Systematic Review of the Impact of COVID-19 on Healthcare Systems and Society—The Role of Diagnostics and Nutrition in Pandemic Response
Abstract
1. Introduction
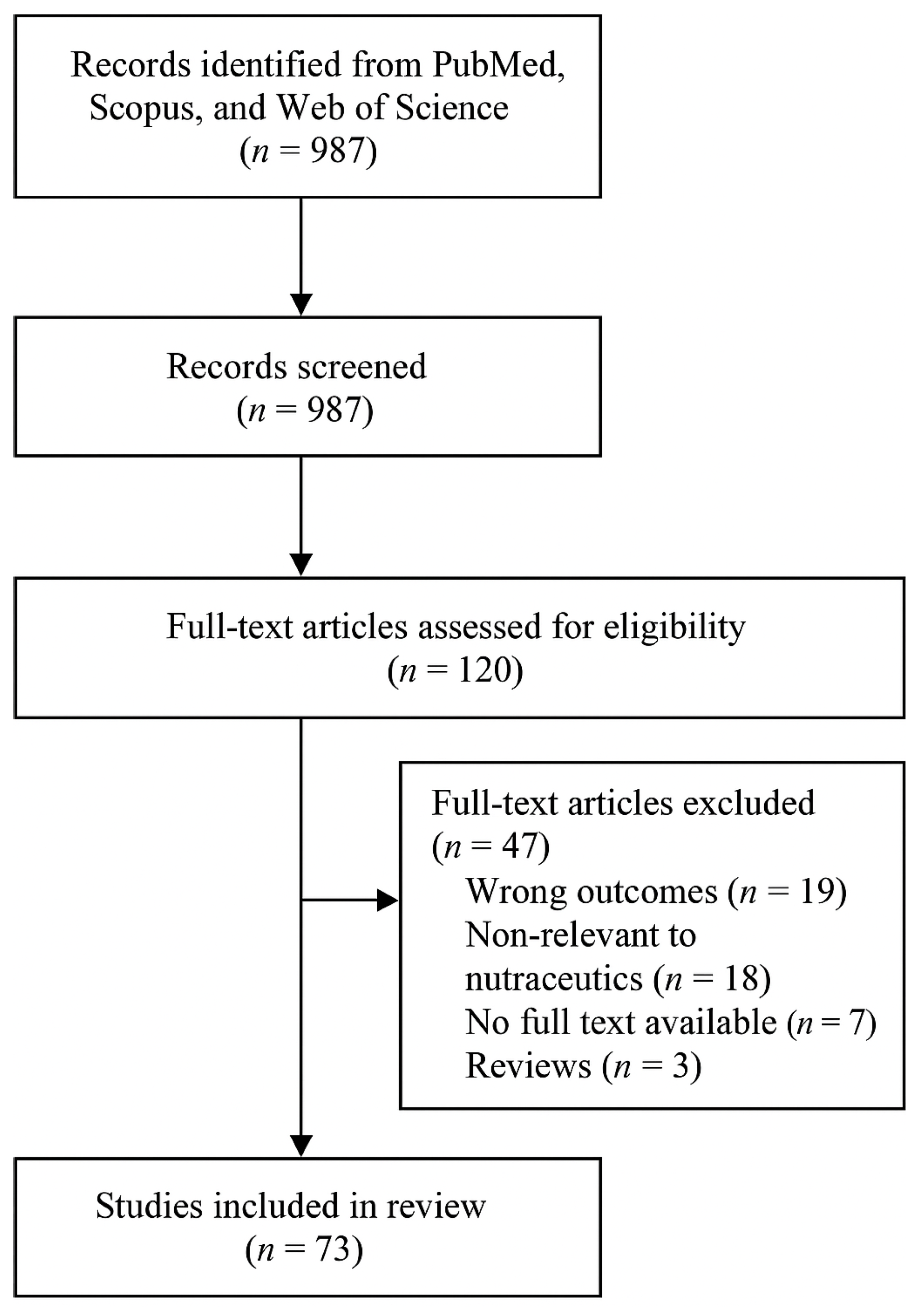
2. Methodology Review
2.1. Literature Review
2.2. Selection Criteria
3. Results
4. Infectivity and Diagnostics of SARS-CoV-2
4.1. Modes of Transmission
- Droplet transmission—the primary mode of human-to-human infection;
- Contact with contaminated surfaces—everyday objects on which the virus has settled, enabling its transfer to the oral and nasal cavities;
- Fecal–oral transmission—via saliva, urine, or feces;
- Ocular transmission—through tears and conjunctival secretions;
- Bloodborne transmission—via direct contact with infected blood [5].
