Clinical, Electrical, and Mechanical Parameters in Potassium Channel-Mediated Congenital Long QT Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. LQTS Mutation Carriers
2.2. Clinical Evaluation
2.3. Resting ECG
2.4. Brisk Standing ECG
2.5. Exercise Stress Test
2.6. Echocardiographical Data
2.7. Statistical Analysis
2.8. Ethical Approval and Informed Consent
3. Results
3.1. Clinical Evaluation
3.2. Resting ECG
3.3. Brisk Standing Test
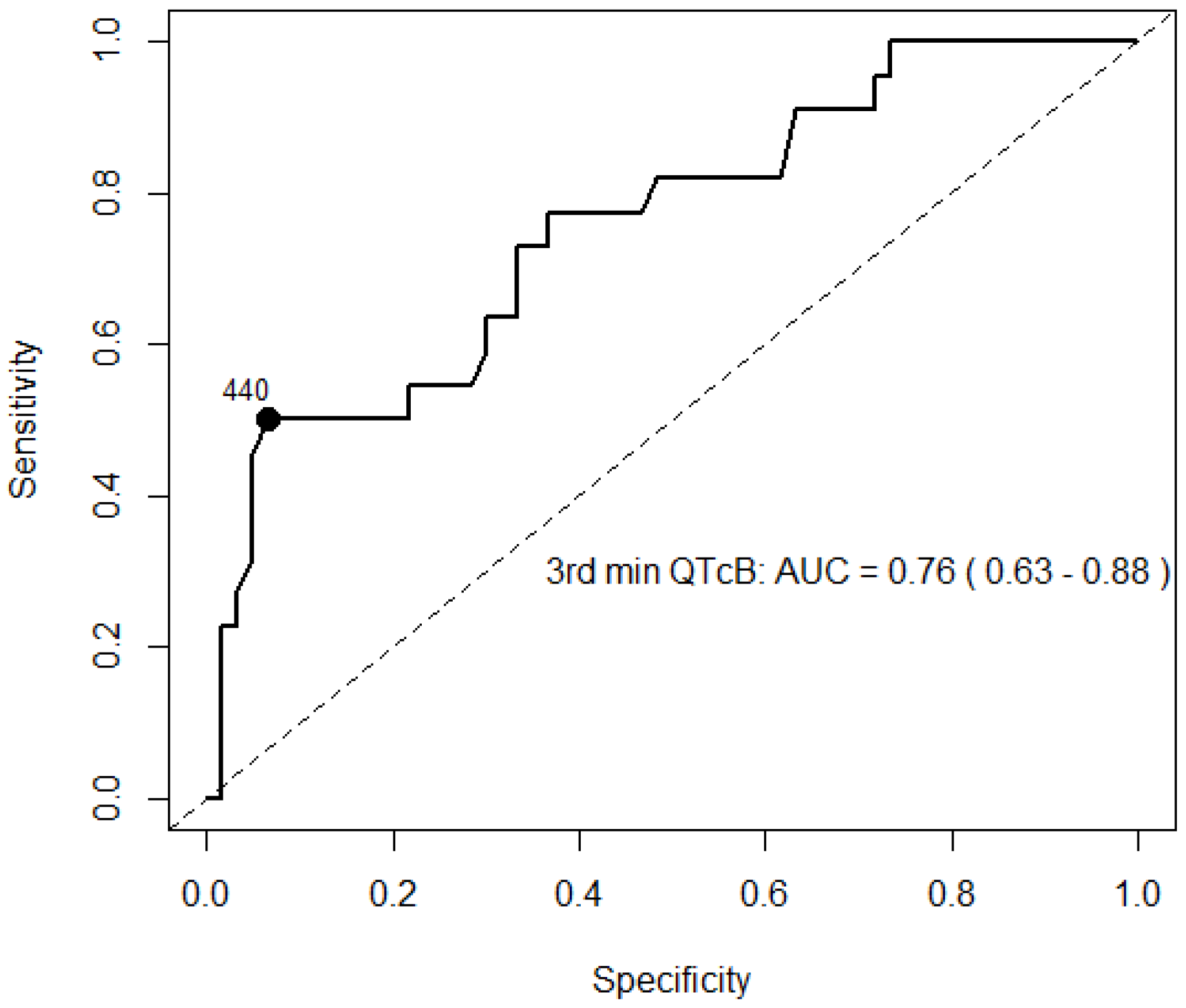
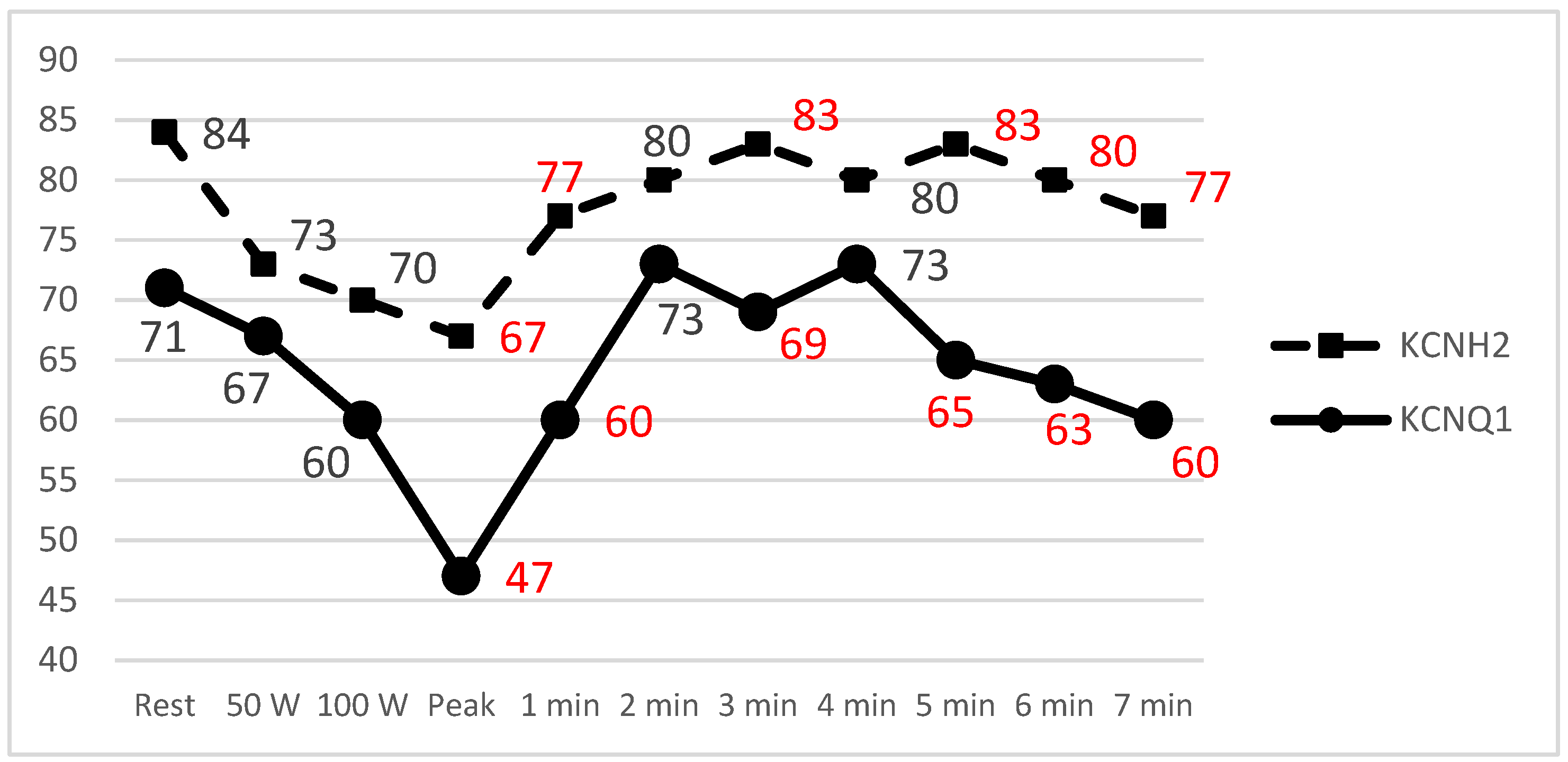
3.4. Exercise Stress Test
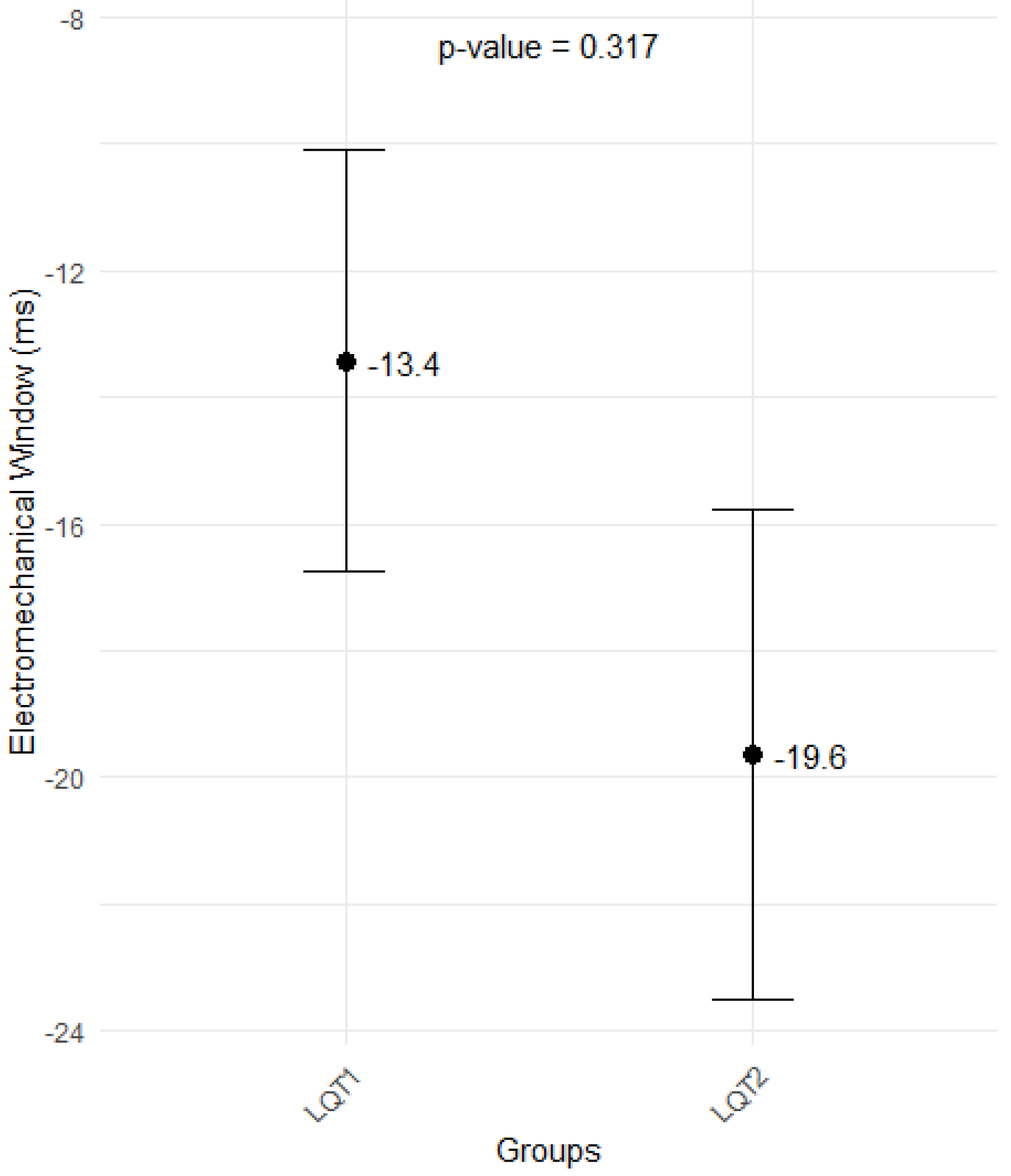
3.5. Echocardiographical Findings
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, P.J.; Stramba-Badiale, M.; Crotti, L.; Pedrazzini, M.; Besana, A.; Bosi, G.; Gabbarini, F.; Goulene, K.; Insolia, R.; Mannarino, S.; et al. Prevalence of the congenital long-QT syndrome. Circulation 2009, 120, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Leren, I.S.; Hasselberg, N.E.; Saberniak, J.; Håland, T.F.; Kongsgård, E.; Smiseth, O.A.; Edvardsen, T.; Haugaa, K.H. Cardiac Mechanical Alterations and Genotype Specific Differences in Subjects With Long QT Syndrome. JACC Cardiovasc. Imaging 2015, 8, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Odening, K.E.; van der Linde, H.J.; Ackerman, M.J.; Volders, P.G.A.; ter Bekke, R.M.A. Electromechanical reciprocity and arrhythmogenesis in long-QT syndrome and beyond. Eur. Heart J. 2022, 43, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, A.; van Zyl, M.; Enger, N.; Mancl, K.; Eidem, B.W.; Oh, J.K.; Bos, J.M.; Asirvatham, S.J.; Ackerman, M.J. Echocardiography-Guided Risk Stratification for Long QT Syndrome. J. Am. Coll. Cardiol. 2020, 76, 2834–2843. [Google Scholar] [CrossRef]
- Deissler, P.M.; Volders, P.G.A.; ter Bekke, R.M.A. The electromechanical window for arrhythmia-risk assessment. Heart Rhythm 2025, 22, 118–127. [Google Scholar] [CrossRef]
