.T.; Tangpricha, V. Bolus Weekly Vitamin D3 Supplementation Impacts Gut and Airway Microbiota in Adults With Cystic Fibrosis: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2018, 103, 564–574. [Google Scholar] [CrossRef]
- Palacios-García, J.; Porras-González, C.; Moreno-Luna, R.; Maza-Solano, J.; Polo-Padillo, J.; Muñoz-Bravo, J.L.; Sánchez-Gómez, S. Role of Fibroblasts in Chronic Inflammatory Signalling in Chronic Rhinosinusitis with Nasal Polyps-A Systematic Review. J. Clin. Med. 2023, 12, 3280. [Google Scholar] [CrossRef]
- Wang, L.F.; Tai, C.F.; Chien, C.Y.; Chiang, F.Y.; Chen, J.Y. Vitamin D decreases the secretion of matrix metalloproteinase-2 and matrix metalloproteinase-9 in fibroblasts derived from Taiwanese patients with chronic rhinosinusitis with nasal polyposis. Kaohsiung J. Med. Sci. 2015, 31, 235–240. [Google Scholar] [CrossRef]
- Wang, L.F.; Chien, C.Y.; Tai, C.F.; Chiang, F.Y.; Chen, J.Y. Vitamin D decreases the secretion of eotaxin and RANTES in nasal polyp fibroblasts derived from Taiwanese patients with chronic rhinosinusitis with nasal polyps. Kaohsiung J. Med. Sci. 2015, 31, 63–69. [Google Scholar] [CrossRef]
- Rostkowska-Nadolska, B.; Sliupkas-Dyrda, E.; Potyka, J.; Kusmierz, D.; Fraczek, M.; Krecicki, T.; Kubik, P.; Zatonski, M.; Latocha, M. Vitamin D derivatives: Calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv. Med. Sci. 2010, 55, 86–92. [Google Scholar] [CrossRef]
- Lee, S.A.; Yang, H.W.; Um, J.Y.; Shin, J.M.; Park, I.H.; Lee, H.M. Vitamin D attenuates myofibroblast differentiation and extracellular matrix accumulation in nasal polyp-derived fibroblasts through smad2/3 signaling pathway. Sci. Rep. 2017, 7, 7299. [Google Scholar] [CrossRef] [PubMed]
- Rostkowska-Nadolska, B.; Fraczek, M.; Gawron, W.; Latocha, M. Influence of vitamin D(3) analogues in combination with budesonid on proliferation of nasal polyp fibroblasts. Acta Biochim. Pol. 2009, 56, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qian, J.; Yu, Z. Budesonide up-regulates vitamin D receptor expression in human bronchial fibroblasts and enhances the inhibitory effect of calcitriol on airway remodeling. Allergol. Immunopathol. 2019, 47, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Sansoni, E.R.; Sautter, N.B.; Mace, J.C.; Smith, T.L.; Yawn, J.R.; Lawrence, L.A.; Schlosser, R.J.; Soler, Z.M.; Mulligan, J.K. Vitamin D3 as a novel regulator of basic fibroblast growth factor in chronic rhinosinusitis with nasal polyposis. Int. Forum Allergy Rhinol. 2015, 5, 191–196. [Google Scholar] [CrossRef]
- Searing, D.A.; Zhang, Y.; Murphy, J.R.; Hauk, P.J.; Goleva, E.; Leung, D.Y. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J. Allergy Clin. Immunol. 2010, 125, 995–1000. [Google Scholar] [CrossRef]
- Xystrakis, E.; Kusumakar, S.; Boswell, S.; Peek, E.; Urry, Z.; Richards, D.F.; Adikibi, T.; Pridgeon, C.; Dallman, M.; Loke, T.K.; et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J. Clin. Investig. 2006, 116, 146–155. [Google Scholar] [CrossRef]