4.2. Types of Diagnostic Tests and Their Effectiveness
- (a)
- High probability of infection based on clinical presentation, epidemiological history, or lung imaging findings (retesting recommended within 24–48 h).
- (b)
- Worsening respiratory symptoms warranting an additional RT-PCR test (within 24–48 h of the first test).
- (c)
- For intubated patients, testing on lower respiratory tract samples may be considered.
- (1)
- ELISA (enzyme-linked immunosorbent assay)—immobilized antigen proteins bind to target antibodies on a microplate surface.
- (2)
- CLIA (chemiluminescent immunoassay)—combines immunochemical reactions with chemiluminescent detection.
- (3)
- LFIA (lateral flow immunoassay)—employs lateral flow technology for rapid antibody detection.
- (1)
- Antigen detection tests—identifying viral protein fragments either on the virus surface or internally, allowing active infection detection within 15 min compared to the several hours required for RT-PCR.
- (2)
- Antibody detection tests—measuring immunoglobulin (IgG and IgM) levels in blood, serum, or plasma to determine whether an individual is actively fighting an infection or has prior exposure to SARS-CoV-2.
- (a)
- ICT (immunochromatographic test)—utilizes colloidal gold-conjugated antibodies to create visible color bands indicating a positive result.
- (b)
- FIA (fluorescent immunoassay)—employs an automated immunofluorescence reader for test interpretation [24].
- (1)
- ACE-2 receptor-based biosensors for detecting virus binding activity.
- (2)
- Gold nanoparticle-based biosensors for enhancing signal detection.
- (3)
- FET (field-effect transistor) biosensors, offering exceptional sensitivity and real-time detection.
- (4)
- ROS (reactive oxygen species)-based biosensors, enabling rapid, cost-effective, and highly sensitive viral detection.
5. Epidemiological Procedures
5.1. National and International Guidelines
- The Act on the Prevention and Control of Infections and Infectious Diseases in Humans [30];
- The Minister of Health’s Regulation of 8 October 2020 regarding organizational standards for healthcare services for patients suspected or confirmed to have SARS-CoV-2 infection [31];
- The Minister of Health’s Regulation of 12 August 2020 on organizational standards for teleconsultation in primary healthcare [31];
- The Minister of Health’s Regulation of 5 March 2021 amending the organizational standards for teleconsultation in primary healthcare [29].
- Facility organization and safety protocols;
- Hygiene and sanitation;
- Cleaning and disinfection procedures;
- Gastronomy-related safety measures;
- Protocols for suspected infections among staff or children.