- Borowiec, K.; Kowalski, M.; Kumor, M.; Duliban, J.; Śmigielski, W.; Hoffman, P.; Biernacka, E.K. Prolonged left ventricular contraction duration in apical segments as a marker of arrhythmic risk in patients with long QT syndrome. EP Eur. 2020, 22, 1279–1286. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Amlie, J.P.; Berge, K.E.; Leren, T.P.; Smiseth, O.A.; Edvardsen, T. Transmural differences in myocardial contraction in long-QT syndrome: Mechanical consequences of ion channel dysfunction. Circulation 2010, 122, 1355–1363. [Google Scholar] [CrossRef]
- Rieder, M.; Kreifels, P.; Stuplich, J.; Ziupa, D.; Servatius, H.; Nicolai, L.; Castiglione, A.; Zweier, C.; Asatryan, B.; Odening, K.E. Genotype-Specific ECG-Based Risk Stratification Approaches in Patients With Long-QT Syndrome. Front. Cardiovasc. Med. 2022, 9, 916036. [Google Scholar] [CrossRef]
- Panikkath, R.; Reinier, K.; Uy-Evanado, A.; Teodorescu, C.; Hattenhauer, J.; Mariani, R.; Gunson, K.; Jui, J.; Chugh, S.S. Prolonged Tpeak to Tend Interval on the Resting Electrocardiogram Is Associated with Increased Risk of Sudden Cardiac Death. Circ. Arrhythm. Electrophysiol. 2011, 4, 441–447. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Shimizu, M.; Ino, H.; Terai, H.; Uchiyama, K.; Oe, K.; Mabuchi, T.; Konno, T.; Kaneda, T.; Mabuchi, H. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: A new index for arrhythmogenicity. Clin. Sci. 2003, 105, 671–676. [Google Scholar] [CrossRef]
- Wong, J.A.; Gula, L.J.; Klein, G.J.; Yee, R.; Skanes, A.C.; Krahn, A.D. Utility of treadmill testing in identification and genotype prediction in long-QT syndrome. Circ. Arrhythm. Electrophysiol. 