- Christensen, J.M.; Cheng, J.; Earls, P.; Sewell, W.; Sacks, R.; Harvey, R.J. Vitamin D pathway regulatory genes encoding 1α-hydroxylase and 24-hydroxylase are dysregulated in sinonasal tissue during chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2017, 7, 169–176. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Sarnowska, E.; Rusetska, N.; Kowalik, K.; Sierdzinski, J.; Siedlecki, J.A.; Badmaev, V.; Stohs, S.J.; Popko, M. Role of Vitamin D and Its Receptors in the Pathophysiology of Chronic Rhinosinusitis. J. Am. Coll. Nutr. 2019, 38, 108–118. [Google Scholar] [CrossRef]
- Mulligan, J.K.; Nagel, W.; O’Connell, B.P.; Wentzel, J.; Atkinson, C.; Schlosser, R.J. Cigarette smoke exposure is associated with vitamin D3 deficiencies in patients with chronic rhinosinusitis. J. Allergy Clin. Immunol. 2014, 134, 342–349. [Google Scholar] [CrossRef]
- Schlosser, R.J.; Carroll, W.W.; Soler, Z.M.; Pasquini, W.N.; Mulligan, J.K. Reduced sinonasal levels of 1α-hydroxylase are associated with worse quality of life in chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2016, 6, 58–65. [Google Scholar] [CrossRef]
- Mulligan, J.K.; Pasquini, W.N.; Carroll, W.W.; Williamson, T.; Reaves, N.; Patel, K.J.; Mappus, E.; Schlosser, R.J.; Atkinson, C. Dietary vitamin D3 deficiency exacerbates sinonasal inflammation and alters local 25(OH)D3 metabolism. PLoS ONE 2017, 12, e0186374. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, A.R.; Shahidi, N.I.; Pourlak, T.A.; Bayat, L.N. Study of relation between vitamin D serum level and biomarkers of human sinonasal fibroblast proliferation in patient with chronic rhinosinusitis with nasal polyposis. Adv. Biosci. Clin. Med. 2020, 31, 11. [Google Scholar] [CrossRef]
- Schlosser, R.J.; Soler, Z.M.; Schmedes, G.W.; Storck, K.; Mulligan, J.K. Impact of vitamin D deficiency upon clinical presentation in nasal polyposis. Int. Forum Allergy Rhinol. 2014, 4, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, Y.; Chen, H. Vitamin D deficiency are associated with subjective disease severity in Chinese patients with chronic rhinosinusitis with nasal polyps. Am. J. Otolaryngol. 2019, 40, 36–39. [Google Scholar] [CrossRef]
- Wang, L.F.; Lee, C.H.; Chien, C.Y.; Chen, J.Y.; Chiang, F.Y.; Tai, C.F. Serum 25-hydroxyvitamin D levels are lower in chronic rhinosinusitis with nasal polyposis and are correlated with disease severity in Taiwanese patients. Am. J. Rhinol. Allergy. 2013, 27, e162–e165. [Google Scholar] [CrossRef]
- Ali, S.A.; Ali, O.A. Vitamin D deficiency contributes to development of nasal polyps in Iraqi patients suffering from chronic rhinosinusitis. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 349–356. [Google Scholar] [CrossRef]
- Zand, V.; Baradaranfar, M.H.; Vaziribozorg, S.; Mandegari, M.; Mansourimanesh, M.; Saeidieslami, N. Correlation of Serum Vitamin D Levels with Chronic Rhinosinusitis Disease Severity. Iran. J. Otorhinolaryngol. 2019, 32, 35–41. [Google Scholar] [CrossRef]
- Bavi, F.; Movahed, R.; Salehi, M.; Hossaini, S.; Bakhshaee, M. Chronic rhinosinusitis with polyposis and serum vitamin D levels. Acta Otorhinolaryngol. Ital. 2019, 39, 336–340. [Google Scholar] [CrossRef]
- Chandrakar, A.K.; Alexander, A.; Rajendiran, K.; Ramasamy, K. 25-Hydroxyl Vitamin D Deficiency in Nasal Polyposis. Int. Arch. Otorhinolaryngol. 2020, 24, e308–e312. [Google Scholar] [CrossRef]