- Handwashing protocols;
- Child drop-off and pick-up procedures;
- Clothing hygiene standards;
- Sector-Specific Guidelines for the Food Service Industry.
- Employee safety measures;
- Customer safety measures;
- Preventive procedures for suspected infections among employees;
5.2. Organization of Healthcare During the Pandemic
Challenges Faced by Healthcare Systems During the Pandemic
6. The Role of Vaccination in Combating the Pandemic—Social Issues
| Area of Impact | Key Observations | Source |
|---|---|---|
| Disruption of immunization services | Over 68 countries experienced moderate to severe disruptions; 80 million+ children at risk of missing essential vaccines | Dinleyici et al. [42] |
| Causes of interruption | Lockdowns, parental fears, workforce reallocation, transport and logistics barriers | Dinleyici et al. [42] |
| Vaccine-preventable disease resurgence | Suspension of campaigns for measles, polio, diphtheria, etc.; outbreaks already observed in countries like Pakistan, Venezuela, and Nepal | Dinleyici et al. [42] |
| Decline in vaccine uptake | US data showed drops up to 63% in children > 2 years old; global trend of missed doses | Santoli et al. [44] |
| Recommendations from global agencies | WHO and UNICEF stressed the need to maintain or resume routine immunizations; emphasized risk–benefit balance | WHO [45] |
| Live vaccines and non-specific immunity | Hypothesized that BCG and oral polio vaccines may offer cross-protection via trained innate immunity | Chumakov et al. [46] |
| Vaccine hesitancy during the pandemic | Anti-vaccine misinformation spread rapidly; reduced trust in immunization programs may affect future uptake | Dinleyici et al. [42] |
| Postponed campaigns and outbreaks | Multiple outbreaks of measles and polio followed campaign suspensions; 178 million people at risk of missing measles vaccine | WHO [46] |
| Disparities in low-income countries | LMICs faced the greatest disruption due to weak health systems and conflict zones, with measles resurgence reported | Roberts et al. [43]; Hoffman et al. [54] |
| Protective potential of existing vaccines | BCG and OPV may offer non-specific protection through trained immunity, but evidence remains inconclusive | Chumakov et al. [46] |
| Call for catch-up programs | WHO recommends enumerating children who missed doses and developing customized catch-up vaccination plans | WHO [45] |
7. Impact of Nutritional Status
7.1. The Importance of Vitamins and Macronutrients in Immunity
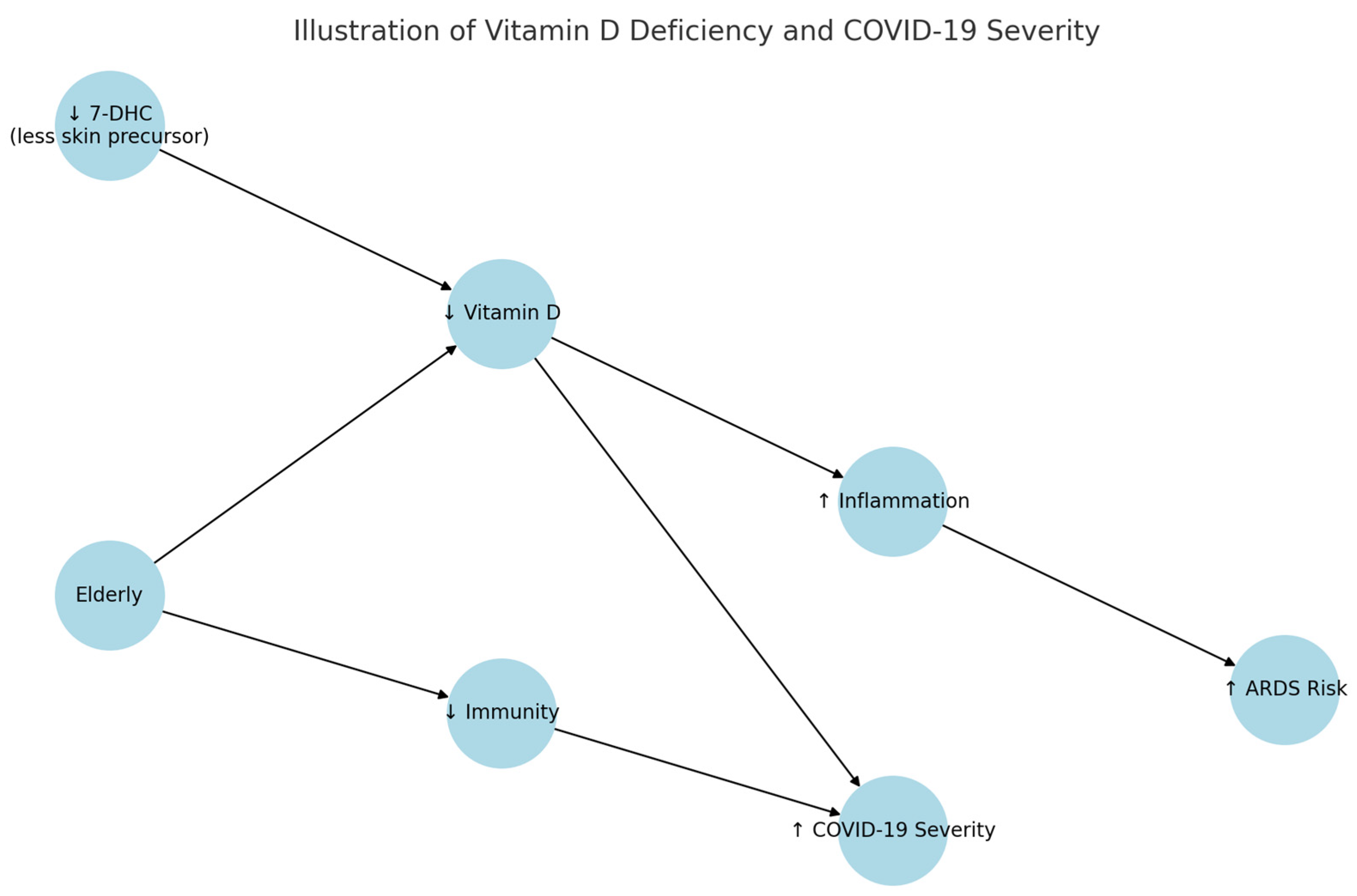
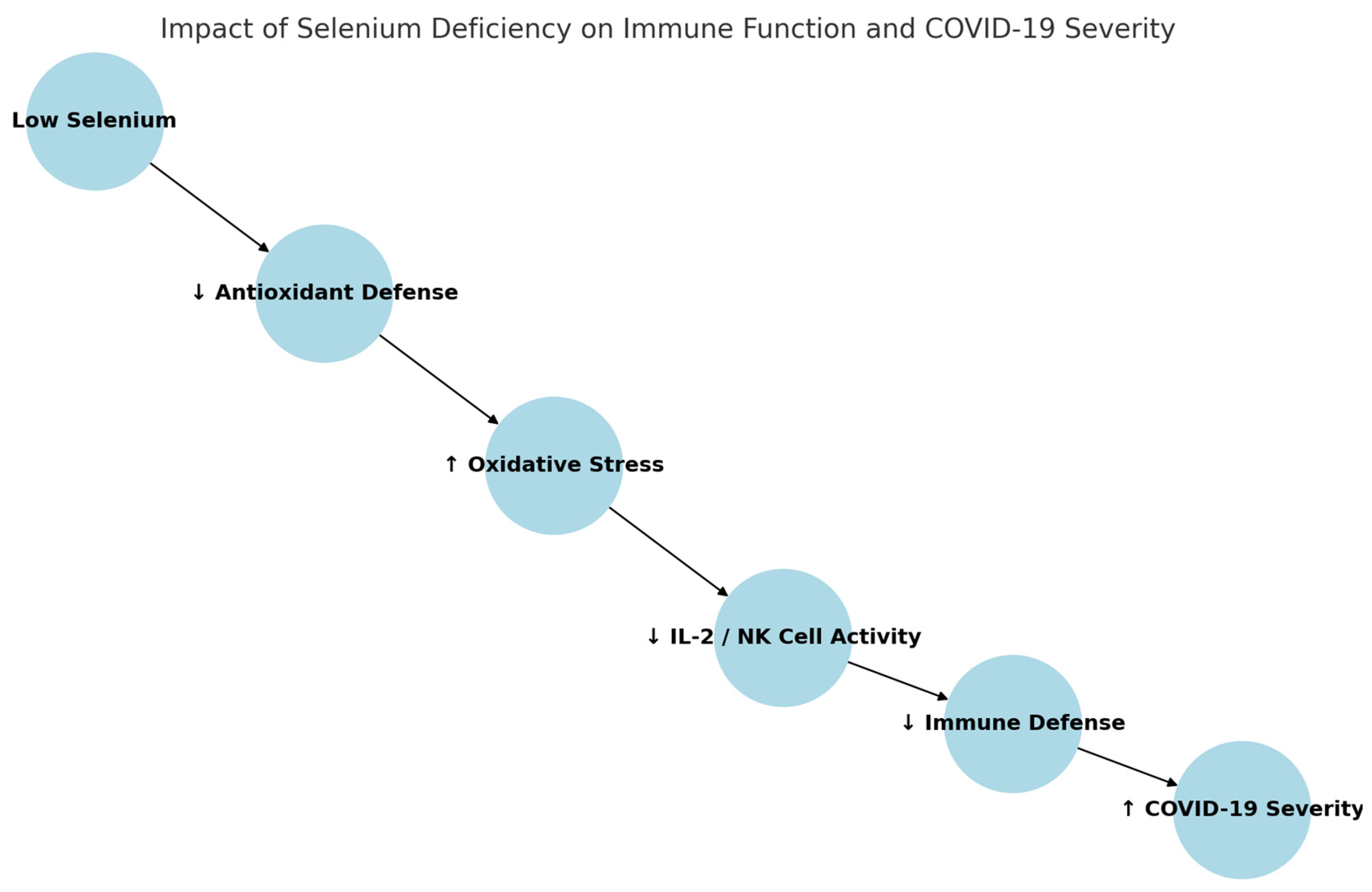
7.2. The Relationship Between Nutritional Deficiencies and the Course of COVID-19