2010, 3, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Horner, J.M.; Horner, M.M.; Ackerman, M.J. The diagnostic utility of recovery phase QTc during treadmill exercise stress testing in the evaluation of long QT syndrome. Heart Rhythm 2011, 8, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.D.; Asaki, S.Y.; Mazzanti, A.; Bos, J.M.; Tuleta, I.; Muir, A.R.; Crotti, L.; Krahn, A.D.; Kutyifa, V.; Shoemaker, M.B.; et al. An International Multicenter Evaluation of Type 5 Long QT Syndrome: A Low Penetrant Primary Arrhythmic Condition. Circulation 2020, 141, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Patel, C.; Patel, H.; Narayanaswamy, S.; Malhotra, B.; Green, J.T.; Yan, G.-X. T(p-e)/QT ratio as an index of arrhythmogenesis. J. Electrocardiol. 2008, 41, 567–574. [Google Scholar] [CrossRef]
- Statters, D.J.; Malik, M.; Ward, D.E.; Camm, A.J. QT Dispersion: Problems of Methodology and Clinical Significance. J. Cardiovasc. Electrophysiol. 1994, 5, 672–685. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Abrahams, T.; Davies, B.; Laksman, Z.; Sy, R.W.; Postema, P.G.; Wilde, A.A.M.; Krahn, A.D.; Han, H.-C. Provocation testing in congenital long QT syndrome: A practical guide. Heart Rhythm 2023, 20, 1570–1582. [Google Scholar] [CrossRef]
- Viskin, S.; Postema, P.G.; Bhuiyan, Z.A.; Rosso, R.; Kalman, J.M.; Vohra, J.K.; Guevara-Valdivia, M.E.; Marquez, M.F.; Kogan, E.; Belhassen, B.; et al. The response of the QT interval to the brief tachycardia provoked by standing: A bedside test for diagnosing long QT syndrome. J. Am. Coll. Cardiol. 2010, 55, 1955–1961. [Google Scholar] [CrossRef]
- Nyberg, J.; Jakobsen, E.O.; Østvik, A.; Holte, E.; Stølen, S.; Lovstakken, L.; Grenne, B.; Dalen, H. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. JACC Cardiovasc. Imaging 2023, 16, 1516–1531. [Google Scholar] [CrossRef]
- Younis, A.; Bos, J.M.; Zareba, W.; Aktas, M.K.; Wilde, A.A.M.; Tabaja, C.; Bodurian, C.; Tobert, K.E.; McNitt, S.; Polonsky, B.; et al. Association Between Syncope Trigger Type and Risk of Subsequent Life-Threatening Events in Patients With Long QT Syndrome. JAMA Cardiol. 2023, 8, 775–783. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Ackerman, M.J. The long QT syndrome: A transatlantic clinical approach to diagnosis and therapy. Eur. Heart J. 2013, 34, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Moennig, G.; Schulze-Bahr, E.; Wedekind, H.; Borggrefe, M.; Funke, H.; Toelle, M.; Kirchhof, P.; Eckardt, L.; Assmann, G.; Breithardt, G.; et al. Clinical value of electrocardiographic parameters in genotyped individuals with familial long QT syndrome. Pacing Clin. Electrophysiol. 2001, 24 Pt 1, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Kutyifa, V.; Daimee, U.A.; McNitt, S.; Polonsky, B.; Lowenstein, C.; Cutter, K.; Lopes, C.; Zareba, W.; Moss, A.J. Clinical aspects of the three major genetic forms of long QT syndrome (LQT1, LQT2, LQT3). Ann. Noninvasive Electrocardiol. 2018, 23, e12537. [Google Scholar] [CrossRef] [PubMed]
- Aziz, P.F.; Wieand, T.S.; Ganley, J.; Henderson, J.; Patel, A.R.; Iyer, V.R.; Vogel, R.L.; McBride, M.; Vetter, V.L.; Shah, M.J. Genotype- and mutation site-specific QT adaptation during exercise, recovery, and postural changes in children with long-QT syndrome. Circ. Arrhythm. Electrophysiol. 2011, 4, 867–873. [Google Scholar] [CrossRef]
- Chua, K.C.M.; Rusinaru, C.; Reinier, K.; Uy-Evanado, A.; Chugh, H.; Gunson, K.; Jui, J.; Chugh, S.S. Tpeak-to-Tend interval corrected for heart rate: A more precise measure of increased sudden death risk? Heart Rhythm 2016, 13, 2181–2185. [Google Scholar] [CrossRef]
- Krijger Juárez, C.; Amin, A.S.; Offerhaus, J.A.; Bezzina, C.R.; Boukens, B.J. Cardiac Repolarization in Health and Disease. JACC Clin. Electrophysiol. 2023, 9, 124–138. [Google Scholar] [CrossRef]
- Viitasalo, M.; Oikarinen, L.; Swan, H.; Väänänen, H.; Glatter, K.; Laitinen, P.J.; Kontula, K.; Barron, H.V.; Toivonen, L.; Scheinman, M.M. Ambulatory electrocardiographic evidence of transmural dispersion of repolarization in patients with long-QT syndrome type 1 and 2. Circulation 2002, 106, 2473–2478. [Google Scholar] [CrossRef]