- Li, B.; Wang, M.; Zhou, L.; Wen, Q.; Zou, J. Association between serum vitamin D and chronic rhinosinusitis: A meta-analysis. Braz. J. Otorhinolaryngol. 2021, 87, 178–187. [Google Scholar] [CrossRef]
- Pantazidou, G.; Papaioannou, I.; Skoulakis, C.; Petinaki, E.; Hajiioannou, J. Vitamin D Levels in Chronic Rhinosinusitis in Patients With or Without Nasal Polyposis: A Systematic Review. Cureus 2023, 15, e46275. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, G.A.; Alzarei, A. The Correlation Between Vitamin D Deficiency and Chronic Rhinosinusitis: A Systematic Review. Cureus 2024, 16, e55955. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, F.; Sadegh, S.; Jahanshahi, J.; Seif Rabiei, M.A.; Hashemian, F. Effects of Vitamin D Supplementation on Recurrence of Nasal Polyposis after Endoscopic Sinus Surgery. Iran. J. Otorhinolaryngol. 2020, 32, 21–28. [Google Scholar] [CrossRef]
- Baruah, B.; Gupta, A.; Kumar, A.; Kumar, A. The role of oral vitamin D3 supplementation in the treatment of Chronic Rhinosinusitis in adults with Vitamin D deficiency. J. Fam. Med. Prim. Care 2020, 9, 2877–2879. [Google Scholar] [CrossRef]
- Kalińczak-Górna, P.; Radajewski, K.; Burduk, P. Relationship between the Severity of Inflammatory Changes in Chronic Sinusitis and the Level of Vitamin D before and after the FESS Procedure. J. Clin. Med. 2021, 10, 2836. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Fotoulaki, M.; Iakovou, I.; Chatziavramidis, A.; Mpalaris, V.; Shobat, K.; Markou, K. Vitamin D3 deficiency and its association with nasal polyposis in patients with cystic fibrosis and patients with chronic rhinosinusitis. Am. J. Rhinol. Allergy 2017, 31, 395–400. [Google Scholar] [CrossRef]
- Abdelaal, A.S.; Elhadidy, S.S.; Ibrahim, M.A. Comparative Study between Vitamin D and Placebo in Patients with Nasal Polyps. Int. J. Med. Arts 2021, 3, 1849–1854. [Google Scholar] [CrossRef]
- G Kazem, N.; Abdallah Ali Elaskarany, A.M.; Abdelazim, H.E.M. Comparative Study of Recurrence of Allergic Nasal Polyposis after Administration of Vitamin D Supplementation. Benha Med. J. 2024, 41, 141–152. [Google Scholar] [CrossRef]
- Ghazavi, H.; Hashemi, S.M.; Jafari, S. The Effect of Vitamin D Supplement on the Relapsing Incidence of Rhinosinusitis with Nasal Polyposis after Fuctional Endoscpoic Sinus Surgery. Adv. Biomed. Res. 2023, 12, 29. [Google Scholar] [CrossRef]
- Faruk, E.M.; Yousef, M.M.; Mohamed, T. Does vitamin D have protective effect on human nasal polyposis: Histological and immunohistochemical study. J. Histol. Histopathol. 2014, 1, 2. [Google Scholar] [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R.Z. Vitamin D: Evidence-Based Health Benefits and Recommendations for Population Guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—Recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Pol. 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Benetti, C.; Piacentini, G.L.; Capristo, C.; Boner, A.L.; Peroni, D.G. Microorganism-induced exacerbations in atopic dermatitis: A possible preventive role for vitamin D? Allergy Asthma Proc. 2015, 36, 19–25. [Google Scholar] [CrossRef]