| Study/Author | Population/Country | Deficiency Observed | Main Findings |
|---|---|---|---|
| Radujkovic et al. [8] | Hospitalized, Germany | Vit D | 21% mortality vs. 3.1% in sufficient group |
| Voelkle et al. [69] | Hospitalized, Switzerland | Selenium, Vit D, Vit A, Zinc | More deficiencies → longer stay and ICU |
| Im et al. [70] | COVID-19 patients, Korea | Vit D, Selenium | Most with respiratory distress were malnourished |
| Hamulka et al. [67] | Polish adults | Inconsistent supplement use | Fear-related spike, later decline |
| Zhang et al. [62] | China | Selenium | Higher selenium areas had better survival |
8. Impact of the Pandemic on Society
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, W.; Sun, B.; Huang, Y.; Glänzel, W. How scientific research reacts to international public health emergencies: A global analysis of response patterns. Scientometrics 2020, 124, 747–773. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Gavrilov, D.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; et al. COVID-19 Pandemic. Our World in Data. 2020. [Google Scholar]
- Chikwem, J.O.; Chikwem, U.J.; Chwikem, S.D.; Omuero, R. Impact of COVID-19 on Healthcare Workers and System; Lincoln University of Pennsylvania: Philadelphia, PA, USA, 2022. [Google Scholar]
- Zawilska, J.B.; Swaczyna, T.; Masiarek, P.; Waligórska, A.; Dominiak, Z. COVID-19: Epidemiology, Pathogenesis, Diagnostics, and Clinical Symptoms. Pathophysiology 2021, 77, 166–177. [Google Scholar]
- Luo, X.; Liao, Q.; Shen, Y.; Li, H.; Cheng, L. Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people. J. Nutr. 2021, 151, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhou, T.; Heianza, Y.; Qi, L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: A prospective study in UK Biobank. Am. J. Clin. Nutr. 2021, 113, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.; van den Worm, S.H.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.T.; Idid, S.Z. Can Zn be a critical element in COVID-19 treatment? Biol. Trace Elem. Res. 2021, 199, 550–558. [Google Scholar]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.; Silva, C.B.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.; et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Razzaque, M.S. COVID-19 pandemic: Can maintaining optimal zinc balance enhance host resistance? Tohoku J. Exp. Med. 2020, 251, 175–181. [Google Scholar]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar]
- Witkowski, J.M.; Bryl, E. Mechanisms of COVID-19 and the Immune System Aging. Kosmos Probl. Biol. Sci. 2021, 70, 407–417. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). SARS-CoV-2 Variant Classifications and Definitions. Available online: https://www.cdc.gov/covid/?CDC_AAref_Val=https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html (accessed on 31 August 2023).