- Pérez-Riera, A.R.; Barbosa-Barros, R.; Shenasa, M. Electrocardiographic Markers of Sudden Cardiac Death (Including Left Ventricular Hypertrophy). Card. Electrophysiol. Clin. 2017, 9, 605–629. [Google Scholar] [CrossRef]
- Kulkarni, V.K.; Pinsky, A.M.; Bos, J.M.; Neves, R.; Bains, S.; Giudicessi, J.R.; Allison, T.G.; Ackerman, M.J. Frequency and Genotype-Dependence of intrinsic chronotropic insufficiency among patients with congenital long QT syndrome. J. Cardiovasc. Electrophysiol. 2025, 36, 17–23. [Google Scholar] [CrossRef]
- Krahn, A.D.; Laksman, Z.; Sy, R.W.; Postema, P.G.; Ackerman, M.J.; Wilde, A.A.M.; Han, H.-C. Congenital Long QT Syndrome. JACC Clin. Electrophysiol. 2022, 8, 687–706. [Google Scholar] [CrossRef]
- Chan, C.-H.; Hu, Y.-F.; Chen, P.-F.; Wu, I.-C.; Chen, S.-A. Exercise Test for Patients with Long QT Syndrome. Acta Cardiol. Sin. 2022, 38, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Hekkala, A.-M.; Viitasalo, M.; Väänänen, H.; Swan, H.; Toivonen, L. Abnormal repolarization dynamics revealed in exercise test in long QT syndrome mutation carriers with normal resting QT interval. Europace 2010, 12, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, W.; Antzelevitch, C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade des pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation 1997, 96, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.J.; Selvaraj, S.; Aguilar, F.G.; Martinez, E.E.; Wilcox, J.E.; Passman, R.; Goldberger, J.J.; Freed, B.H.; Shah, S.J. Relationship Between Repolarization Heterogeneity and Abnormal Myocardial Mechanics. Int. J. Cardiol. 2014, 172, 289–291. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Rider, O.; Cvijic, M.; Valkovič, L.; Remme, E.W.; Voigt, J.-U. Myocardial Strain Imaging: Theory, Current Practice, and the Future. JACC Cardiovasc. Imaging 2025, 18, 340–381. [Google Scholar] [CrossRef]