- Kawada, K.; Sato, C.; Ishida, T.; Nagao, Y.; Yamamoto, T.; Jobu, K.; Hamada, Y.; Izawa Ishizawa, Y.; Ishizawa, K.; Abe, S. Vitamin D Supplementation and Allergic Rhinitis: A Systematic Review and Meta-Analysis. Medicina 2025, 61, 355. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, C.; Mei, J.; Zhu, L.; Kong, H. Vitamin D can safely reduce asthma exacerbations among corticosteroid-using children and adults with asthma: A systematic review and meta-analysis of randomized controlled trials. Nutr. Res. 2021, 92, 49–61. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Wang, C.; Xiao, Y.; An, T.; Zou, M.; Cheng, G. Association between vitamin D status and asthma control: A meta-analysis of randomized trials. Respir. Med. 2019, 150, 85–94. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Griffiths, C.J.; Camargo, C.A., Jr.; Kerley, C.P.; Jensen, M.E.; Mauger, D.; Stelmach, I.; Urashima, M.; et al. Vitamin D supplementation to prevent asthma exacerbations: A systematic review and meta-analysis of individual participant data. Lancet Respir. Med. 2018, 6, e27. [Google Scholar] [CrossRef] [PubMed]
- Aryan, Z.; Rezaei, N.; Camargo, C.A., Jr. Vitamin D status, aeroallergen sensitization, and allergic rhinitis: A systematic review and meta-analysis. Int. Rev. Immunol. 2017, 36, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Bakhshaee, M.; Sharifian, M.; Esmatinia, F.; Rasoulian, B.; Mohebbi, M. Therapeutic effect of vitamin D supplementation on allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2019, 276, 2797–2801. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef]
- Cannell, J.J.; Grant, W.B.; Holick, M.F. Vitamin D and inflammation. Dermatoendocrinol 2015, 6, e983401. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Mitra, A.; Datta-Mitra, A. Immunomodulatory mechanisms of action of calcitriol in psoriasis. Indian J. Dermatol. 2014, 59, 116–122. [Google Scholar] [CrossRef]
- Psomadakis, C.E.; Han, G. New and Emerging Topical Therapies for Psoriasis and Atopic Dermatitis. J. Clin. Aesthetic Dermatol. 2019, 12, 28–34. [Google Scholar]
- Safarov, R.; Fedotova, O.; Uvarova, A.; Gordienko, M.; Menshutina, N. Review of Intranasal Active Pharmaceutical Ingredient Delivery Systems. Pharmaceuticals 2024, 17, 1180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bizzaro, G.; Antico, A.; Fortunato, A.; Bizzaro, N. Vitamin D and Autoimmune Diseases: Is Vitamin D Receptor (VDR) Polymorphism the Culprit? Isr. Med. Assoc. J. 2017, 19, 438–443. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippart, A.; Eloy, P. Vitamin D and Chronic Rhinosinusitis with Nasal Polyps: A Narrative Review and Perspectives. J. Clin. Med. 2025, 14, 2467. https://doi.org/10.3390/jcm14072467
Philippart A, Eloy P. Vitamin D and Chronic Rhinosinusitis with Nasal Polyps: A Narrative Review and Perspectives. Journal of Clinical Medicine. 2025; 14(7):2467. https://doi.org/10.3390/jcm14072467
Chicago/Turabian StylePhilippart, Adrien, and Philippe Eloy. 2025. "Vitamin D and Chronic Rhinosinusitis with Nasal Polyps: A Narrative Review and Perspectives" Journal of Clinical Medicine 14, no. 7: 2467. https://doi.org/10.3390/jcm14072467
APA StylePhilippart, A., & Eloy, P. (2025). Vitamin D and Chronic Rhinosinusitis with Nasal Polyps: A Narrative Review and Perspectives. Journal of Clinical Medicine, 14(7), 2467. https://doi.org/10.3390/jcm14072467