- Gao, S.J.; Guo, H.; Luo, G. Omicron Variant (B.1.1.529) of SARS-CoV-2: A Global Urgent Public Health Alert! J. Med. Virol. 2021, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F.; Tang, Y.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron Variant of SARS-CoV-2: Genomics, Transmissibility, and Responses to Current COVID-19 Vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Rio Del, C.; Omer, S.B.; Malani, P.N. Winter of Omicron—The Evolving COVID-19 Pandemic. JAMA 2021, 327, 319–320. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Tracking SARS-CoV-2 Variants, January 2022. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 31 August 2023).
- Parums, D.V. Editorial: The 2022 World Health Organization (WHO) Priority Recommendations and Response to the Omicron Variant (B.1.1.529) of SARS-CoV-2. Med. Sci. Monit. 2022, 28, e936199-1. [Google Scholar] [CrossRef] [PubMed]
- Agency for Health Technology Assessment and Tariff System. COVID-19 Diagnostics: Updated Recommendations, Version 3.0; Agency for Health Technology Assessment and Tariff System: Warsaw, Poland, 2022.
- Van Elslande, J.; Houben, E.; Depypere, M.; Brackenier, A.; Desmet, S.; André, E.; Van Ranst, M.; Lagrou, K.; Vermeersch, P. Diagnostic Performance of Seven Rapid IgG/IgM Antibody Tests and the Euroimmun IgA/IgG ELISA in COVID-19 Patients. Clin. Microbiol. Infect. 2020, 26, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Olliaro, P.L.; Boeras, D.I.; Fongwen, N. Scaling Up COVID-19 Rapid Antigen Tests: Promises and Challenges. Lancet Infect. Dis. 2021, 21, e290–e295. [Google Scholar] [CrossRef]
- Giri, B.; Pandey, S.; Shrestha, R.; Pokharel, K.; Ligler, F.S.; Neupane, B.B. Review of Analytical Performance of COVID-19 Detection Methods. Anal. Bioanal. Chem. 2021, 413, 35–48. [Google Scholar] [CrossRef]
- Maia, R.; Carvalho, V.; Faria, B.; Miranda, I.; Catarino, S.; Teixeira, S.; Lima, R.; Minas, G.; Ribeiro, J. Diagnosis Methods for COVID-19: A Systematic Review. Micromachines 2022, 13, 1349. [Google Scholar] [CrossRef]
- European Commission—Directorate-General for Health and Food Safety. EU Common List of COVID-19 Antigen Tests, EU Digital COVID Certificate Regulation; European Commission—Directorate-General for Health and Food Safety: Brussels, Belgium, 2023. [Google Scholar]
- Ministry of Health. Updated Guidelines for Primary Care Nurses During the SARS-CoV-2 Epidemic. Available online: https://www.gov.pl/web/zdrowie/aktualizacja-wytycznych-do-stosowania-przez-pielegniarki-poz-w-czasie-epidemii-wirusa-sars-cov-2 (accessed on 31 August 2023).