- Abdelsayed, M.; Bytyçi, I.; Rydberg, A.; Henein, M.Y. Left Ventricular Contraction Duration Is the Most Powerful Predictor of Cardiac Events in LQTS: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2820. [Google Scholar] [CrossRef]


| Gene | Mutation Type | Nucleotide Change | No. of Participants |
|---|---|---|---|
| KCNQ1 (NM_000218.3) | Missense | c.701A>C | 1 |
| Missense | c.1831G>A | 1 | |
| Missense | c.568C>T | 1 | |
| Missense | c.1111G>C | 9 | |
| Splicing | c.477+1G>A | 34 | |
| Deletion | c.1265del | 8 | |
| Missense | c.502G>A | 1 | |
| Missense | c.674C>T | 2 | |
| Deletion | c.790delA | 3 | |
| Nonsense | c.513C>A | 2 | |
| KCNH2 (NM_000238.4) | Missense | c.243G>C | 2 |
| Deletion | c.321_322del | 2 | |
| Missense | c.526C>T | 2 | |
| Deletion | c.681delC | 2 | |
| Missense | c.859G>T | 1 | |
| Missense | c.1141G>T | 1 | |
| Missense | c.1600C>T | 6 | |
| Missense | c.1750G>A | 2 | |
| Missense | c.1832A>T | 1 | |
| Missense | c.1849T>G | 1 | |
| Missense | c.1891T>C | 2 | |
| Missense | c.2453C>T | 2 | |
| Deletion | c.3103_3152+6del | 1 |
| LQT1 (n = 62) | LQT2 (n = 25) | p Value | |
|---|---|---|---|
| Age, y | 37.0 (23.0, 44.8) | 40.0 (28.0, 46.0) | 0.6 |
| Female, n (%) | 36 (58.1%) | 17 (68.0%) | 0.4 |
| Probands, n (%) | 21 (33.9%) | 11 (44.0%) | 0.4 |
| HR, bpm | 72 (64, 78) | 73 (65, 83) | 0.8 |
| Hypertension, n (%) | 10 (16.1%) | 5 (20.0%) | 0.8 |
| Systolic BP, mmHg | 130 ± 15 | 127 ± 12 | 0.3 |
| Diastolic BP, mmHg | 83 ± 11 | 82 ± 8 | 0.6 |
| Premature ventricular contractions, n (%) | 12 (19.4%) | 11 (44.0%) | 0.02 |
| Palpitations, n (%) | 13 (21.0%) | 9 (36.0%) | 0.1 |
| Penetration ECG, n (%) | 9 (14.5%) | 5 (20.0%) | 0.5 |
| Syncope, n (%) | 20 (32.3%) | 17 (68.0%) | 0.002 |
| Presyncope, n (%) | 11 (17.7%) | 12 (48.0%) | 0.004 |
| Aborted cardiac arrest, n (%) | 1 (1.6%) | 2 (8.0%) | 0.2 |
| Ventricular tachycardia, n (%) | 3 (12.0%) | 1 (1.6%) | 0.07 |