- Ministry of Health. Guidelines for Specific Healthcare Services and Types of Medical Care. Available online: https://www.gov.pl/web/zdrowie/wytyczne-dla-poszczegolnych-zakresow-i-rodzajow-swiadczen (accessed on 31 August 2023).
- Mastelerz-Migas, A. Recommendations of the National Consultant in Family Medicine on October 29, 2021, Regarding Diagnostic Testing for SARS-CoV-2 in Primary Healthcare Facilities; Department and Chair of Family Medicine, Medical University of Wrocław: Wrocław, Poland, 2021. [Google Scholar]
- Act of 5 December 2008 on the prevention and combating of infections and infectious diseases in humans. Journal of Laws of the Republic of Poland, 2008, No. 234, item 1570, as amended.
- Announcement of the Minister of Justice of 21 October 2020 on the announcement of the uniform text of the regulation of the Minister of Justice on conducting a physical fitness test in the Prison Service. Journal of Laws of the Republic of Poland, 2020, item 1973, Poland.
- Announcement of the Speaker of the Sejm of the Republic of Poland of 20 January 2021 on the announcement of the uniform text of the Act on the State Sanitary Inspectorate. Journal of Laws of the Republic of Poland, 2021, item 195, as amended.
- Epidemic Prevention Guidelines—SARS-CoV-2. Available online: https://www.gov.pl/web/gis/wytyczne-przeciwepidemiczne--koronawirus-sars-cov-2 (accessed on 31 August 2023).
- Announcement of the Marshal of the Sejm of the Republic of Poland of 5 December 2018 on the announcement of the uniform text of the Act on the State Sanitary Inspectorate. Journal of Laws of the Republic of Poland, 2019, item 59.
- Closing Borders Due to Coronavirus. Available online: https://www.gov.pl/web/koronawirus/zamykamy-granice-przed-koronawirusem (accessed on 31 August 2023).
- Marcinkiewicz, K.; Nowak, P.; Popielec, D.; Wilk, M. Coronavirus as a Challenge for Modern Society: Media and Social Communication; Polish Society for Social Communication: Wrocław, Poland, 2020. [Google Scholar]
- “Final Stretch”! New Campaign of the National Vaccination Program. Available online: https://www.gov.pl/web/szczepimysie/ostatnia-prosta-nowa-kampania-narodowego-programu-szczepien (accessed on 31 August 2023).
- “COVID Passports” Until Mid-2023. Available online: https://poland.representation.ec.europa.eu/news/paszpoty-covidowe-do-polowy-2023-2022-02-03_pl (accessed on 31 August 2023).
- Diagnostic Guidelines for Reducing Epidemic Risk Related to COVID-19. Available online: https://www.mp.pl/covid19/diagnostyka/294913,zalecenia-postepowania-diagnostycznego-w-sytuacji-zmniejszenia-zagrozenia-epidemicznego-zwiazanego-z-covid-19 (accessed on 23 September 2023).
- Dymecka, J. Psychosocial Effects of the COVID-19 Pandemic. Neuropsychiatry Neuropsychol. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Du Laing, G.; Petrovic, M.; Lachat, C.; De Boevre, M.; Klingenberg, G.J.; Sun, Q.; De Saeger, S.; De Clercq, J.; Ide, L.; Vandekerckhove, L.; et al. Course and Survival of COVID-19 Patients with Comorbidities in Relation to the Trace Element Status at Hospital Admission. Nutrients 2021, 13, 3304. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Borrow, R.; Safadi, M.A.P.; van Damme, P.; Munoz, F.M. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum. Vaccines Immunother. 2021, 17, 400–407. [Google Scholar] [CrossRef]
- Roberts, L. Why measles deaths are surging—And coronavirus could make it worse. Nature 2020, 580, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Santoli, J.M. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 591–593. [Google Scholar] [CrossRef]
- WHO. Immunization in the Context of COVID-19 Pandemic. 2020. Available online: https://www.who.int/publications/i/item/immunization-in-the-context-of-covid-19-pandemic (accessed on 1 January 2025).
- Chumakov, K.; Benn, C.S.; Aaby, P.; Kottilil, S.; Gallo, R. Can existing live vaccines prevent COVID-19? Science 2020, 368, 1187–1188. [Google Scholar] [CrossRef] [PubMed]
- Okabe-Miyamoto, K.; Lyubomirsky, S. Social Connection and Well-Being During COVID-19; University of California: Riverside, CA, USA, 2021. [Google Scholar]
- VanderWeele, T.J.; Fulks, J.; Plake, J.F.; Lee, T. National Well-being Measures Before and During the COVID-19 Pandemic in Online Samples. J. Gen. Intern. Med. 2020, 3, 3–5. [Google Scholar]
- Fao, R.; Gilbert, S.; Fabian, M.O. COVID-19 and Subjective Well-Being: Separating the Effects of Lockdowns from the Pandemic. SSRN 2020. Available online: https://www.repository.cam.ac.uk/handle/1810/342845 (accessed on 1 January 2025).