| SCD in 1st-degree relatives, n (%) | 17 (27.4%) | 8 (32.0%) | 0.7 |
| Syncope in 1st-degree relatives, n (%) | 12 (19.4%) | 13 (52.0%) | 0.01 |
| Seizures in family members, n (%) | 3 (4.8%) | 3 (12.0%) | 0.3 |
| LQTS in relatives, n (%) | 40 (64.5%) | 14 (56.0%) | 0.5 |
| BNP, ng/L | 12.5 (0.0, 19.2) | 14.0 (0.0, 23.0) | 0.2 |
| Potassium, mmol/L | 4.5 (4.3, 4.7) | 4.6 (4.3, 4.8) | 0.5 |
| Without beta-blocker during clinical evaluation, n (%) | 55 (89%) | 25 (100%) | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bileišienė, N.; Mikštienė, V.; Preikšaitienė, E.; Kažukauskienė, I.; Tarutytė, G.; Zakarkaitė, D.; Kramena, R.; Marinskis, G.; Aidietis, A.; Barysienė, J. Clinical, Electrical, and Mechanical Parameters in Potassium Channel-Mediated Congenital Long QT Syndrome. J. Clin. Med. 2025, 14, 2540. https://doi.org/10.3390/jcm14082540
Bileišienė N, Mikštienė V, Preikšaitienė E, Kažukauskienė I, Tarutytė G, Zakarkaitė D, Kramena R, Marinskis G, Aidietis A, Barysienė J. Clinical, Electrical, and Mechanical Parameters in Potassium Channel-Mediated Congenital Long QT Syndrome. Journal of Clinical Medicine. 2025; 14(8):2540. https://doi.org/10.3390/jcm14082540
Chicago/Turabian StyleBileišienė, Neringa, Violeta Mikštienė, Eglė Preikšaitienė, Ieva Kažukauskienė, Gabrielė Tarutytė, Diana Zakarkaitė, Rita Kramena, Germanas Marinskis, Audrius Aidietis, and Jūratė Barysienė. 2025. "Clinical, Electrical, and Mechanical Parameters in Potassium Channel-Mediated Congenital Long QT Syndrome" Journal of Clinical Medicine 14, no. 8: 2540. https://doi.org/10.3390/jcm14082540
APA StyleBileišienė, N., Mikštienė, V., Preikšaitienė, E., Kažukauskienė, I., Tarutytė, G., Zakarkaitė, D., Kramena, R., Marinskis, G., Aidietis, A., & Barysienė, J. (2025). Clinical, Electrical, and Mechanical Parameters in Potassium Channel-Mediated Congenital Long QT Syndrome. Journal of Clinical Medicine, 14(8), 2540. https://doi.org/10.3390/jcm14082540