- Recchi, E.; Ferragina, E.; Helmeid, E.; Pauly, S.; Safi, M.; Sauger, N.; Schradie, J. The “Eye of the Hurricane” Paradox: An Unexpected and Unequal Rise of Well-Being During the COVID-19 Lockdown in France. Res. Soc. Stratif. Mobil. 2020, 68, 100508. [Google Scholar]
- Folk, D.; Okabe-Miyamoto, K.; Dunn, E.; Lyubomirsky, S. Did Social Connection Decline During the First Wave of COVID-19? The Role of Extraversion. Collabra Psychol. 2020, 6, 37. [Google Scholar] [CrossRef]
- Hawryluck, L.; Gold, W.L.; Robinson, S.; Pogorski, S.; Galea, S.; Styra, R. SARS Control and Psychological Effects of Quarantine, Toronto, Canada. Emerg. Infect. Dis. 2004, 10, 1206–1212. [Google Scholar]
- Zhu, X.; Wu, S.; Miao, D.; Li, Y. Changes in Emotion of the Chinese Public in Regard to the SARS Period. Behav. Pers. Int. J. 2008, 36, 447–454. [Google Scholar] [CrossRef]
- Hoffmann, K.; Paczkowska, A.; Bońka, A.; Michalak, M.; Bryl, W.; Kopciuch, D.; Zaprutko, T.; Ratajczak, P.; Nowakowska, E.; Kus, K. Assessment of the Impact of the COVID-19 Pandemic on the Pro-Health Behavior of Poles. Int. J. Environ. Res. Public Health 2022, 19, 1299. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, O.W.; Czyżniewski, B.; Żukiewicz-Sobczak, W.; Gibas-Dorna, M. Nutritional Behavior in European Countries During the COVID-19 Pandemic—A Review. Nutrients 2023, 15, 3451. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-Boosting Role of Vitamins D, C, E, Zinc, Selenium, and Omega-3 Fatty Acids: Could They Help Against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Mikkelsen, K.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Stojanovska, L.; Apostolopoulos, V. Be well: A potential role for vitamin B in COVID-19. Maturitas 2021, 144, 108–111. [Google Scholar] [CrossRef]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID 19. Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef]
- Perera, M.; El Khoury, J.; Chinni, V.; Bolton, D.; Qu, L.; Johnson, P.; Trubiano, J.; McDonald, C.F.; Jones, D.; Bellomo, R.; et al. Randomised controlled trial for high-dose intravenous zinc as adjunctive therapy in SARS-CoV-2 (COVID-19) positive critically ill patients: Trial protocol. BMJ Open 2020, 10, e040580. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [PubMed]
- Rogero, M.M.; Leao, M.D.C.; Santana, T.M.; de MBPimentel, M.V.; Carlini, G.C.; da Silveira, T.F.; Goncalves, R.C.; Castro, I.A. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free. Radic. Biol. Med. 2020, 156, 190–199. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar]
- Wu, D.; Meydani, S.N. Vitamin E, immune function, and protection against infection. In Vitamin E in Human Health; Springer: Berlin/Heidelberg, Germany, 2019; pp. 371–384. [Google Scholar]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef] [PubMed]
- Sekulic, M.; Stajic, D.; Jurisic Skevin, A.; Kocovic, A.; Zivkovic Zaric, R.; Djonovic, N.; Vasiljevic, D.; Radmanovic, B.; Spasic, M.; Janicijevic, K.; et al. Lifestyle, Physical Activity, Eating, and Hygiene Habits: A Comparative Analysis Before and During the COVID-19 Pandemic in the Student Population. Front. Public Health 2022, 10, 862816. [Google Scholar] [CrossRef]
- Hamulka, J.; Jeruszka-Bielak, M.; Górnicka, M.; Drywień, M.E.; Zielinska-Pukos, M.A. Dietary Supplements During the COVID-19 Outbreak: Results of Google Trends Analysis Supported by PLifeCOVID-19 Online Studies. Nutrients 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Matacz, M. Factors Influencing Severe COVID-19 Progression—Increasing Knowledge. Pulmonology—Termedia 2021.
- Voelkle, M.; Gregoriano, C.; Neyer, P.; Koch, D.; Kutz, A.; Bernasconi, L.; Conen, A.; Mueller, B.; Schuetz, P. Prevalence of micronutrient deficiencies in patients hospitalized with COVID-19: An observational cohort study. Nutrients 2022, 14, 1862. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Je, Y.S.; Baek, J.; Chung, M.H.; Kwon, H.Y.; Lee, J.S. Nutritional Status of Patients with COVID-19. Int. J. Infect. Dis. 2020, 100, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Mullie, P.; Macacu, A.; Dragomir, M.; Boniol, M.; Coppens, K.; Pizot, C.; Boniol, M. Effect of Vitamin D Supplementation on Non-Skeletal Disorders: A Systematic Review of Meta-Analyses and Randomized Trials. Lancet Diabetes Endocrinol. 2017, 5, 986–1004. [Google Scholar] [CrossRef]
- Matacz, R.; Byrne, S.; Nosaka, K.; Priddis, L.; Finlay-Jones, A.; Lim, I.; Bloxsome, D.; Newman-Morris, V. Evaluation of the Pregnancy to Parenthood program: A dyadic intervention for mothers with perinatal mental disorders and their infants. Infant Ment. Health J. 2025, 46, 70–84. [Google Scholar] [PubMed]
- Długosz, P. Social Consequences of the COVID-19 Pandemic Among Poles; Pedagogical University of Kraków: Kraków, Poland, 2020. [Google Scholar]
- Gröber, U.; Holick, M.F. The coronavirus disease (COVID-19)–A supportive approach with selected micronutrients. Int. J. Vitam. Nutr. Res. 2021, 25, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Dowling, K.; Florentine, S. Can optimum solar radiation exposure or supplemented vitamin D intake reduce the severity of COVID-19 symptoms? Int. J. Environ. Res. Public Health 2021, 18, 740. [Google Scholar] [CrossRef] [PubMed]
| Test Type | Target | Sensitivity | Time to Result | Notes |
|---|---|---|---|---|
| RT-PCR | Viral RNA | High | Several hours | Gold standard, requires lab |
| Rapid Antigen | Viral proteins | Moderate | 15–30 min | Useful for symptomatic screening |
| ELISA/CLIA | IgG, IgM antibodies | Variable | 1–3 h | Used for seroprevalence |
| LFIA | IgG, IgM antibodies | Moderate | 15–30 min | Point-of-care serology |
| FET Biosensor | Virus activity | Very high | Rapid | Experimental, high sensitivity |
| Nutrient | Mechanism in Immunity | COVID-19 Impact | Sources |
|---|---|---|---|
| Vitamin D | Regulates cytokine response | Linked to ARDS, inflammatory control, low levels linked to higher severity | Sunlight, fatty fish |
| Vitamin C | Antioxidant, supports epithelial barrier | Reduces severity of ARDS, improves biomarkers, sepsis | Citrus fruits, vegetables |
| Zinc | Supports T cells and NK cells | Inhibits viral replication, enhances IL-2, linked to better outcomes | Meat, legumes |
| Selenium | Antioxidant, NK cell activation | Correlated with recovery rates, viral defense, deficiency associated with worse prognosis | Brazil nuts, fish |
| Omega-3 | Anti-inflammatory | Improves oxygenation, potential risks in excess | Fish oils, flaxseeds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olesińska, W.; Biernatek, M.; Lachowicz-Wiśniewska, S.; Piątek, J. Systematic Review of the Impact of COVID-19 on Healthcare Systems and Society—The Role of Diagnostics and Nutrition in Pandemic Response. J. Clin. Med. 2025, 14, 2482. https://doi.org/10.3390/jcm14072482
Olesińska W, Biernatek M, Lachowicz-Wiśniewska S, Piątek J. Systematic Review of the Impact of COVID-19 on Healthcare Systems and Society—The Role of Diagnostics and Nutrition in Pandemic Response. Journal of Clinical Medicine. 2025; 14(7):2482. https://doi.org/10.3390/jcm14072482
Chicago/Turabian StyleOlesińska, Wanda, Małgorzata Biernatek, Sabina Lachowicz-Wiśniewska, and Jacek Piątek. 2025. "Systematic Review of the Impact of COVID-19 on Healthcare Systems and Society—The Role of Diagnostics and Nutrition in Pandemic Response" Journal of Clinical Medicine 14, no. 7: 2482. https://doi.org/10.3390/jcm14072482
APA StyleOlesińska, W., Biernatek, M., Lachowicz-Wiśniewska, S., & Piątek, J. (2025). Systematic Review of the Impact of COVID-19 on Healthcare Systems and Society—The Role of Diagnostics and Nutrition in Pandemic Response. Journal of Clinical Medicine, 14(7), 2482. https://doi.org/10.3390/jcm14072482







